Abstract
Aim
To test the hypothesis that hyper-caloric diet on mechanisms of angiotensin (Ang)II-induced vasoconstriction in Dahl salt-sensitive (SS) rats and genetic control SS-13BN rats.
Methods
Aortic function was assessed using wire myography in 16-week-old rats maintained on a normal diet or started on a 4-week hyper-caloric diet at 12-weeks-old.
Results
On normal diet, AngII vasoconstriction was greater in SS rats than SS-13BN rats. Intriguingly, the AngII response was reduced in aortic rings from SS rats on hyper-caloric diet versus normal diet, whereas this response was not altered by hyper-caloric diet in SS-13BN rats. We probed whether O2−, H2O2, or NO mediate the AngII response. PEG-SOD reduced the AngII response in all 4 groups. Catalase treatment of aortic rings did not alter aortic AngII response from SS rats on hyper-caloric but reduced the aortic AngII response of SS rats on normal diet; the exact opposite finding was observed with catalase in SS-13BN rats. L-NAME increased AngII response in SS rats on hyper-caloric but was without effect in the normal diet group. In stark contrast, L-NAME did not alter the AngII response in SS-13BN rats on hyper-caloric diet, yet L-NAME enhanced AngII responsiveness in the normal diet group. In SS rats, hyper-caloric diet increased aortic NOS3 activity and expression corresponding with increased endothelial-dependent vasorelaxation.
Conclusion
A short-term hyper-caloric diet elicits a vasodilatory phenotype in SS rats, but not in SS-13BN rats, by increasing NOS3 expression and function as well as reducing H2O2 function.
Keywords: Angiotensin, Dahl salt-sensitive, nitric oxide synthase, SS-13BN, reactive oxygen species
Introduction
Angiotensin (Ang)II plays a dual role in regulating vascular tone (Zhang et al., 2003). Classically, AngII mediates vasoconstriction via the AngII type 1 receptor (AT1R) (Loria et al., 2011b, Oddie et al., 1993, Ryan et al., 2004, Zhou et al., 2003b, Moltzer et al., 2010, Sparks et al., 2011) and vasorelaxation when signaling through the AT2R (Tsutsumi et al., 1999, Loria et al., 2011b). Regulation of nitric oxide synthase (NOS) function links the vasoconstrictor and vasodilator actions of AngII. AT1R activation produces reactive oxygen species (ROS) i.e., superoxide (O2·−) and hydrogen peroxide (H2O2) from the endothelium, vascular smooth muscle cells, and fibroblasts (Rueckschloss et al., 2002, Zafari et al., 1998), which induces a pro-oxidant environment in the vessel wall that subsequently quenches NO-mediated vasodilation (Dikalova et al., 2010). Both the AT1R (Suzuki et al., 2006) and AT2R (Zhang et al., 2003) activate NOS. Because NOS functionally buffers vasoconstriction in healthy blood vessels (Loria et al., 2011b, Sauzeau et al., 2010), it is critical to understand the functional regulation of AngII-NOS signaling on vascular homeostasis in the pathogenesis of cardiovascular disease.
The large artery disease, atherosclerosis, is a causative factor in cardiovascular disease, the leading cause of death worldwide (Pepine, 2001, Vaartjes et al., 2009). In experimental settings of pro-atherosclerosis models, such as hyper-caloric diet feeding in ApoE−/− or LDLr−/− mice, AT1R promotes (Koga et al., 2008, Wassmann et al., 2004) and the AT2R protects against atherosclerosis (Iwai et al., 2005, Tesanovic et al., 2010). Additionally in these models, the expression of anti-atherosclerotic endothelial NOS (NOS3) is reduced (Wever et al., 1998, Tesanovic et al., 2010). Reports of hyper-caloric feeding in non-genetically manipulated animal models demonstrate a pro-contractile vascular phenotype. In Sprague-Dawley rats, 4 weeks of hyper-caloric diet increased AngII-mediated aortic vasoconstriction (Ghatta and Ramarao, 2004). In dogs, 6 weeks of hyper-caloric diet enhances coronary arteriolar AngII response with no change in AT1R or AT2R expression (Zhang et al., 2005). Moreover, C57BL/6 mice on hyper-caloric diet for 15–30 weeks have increased aortic AngII response linked to increased AT1R expression and reduced NOS buffering capacity (Yang et al., 1998, Mundy et al., 2007, Zhang et al., 2005, Bhattacharya et al., 2008, Barton et al., 2000).
The Dahl salt-sensitive (SS) rat strain is a model of polygenetic cardiovascular disease (Mattson et al., 2008) and mimics the progression of salt-sensitive hypertension in humans (Herrera et al., 2001, Wendt et al., 2009). This rat strain presents heightened cardiovascular disease risk under normal diet conditions (Poyan Mehr et al., 2003, Garrett et al., 2003). On a normal diet, we found greater AngII reactivity along with a lack of NOS buffering of AngII response in aortas from SS rats compared to SS-13BN genetic control rats. Based on this finding, we hypothesized that hyper-caloric diet induces an increase in the vasoconstrictive action of AngII via increased ROS function and/or reduced NOS buffering capacity in SS rats but not SS-13BN rats. Contrary to this hypothesis, a 4-week hyper-caloric diet drastically blunted AngII-mediated aortic constriction in SS rats but no change in SS-13BN rats. This finding changed the scope of our work. Our hypothesis was redirected to examine if reductions in pro-contractile ROS and anti-contractile NOS mediate blunted aortic AngII reactivity in SS rats on a hyper-caloric diet.
Methods
Animals
Our studies conformed to the Good Publishing Practice in Physiology (Persson and Henriksson, 2011). Male Dahl salt-sensitive (SS) rats and SS-13BN rats were purchased from Charles River Laboratories (Wilmington, MA). Upon arrival at our institution at 10–11 weeks old, both rat strains were started on Teklad 8604 rodent diet and water provided ad libitum. The Teklad diet was used as the normal diet in our study and consisted of calories from: 33% protein (source: soy, fish meal, wheat, corn, yeast, molasses, whey), 53% carbohydrates (source: corn, wheat, soy, molasses, whey, yeast), and 14% fat (source: soy, corn, wheat, fish) with 3.93 kcal/g gross energy and 0.4% sodium. At 12 weeks old, rats from each strain were randomly placed on hyper-caloric (BioServ F2685, Frenchtown, NJ) or selected to remain on the normal diet. The hyper-caloric diet consisted of calories from: 15% protein (source: casein), 27% carbohydrates (source: corn and sucrose) and 59% fat (source: lard) with 5.45 kcal/g gross energy and 0.4% sodium.
At 16 weeks old, rats were euthanized with pentobarbital sodium (Nembutal; 0.5 mg/kg; Abbott Laboratories, Abbott Park, IL). Whole blood was collected in ice-cold syringes primed with EDTA (Sigma, St. Louis, MO), centrifuged at 3000xg at 4°C for 10 minutes, and plasma was snap-frozen in liquid N2 and stored at −80°C until analyzed. Thoracic aortas were isolated for vascular reactivity studies or snap frozen for Western blotting as detailed below.
Systolic blood pressure measurements
Tail-cuff plethysmyography was utilized in 16-week old SS-13BN rats and SS rats on normal diet and hyper-caloric diet groups as previously described (Kang et al., 2011).
Plasma assays
Colorimetric assays were used to assess plasma total cholesterol (Cayman Chemical, Ann Arbor, MI), triglycerides (Cayman), and free fatty acids (Zen-Bio, Inc., Research Triangle Park, NC). Plasma renin activity (PRA) was assessed via radioimmunoassay (DiaSorin, Saluggia, Italy).
Aortic vascular reactivity
Thoracic aortas were prepared for wire myography (Danish Myo Technology A/S, Aarhus, Denmark) as previously described (Spradley et al., 2011). Rings were incubated for 15 minutes in the presence or absence of polyethylene glycol superoxide dismutase (PEG-SOD; 200 U/mL; Sigma), catalase (1000 U/mL; Roche Diagnostics, Indianapolis, IN) or the non-selective NOS inhibitor Nω-Nitro-L-arginine methyl ester (L-NAME; 100 μM; Sigma) followed by generation of cumulative-concentration response curves to AngII (1 x 10−11 to 1 x 10−7 M; Phoenix Pharmaceuticals, Inc., Burlingame, CA). We previously demonstrated this concentration response range elicits a maximal response to AngII-mediated aortic vasoconstriction (Loria et al., 2011a), and a pilot study for our present investigation showed this concentration response range elicits maximal AngII response without provoking tachyphylaxis (data not shown). Vessel segments were washed and stabilized for 30 min with PSS and KCl (8 x 10−3 to 100 x 10−3 M) response curves performed as previously described (Spradley et al., 2011). Separate rings were incubated in the presence or absence of L-NAME and response curves generated to acetylcholine (ACh; 1 x 10−9 to 3 x 10−5 M; Sigma); vessel segments were washed and stabilized for 30 min with PSS and sodium nitroprusside (SNP; 1 x 10−10 to 3 x 10−5 M) response curves performed.
AT1R and NOS3 expression and NOS activity measurements
In a different set of SS rats on normal diet or hyper-caloric diet, whole thoracic aortas were used for Western blotting as previously described (Spradley et al., 2011). Membranes were probed using antibodies for anti-NOS3 (1:500; BD Biosciences, San Jose, CA), anti-AT1R (1:5000; Santa Cruz Biotechnology, CA), and anti-β-actin (1:20,000; Sigma). Secondary antibodies were used to detect AT1R and NOS3 antibody (goat anti-mouse 1:1000; Invitrogen, Carlsbad, CA) and the β-actin antibody (goat anti-rabbit 1:10,000; Invitrogen). When analyzed, AT1R and NOS expression was normalized to corresponding β-actin densities.
In a different set of SS rats on normal diet or hyper-caloric diet, thoracic aortas were processed for the L-[3H]arginine-to-L-[3H]citrulline conversion assay and NOS isoform activities defined as described previously (Loria et al., 2011b).
Statistical analyses
Data are expressed as mean±standard error. Data were graphed and statistically analyzed using GraphPad Prism (La Jolla, CA). The metabolic parameters in Table 1 were analyzed using 2-way ANOVA. The remainder of the data was analyzed using Student’s t-test or 2-way ANOVA. P<0.05 was considered statistically significant.
Table 1.
Metabolic parameters measured at 16 weeks old in SS-13BN rats and SS rats on normal diet or 4 weeks of hyper-caloric diet.
| Parameter | SS-13BN
|
SS
|
||
|---|---|---|---|---|
| Normal | Hyper-caloric | Normal | Hyper-caloric | |
| Body Weight (g) | 368±16 (6) | 393±12 (6)* | 366±7 (6) | 402±12 (6)† |
| Epididymal Fat/BW (mg/g) | 9±1 (4) | 19±2 (6)* | 3±0.1 (3)* | 5±0.4 (5)† |
| Total Cholesterol (mg/dL) | 127±12 (6) | 171±6 (6)* | 168±9(6)* | 168±8 (6) |
| Triglycerides | 120±12 (6) | 211±27 (5)* | 132±11 (5) | 190±17 (5)† |
| Free Fatty Acids | 15±1 (6) | 42±8 (5)* | 17±2 (6) | 46±7 (6)† |
| PRA (ng Ang1/mL/h) | 4.8±0.6 (4) | 9.7±2.7 (4)* | 6.9±1.0 (5) | 5.5±0.4 (6) |
| Food Intake (g/day) | 24±1 (6) | 12±0.4 (6)* | 23±1 (6) | 12±1 (6)† |
| SBP (mmHg, Tail-Cuff) | 135±6 (6) | 136±3 (6) | 174±5 (6)* | 176±6 (6) |
PRA = plasma renin activity; SBP = systolic blood pressure.
P<0.05 vs. SS-13BN rats on normal diet;
P<0.05 vs. SS rats on normal diet. Number of rats is in parentheses.
Results
Metabolic parameters
Table 1 details the metabolic parameters of SS-13BN rats and SS rats maintained on a normal diet until 16 weeks old or a 4-week hyper-caloric diet started at 12 weeks old. Body weight increased similarly in SS-13BN and SS rats with hyper-caloric diet and was not different under normal diet conditions in both rat strains. Epididymal fat mass was increased with hyper-caloric diet in both rat strains but fat mass was greater in SS-13BN rats than SS rats in both diet groups. Hyper-caloric diet increased plasma total cholesterol only in SS-13BN rats, whereas free fatty acids and triglycerides were increased in both rat strains. Hyper-caloric diet increased plasma renin activity in SS-13BN rat but not SS rats. Food intake was less in hyper-caloric diet groups but was not different between rat strains or in normal diet groups.
Systolic blood pressure
SBP was assessed at 16 weeks old in SS-13BN rats and SS rats on normal diet or hyper-caloric diet (Table 1). In both rat strains, SBP was similar in normal diet and hyper-caloric groups; however under normal diet conditions, SBP was greater in SS rats than SS-13BN rats.
Assessment of AngII function using aortic rings
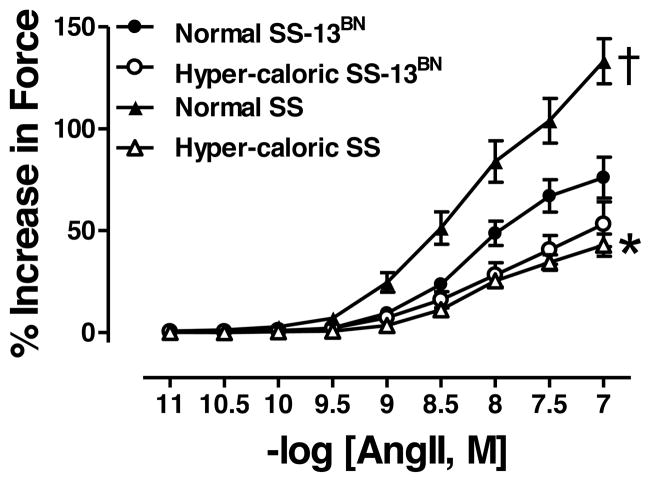
Maximum response (Emax) to AngII was similar in SS-13BN rats on normal diet or hyper-caloric diet (Figure 1, Table 2). In contrast, Emax was reduced in aortas from SS rats on hyper-caloric diet compared to normal diet counterparts (Figure 1, Table 2). On normal diet, Emax response to AngII was greater in SS rats than SS-13BN rats (Figure 1, Table 2). Sensitivity (logEC50) to AngII was similar in all 4 groups (Table 2).
Figure 1.
Angiotensin (Ang)II-mediated vasoconstriction in aortas from SS-13BN rats normal diet (N=12) or hyper-caloric diet (N=10) or SS rats on normal diet (N=8) or hyper-caloric diet (n=14). *P<0.05 for Emax in aortic segments from SS rats on normal diet vs. hyper-caloric diet; †P<0.05 for Emax in aortic segments SS rats vs. SS-13BN rats on normal diet.
Table 2.
Maximum response (Emax) and sensitivity (logEC50) to angiotensin II (ANGII)-, phenylephrine (PE)-, and KCl-mediated vasoconstriction in aortas from SS-13BN rats and SS rats on normal diet or hyper-caloric diet.
| SS-13BN
|
SS
|
|||
|---|---|---|---|---|
| Normal | Hyper-caloric | Normal | Hyper-caloric | |
| Emax, % Increase in Force | ||||
| AngII | 71±10 (10) | 53±11(10) | 133±11 (8)* | 43±5 (14)† |
| +PEG-SOD | 55±6 (6)‡ | 27±7 (3)‡ | 53±17 (6)‡ | 16±2 (9)‡ |
| +CATALASE | 88±13 (4) | 25±5 (4)‡ | 73±21 (5)‡ | 46±6 (12) |
| +L-NAME | 114±15 (6)‡ | 51±6 (5) | 117±13 (5) | 91±11 (11)‡ |
| KCl | 140±4 (12) | 114±9 (10)* | 156±9 (9) | 148±6 (14) |
|
| ||||
| logEC50, [M] for AngII; EC50, [mM] for KCl | ||||
|
| ||||
| AngII | −8.1±0.01 (11) | −8.2±0.1 (10) | −8.4±0.1 (11) | −8.1±0.1 (14) |
| +PEG-SOD | −7.8±0.2 (6)‡ | −7.7±0.1 (3)‡ | −8.0±0.2 (5) | −7.9±0.1 (9) |
| +CATALASE | −8.2±0.1 (4) | −8.1±0.2 (4) | −8.3±0.4 (5) | −8.1±0.1 (11) |
| +L-NAME | −8.4±0.1 (6)‡ | −7.9±0.1 (5) | −8.7±0.3 (6) | −8.2±0.04 (11) |
| KCl | 17.6±1.1 (10) | 28.8±3.1 (6)* | 20.3±1.3 (13) | 25.9±1.4 (8)† |
P<0.05 vs. untreated aortas from SS-13BN rats on normal diet;
P<0.05 vs. untreated aortas from SS rats on normal diet;
P<0.05 vs. corresponding untreated aortas. Number of rats is in parentheses.
When KCl responses were assessed, Emax was reduced solely in SS-13BN rats with hyper-caloric diet, whereas Emax was similar between rat strains on normal diet (Table 2). Sensitivity to KCl was similarly increased in both rat strains with hyper-caloric diet with no difference between rat strains on normal diet (Table 2).
Aortic ROS function during AngII vasoconstriction
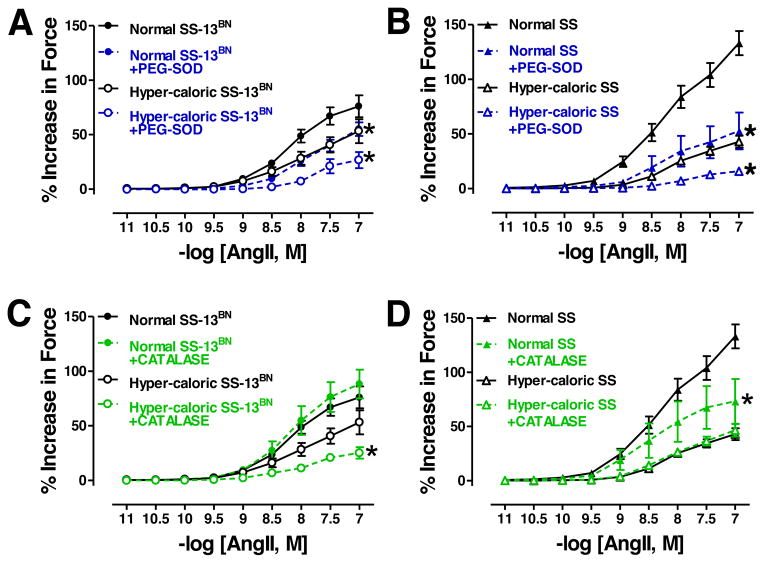
Treatment of aortic rings with PEG-SOD significantly reduced Emax and logEC50 to AngII constriction in SS-13BN rats on either diet (Figure 2A, Table 2), whereas, PEG-SOD treatment reduced Emax with no change in logEC50 to AngII in aortas from SS rats on either diet (Figure 2B, Table 2).
Figure 2.
Angiotensin (Ang)II-mediated vasoconstriction in aortas from SS-13BN rats (A: normal diet: untreated N=11, +PEG-SOD N=6; hyper-caloric diet: untreated N=10, +PEG-SOD N=3) (C: normal diet: untreated N=11, +CATALASE N=4; hyper-caloric diet: untreated N=10, +CATALASE N=4). AngII vasoconstriction in aortas from SS rats (B: normal diet: untreated N=8, +PEG-SOD N=6; hyper-caloric diet: untreated N=14, +PEG-SOD N=9). (D: normal diet: untreated N=8, +CATALASE N=5; hyper-caloric diet: untreated N=14, +CATALASE N=12). *P<0.05 for Emax in aortic segments in corresponding untreated groups.
In SS-13BN rats, catalase reduced Emax with no change in logEC50 to AngII in aortic rings from the hyper-caloric diet group but no effect on rings from the normal diet groups (Figure 2C, Table 2). In contrast, catalase had no affect on Emax or logEC50 to AngII in aortic rings from SS rats on hyper-caloric diet but reduced on Emax in rings from SS rats on normal diet (Figure 2D, Table 2).
Aortic NOS function during AngII vasoconstriction
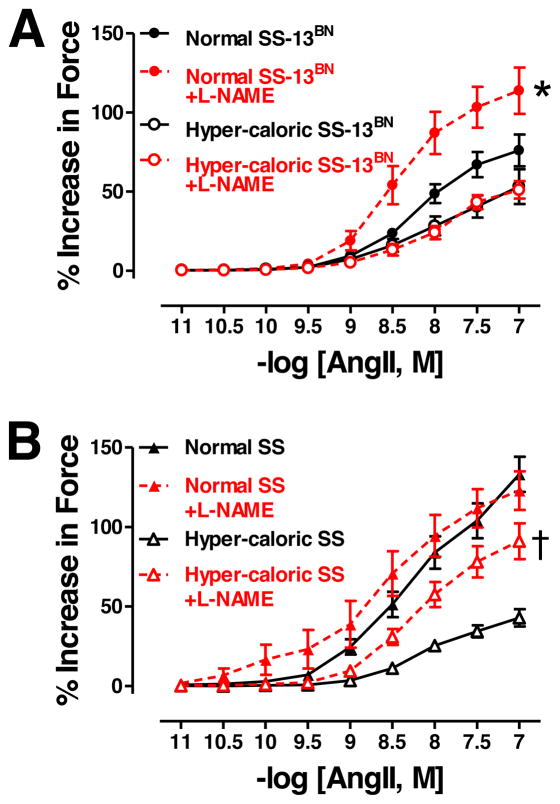
L-NAME, a non-selective NOS inhibitor, increased Emax and reduced logEC50 to AngII in SS-13BN rats on normal diet but not on hyper-caloric diet (Figure 3A, Table 2). In stark contrast, L-NAME increased Emax without altering logEC50 to AngII in SS rats on hyper-caloric diet but not on normal diet (Figure 3B, Table 2).
Figure 3.
A: Angiotensin (Ang)II-mediated vasoconstriction in aortas from SS-13BN rats on normal diet (untreated N=12, +L-NAME N=6) or hyper-caloric diet (untreated N=10, +L-NAME N=5). B: AngII vasoconstriction in aortas from SS rats on normal diet (untreated N=8, +L-NAME N=6) or hyper-caloric diet (untreated N=14, +L-NAME N=11). *P<0.05 for Emax in L-NAME-treated vs. untreated aortic segments from SS-13BN rats on normal diet. †P<0.05 for Emax in L-NAME-treated vs. untreated aortic segments from SS rats on hyper-caloric diet.
Aortic vasorelaxation
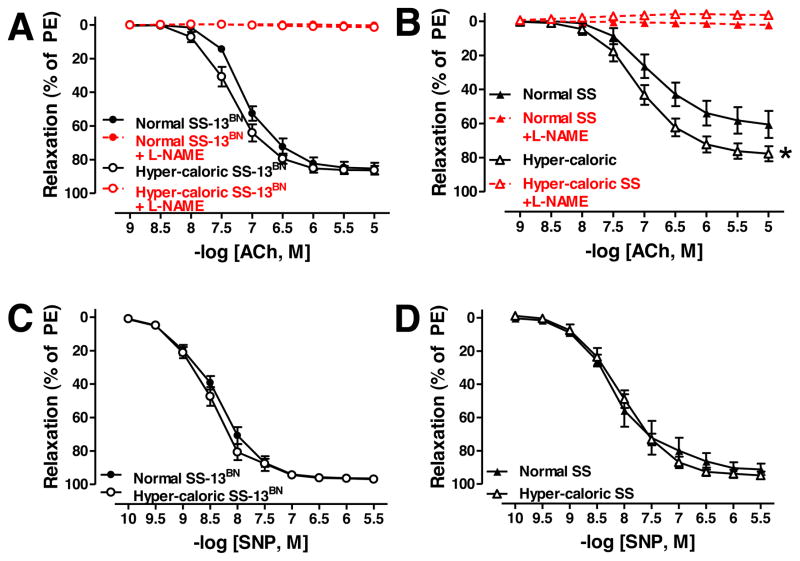
Emax and logEC50 to ACh-mediated vasorelaxation was similar in SS-13BN rats on normal diet and hyper-caloric diet (Figure 4A, Table 3); ACh response was totally blocked with L-NAME (Figure 4A, Table 3). In contrast, Emax but not logEC50 to ACh-mediated vasorelaxation was greater in aortas from SS rats on hyper-caloric diet normal diet (Figure 4B, Table 3); ACh response in both diet groups of SS rats was totally blocked with L-NAME (Figure 4B, Table 3). SNP response was similar in SS-13BN rat (Figure 4C, Table 3) and SS rat diet groups (Figure 4D, Table 3).
Figure 4.
Acetylcholine (ACh)- and sodium nitroprusside (SNP)-mediated vasorelaxation in SS-13BN rats (A and B, respectively; normal diet: untreated N=10, +L-NAME N=10; hyper-caloric diet: untreated N=9, +L-NAME N=9) and SS rats (C and D, respectively; normal diet: untreated N=8, +L-NAME N=8; hyper-caloric diet: untreated N=8–14, +L-NAME N=14). *P<0.05 Emax vs. SS rats on normal diet.
Table 3.
Maximum response (Emax) and sensitivity (logEC50) to acetylcholine (ACh)- and sodium nitroprusside (SNP)-mediated vasorelaxation in aortas from SS rats on normal diet or hyper-caloric diet.
| SS-13BN
|
SS
|
|||
|---|---|---|---|---|
| Normal | Hyper-caloric | Normal | Hyper-caloric | |
|
|
||||
| Emax, % of PE | ||||
| ACh | 85±3 (10) | 86±3 (10) | 56±7 (7)* | 78±4 (14)† |
| +L-NAME | 0.3±0.9 (10)‡ | 1.4±0.9 (10)‡ | 2.1±0.7 (8)‡ | -3.7±2.3 (14)‡ |
| SNP | 95.0±1.5 (10) | 96.0±0.8 (10) | 91.2±3.5 (8) | 94.8±2.3 (14) |
|
| ||||
|
logEC50, [M]
|
||||
| ACh | −7.1±0.1 (10) | −7.3±0.1 (10) | −6.9±0.1 (8) | −6.9±0.2 (13) |
| SNP | −8.37±0.1 (9) | −8.49±0.1 (9) | −8.0±0.2 (8) | −7.9±0.1 (13) |
P<0.05 vs. aortic artery segments from SS-13BN rats on normal diet,
P<0.05 vs. aortic artery segments from SS rats on normal diet,
P<0.05 vs. corresponding untreated aortic artery segments. Number of rats is in parentheses.
AT1R and NOS3 expression and NOS activity in aortas from SS rats
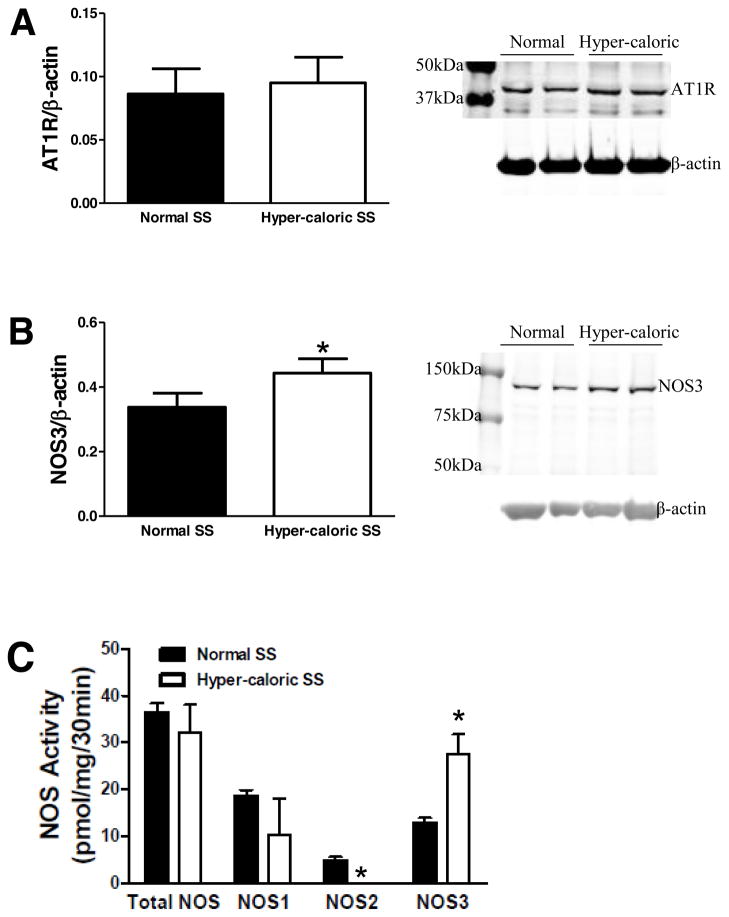
Aortic AT1R expression was similar in SS rats on normal diet or hyper-caloric diet (Figure 5A), whereas NOS3 protein expression was increased in SS rats on hyper-caloric diet (Figure 5B).
Figure 5.
In aortic tissue from SS rats, A: AT1R expression (normal diet N=8; hyper-caloric diet N=8), B: NOS3 protein expression (normal diet N=7; hyper-caloric diet N=7), and C: NOS activity (normal diet N=3; hyper-caloric diet N=4). *P<0.05 vs. SS rats on normal diet.
Total NOS activity and NOS1 specific activity were similar in aortas from SS rats on normal diet or hyper-caloric diet; however, hyper-caloric diet elicited a reduction in NOS2 activity and an increase in NOS3 activity in aortas from SS rats (Figure 5C).
Discussion
Our study elucidated the interaction between AngII and NOS signaling in the vasculature of Dahl salt-sensitive (SS) rats and the extent to which this interaction is modulated by a hyper-caloric diet. Preliminary studies showed under normal diet conditions that a lack of NOS function resulted in greater AngII reactivity in aortas from SS rats than SS-13BN rats. Because a hyper-caloric diet elicits exaggerated AngII reactivity in large arteries when NOS function is reduced (Bhattacharya et al., 2008), we tested the hypothesis that a 4-week hyper-caloric diet promotes greater AngII reactivity in aortas from SS rats than SS-13BN rats. Contrary to this hypothesis, hyper-caloric did not increase AngII reactivity in aortic rings from SS-13BN rats and dramatically blunted this response in SS rats compared to rats on normal diet. Therefore, we redirected our hypothesis to examine if the apparent imbalance between AngII and NOS signaling in the SS rats on normal diet is attenuated by a hyper-caloric diet. Our main findings are congruent with this hypothesis as NOS3 expression and function was increased in SS rats on hyper-caloric diet.
Classical studies use the SS rat to model salt-sensitive cardiovascular disease in humans. Even on a normal-salt diet, SS rats are susceptible to cardiovascular-renal disease (Garrett et al., 2003). In fact, we showed on normal diet that SS rats but not salt-resistant SS-13BN rats had greater aortic responsiveness to AngII linked to reduced NOS function. It is established that local AngII disrupts NO’s regulation of vascular tone through reactive oxygen species (ROS) signaling in aortas from SS rats on high-salt diet (Zhou et al., 2003a); this had not been examined under normal diet circumstances. Although O2·− similarly mediated aortic response to AngII in both rat strains, H2O2-mediated AngII reactivity was greater in SS rats than SS-13BN rats. These data highlight the susceptibility of cardiovascular disease via pro-oxidant mechanisms in SS rats under normal diet conditions.
In SS-13BN rats, hyper-caloric diet did not significantly alter aortic AngII reactivity. However, the mechanisms of AngII reactivity were different between the SS-13BN diet groups. It is well known in the blood vessel wall that AngII is a potent stimulator of ROS including O2·− (Berry et al., 2000) and H2O2 (Zafari et al., 1998). Many studies show these two ROS mediate AngII-induced vasoconstriction (Bagi et al., 2008, Puntmann et al., 2005, Hussain et al., 2006, de Groot et al., 2004, Shastri et al., 2002, Torrecillas et al., 2001, Kawazoe et al., 1999, Kim et al., 2010, Pfister et al., 2011). Quenching of O2·− with PEG-SOD blunted AngII reactivity similarly in SS-13BN rats on normal diet and hyper-caloric diet, whereas H2O2-mediated AngII reactivity was only present in aortas from SS-13BN rats on hyper-caloric diet. Our data in SS-13BN rats are reminiscent of studies utilizing salt-resistant Fisher rats whereby 7 months of hyper-caloric diet increased plasma levels of H2O2 without altering aortic catalase expression (Roberts et al., 2006, Roberts et al., 2005). Our data confirm that hyper-caloric diet promotes a pro-oxidant environment in the vasculature in salt-resistant rats.
In contrast to SS-13BN rats, we propose that short-term hyper-caloric feeding promotes an anti-oxidant environment in the blood vessel wall in SS rats. Foremost, H2O2-mediated AngII vasoconstriction in aortas from SS rats on a normal diet was not present following the hyper-caloric diet, possibly due to the scavenging effect of NO through the increased NOS3 activity. Hyper-caloric diet increased aortic NOS3-specific activity and expression in addition to reducing aortic NOS2 activity in SS rats. Studies show that activation of the peroxisome proliferator-activated receptors (PPARs) class of transcription factors, in particular PPAR-α, increases endothelial cell NOS3 expression (Wang et al., 2006) and activity (Okayasu et al., 2008). It has been shown that PPAR-α reduces NOS2 activity and expression in macrophages (Colville-Nash et al., 1998). Interestingly, a 12-week low-carbohydrate/high-fat diet increases PPAR-α protein expression in whole hearts from SS rats (Okere et al., 2006). Further research will examine if hyper-caloric diet-mediated enhancement of aortic endothelial function in SS rats is mediated by activation of PPAR-α.
AngII-mediated vasoconstriction in aortic tissue has been utilized as a surrogate measure of large artery disease. In aortic ring preparations from rabbits, a 10-week hypercholesterolemic diet increased AT1R mRNA and protein expressions corresponding to exaggerated AngII reactivity and endothelial dysfunction; importantly, these changes in aortic reactivity paralleled the formation of atherosclerotic plaques (Yang et al., 1998). Because circulating AngII levels are increased from picomolar to nanomolar concentrations in diet-induced obese mice (Gupte et al., 2008), we propose that SS rats are protected against endothelial pathologies linked to atherogenesis following a short-term hyper-caloric diet. This proposal is supported by findings from Lombard’s group demonstrating that not only is middle cerebral arterial response to acetylcholine enhanced in SS rats fed a 16–20 hyper-caloric diet, but that AT1R protein expression is reduced in these arteries as well (Beyer et al., 2012).
The choice of utilizing Teklad 8604 as the normal diet in the present study was based on our previous studies (Spradley et al., 2011). SS rats were maintained on the American Institutes of Nutrition (AIN)-76A diet since weaning at 3-weeks-old at Charles River Laboratories but started on Teklad diet at 12-weeks-old at our institution. When assessing acetylcholine-mediated vasorelaxation and phenylephrine-mediated vasoconstriction, we showed this diet protocol did not alter aortic NOS function but promoted NOS dysfunction in small mesenteric arteries (Spradley et al., 2011). We now demonstrate aortic NOS dysfunction in SS rats on this diet regimen in response to AngII. However, we can only speculate about using a conductance vessel as a surrogate measure of AngII function in systemic resistance blood vessels.
There are limitations to using the Teklad diet as the control diet in our hyper-caloric diet studies. As detailed in the Methods, the Teklad diet is proprietary and consists of multiple components for each macronutrient whereas the hyper-caloric diet is a purified diet. Because of the complex nature of the Teklad diet, future studies should use a proper control diet to solidify our findings. Furthermore, we highlight that both rat strains consumed approximately half as much of the hyper-caloric diet, and thus salt as well, when compared to the normal diet groups. Lombard’s laboratory showed that endothelial dysfunction is present in cerebral arteries from SS rats not SS-13BN rats when on a low salt (0.01% NaCl) or normal salt (0.4% NaCl) via reduced AngII signaling; in fact, a subpressor infusion of AngII for 3 days restored endothelium-dependent vasorelaxation in SS rats on normal-salt diet. (Drenjancevic-Peric and Lombard, 2005, Durand et al., 2010, Durand and Lombard, 2011). The reduced salt intake in the SS-13BN rats on hyper-caloric diet was echoed by increased plasma renin activity, indicating that the renin-angiotensin system is increased in this diet group. In contrast, PRA was not altered with 4-week hyper-caloric diet in SS rats, indicating that systemic activation of the RAS is not increased in this diet group. We speculate that the increased vasodilator mechanisms in SS rats on hyper-caloric diet is not due to a reduction in salt intake.
In perspective, classic monogenetic models of hyper-caloric diet-induced cardiovascular diseases have yielded invaluable mechanistic insights, yet most cardiovascular diseases are polygenetic (Dominiczak et al., 2005). The SS rat is a polygenetic model of cardiovascular disease (Mattson et al., 2008). SS rats responded to the short-term hyper-caloric diet by reduced H2O2-mediated vasoconstriction and increased aortic NOS3 activity and endothelial function as well. Whereas, SS-13BN rats showed reduced NOS function response to hyper-caloric diet. NO is protective against atherosclerosis, a slowly progressing large artery disease associated with high caloric intake (Mainous et al., 2011). Future research will be directed at elucidating the molecular mechanism(s) involved with a short-term hyper-caloric diet to induce vascular NOS3 activity and expression possibly revealing novel vasoprotective target(s) in the salt-sensitive population.
Acknowledgments
We are grateful to David M. Pollock, PhD for his insightful discussions regarding this study. We thank Jacqueline B. Musall for her expert technical assistance.
Grants
This study was supported by NIH P01 HL69999 (JSP), NIH R01 HL60653 (JSP), T32 NHLBI Predoctoral Training Fellowship (FTS), and AHA Predoctoral Fellowship (FTS).
Footnotes
Disclosures
None
References
- Bagi Z, Erdei N, Koller A. High intraluminal pressure via H2O2 upregulates arteriolar constrictions to angiotensin II by increasing the functional availability of AT1 receptors. Am J Physiol Heart Circ Physiol. 2008;295:H835–41. doi: 10.1152/ajpheart.00205.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M, Carmona R, Morawietz H, d’Uscio LV, Goettsch W, Hillen H, Haudenschild CC, Krieger JE, Munter K, Lattmann T, Luscher TF, Shaw S. Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin. Hypertension. 2000;35:329–36. doi: 10.1161/01.hyp.35.1.329. [DOI] [PubMed] [Google Scholar]
- Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, Dominiczak AF. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101:2206–12. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- Beyer AM, Raffai G, Weinberg B, Fredrich K, Lombard JH. Dahl Salt-Sensitive Rats Are Protected Against Vascular Defects Related to Diet-Induced Obesity. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.112.191551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya I, Mundy AL, Widmer CC, Kretz M, Barton M. Regional heterogeneity of functional changes in conduit arteries after high-fat diet. Obesity (Silver Spring) 2008;16:743–8. doi: 10.1038/oby.2007.111. [DOI] [PubMed] [Google Scholar]
- Colville-Nash PR, Qureshi SS, Willis D, Willoughby DA. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J Immunol. 1998;161:978–84. [PubMed] [Google Scholar]
- de Groot AA, van Zwieten PA, Peters SL. Involvement of reactive oxygen species in angiotensin II-induced vasoconstriction. J Cardiovasc Pharmacol. 2004;43:154–9. doi: 10.1097/00005344-200401000-00023. [DOI] [PubMed] [Google Scholar]
- Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–9. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominiczak AF, Graham D, McBride MW, Brain NJ, Lee WK, Charchar FJ, Tomaszewski M, Delles C, Hamilton CA. Corcoran Lecture. Cardiovascular genomics and oxidative stress. Hypertension. 2005;45:636–42. doi: 10.1161/01.HYP.0000154253.53134.09. [DOI] [PubMed] [Google Scholar]
- Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension. 2005;45:687–91. doi: 10.1161/01.HYP.0000154684.40599.03. [DOI] [PubMed] [Google Scholar]
- Durand MJ, Lombard JH. Introgression of the Brown Norway renin allele onto the Dahl salt-sensitive genetic background increases Cu/Zn SOD expression in cerebral arteries. Am J Hypertens. 2011;24:563–8. doi: 10.1038/ajh.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand MJ, Moreno C, Greene AS, Lombard JH. Impaired relaxation of cerebral arteries in the absence of elevated salt intake in normotensive congenic rats carrying the Dahl salt-sensitive renin gene. Am J Physiol Heart Circ Physiol. 2010;299:H1865–74. doi: 10.1152/ajpheart.00700.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett MR, Dene H, Rapp JP. Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J Am Soc Nephrol. 2003;14:1175–87. doi: 10.1097/01.asn.0000060572.13794.58. [DOI] [PubMed] [Google Scholar]
- Ghatta S, Ramarao P. Increased contractile responses to 5-Hydroxytryptamine and Angiotensin II in high fat diet fed rat thoracic aorta. Lipids Health Dis. 2004;3:19. doi: 10.1186/1476-511X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–8. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera VM, Didishvili T, Lopez LV, Zander K, Traverse S, Gantz D, Herscovitz H, Ruiz-Opazo N. Hypertension exacerbates coronary artery disease in transgenic hyperlipidemic Dahl salt-sensitive hypertensive rats. Mol Med. 2001;7:831–44. [PMC free article] [PubMed] [Google Scholar]
- Hussain MB, Puntmann VO, Mayr M, Khong T, Singer DR. The role of oxidant stress in angiotensin II-mediated contraction of human resistance arteries in the state of health and the presence of cardiovascular disease. Vascul Pharmacol. 2006;45:395–9. doi: 10.1016/j.vph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Iwai M, Chen R, Li Z, Shiuchi T, Suzuki J, Ide A, Tsuda M, Okumura M, Min LJ, Mogi M, Horiuchi M. Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation. 2005;112:1636–43. doi: 10.1161/CIRCULATIONAHA.104.525550. [DOI] [PubMed] [Google Scholar]
- Kang KT, Sullivan JC, Spradley FT, d’Uscio LV, Katusic ZS, Pollock JS. Antihypertensive therapy increases tetrahydrobiopterin levels and NO/cGMP signaling in small arteries of angiotensin II-infused hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300:H718–24. doi: 10.1152/ajpheart.00393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazoe T, Kosaka H, Yoneyama H, Hata Y. Involvement of superoxide in acute reaction of angiotensin II in mesenteric microcirculation. Jpn J Physiol. 1999;49:437–43. doi: 10.2170/jjphysiol.49.437. [DOI] [PubMed] [Google Scholar]
- Kim JI, Jung SW, Yang E, Park KM, Eto M, Kim IK. Heat shock augments angiotensin II-induced vascular contraction through increased production of reactive oxygen species. Biochem Biophys Res Commun. 2010;399:452–7. doi: 10.1016/j.bbrc.2010.07.115. [DOI] [PubMed] [Google Scholar]
- Koga J, Egashira K, Matoba T, Kubo M, Ihara Y, Iwai M, Horiuchi M, Sunagawa K. Essential role of angiotensin II type 1a receptors in the host vascular wall, but not the bone marrow, in the pathogenesis of angiotensin II-induced atherosclerosis. Hypertens Res. 2008;31:1791–800. doi: 10.1291/hypres.31.1791. [DOI] [PubMed] [Google Scholar]
- Loria AS, Kang KT, Pollock DM, Pollock JS. Early Life Stress Enhances Angiotensin II-Mediated Vasoconstriction by Reduced Endothelial Nitric Oxide Buffering Capacity. Hypertension. 2011a doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension. 2011b;58:619–26. doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous AG, 3rd, Everett CJ, Diaz VA, Player MS, Gebregziabher M, Smith DW. Life stress and atherosclerosis: a pathway through unhealthy lifestyle. Int J Psychiatry Med. 2011;40:147–61. doi: 10.2190/PM.40.2.b. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW., Jr Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol. 2008;295:F837–42. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltzer E, Verkuil AV, van Veghel R, Danser AH, van Esch JH. Effects of angiotensin metabolites in the coronary vascular bed of the spontaneously hypertensive rat: loss of angiotensin II type 2 receptor-mediated vasodilation. Hypertension. 2010;55:516–22. doi: 10.1161/HYPERTENSIONAHA.109.145037. [DOI] [PubMed] [Google Scholar]
- Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Hofmann-Lehmann R, Minotti R, Barton M. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: implications for diet-induced obesity. Cardiovasc Res. 2007;73:368–75. doi: 10.1016/j.cardiores.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Oddie CJ, Dilley RJ, Kanellakis P, Bobik A. Chronic angiotensin II type 1 receptor antagonism in genetic hypertension: effects on vascular structure and reactivity. J Hypertens. 1993;11:717–24. doi: 10.1097/00004872-199307000-00006. [DOI] [PubMed] [Google Scholar]
- Okayasu T, Tomizawa A, Suzuki K, Manaka K, Hattori Y. PPARalpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci. 2008;82:884–91. doi: 10.1016/j.lfs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–23. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- Pepine CJ. Why vascular biology matters. Am J Cardiol. 2001;88:5K–9K. doi: 10.1016/s0002-9149(01)01923-3. [DOI] [PubMed] [Google Scholar]
- Persson PB, Henriksson J. Good publishing practice in physiology. Acta Physiol (Oxford) 2011;203:403–407. [Google Scholar]
- Pfister SL, Nithipatikom K, Campbell WB. Role of superoxide and thromboxane receptors in acute angiotensin II-induced vasoconstriction of rabbit vessels. Am J Physiol Heart Circ Physiol. 2011;300:H2064–71. doi: 10.1152/ajpheart.01135.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyan Mehr A, Siegel AK, Kossmehl P, Schulz A, Plehm R, de Bruijn JA, de Heer E, Kreutz R. Early onset albuminuria in Dahl rats is a polygenetic trait that is independent from salt loading. Physiol Genomics. 2003;14:209–16. doi: 10.1152/physiolgenomics.00053.2003. [DOI] [PubMed] [Google Scholar]
- Puntmann VO, Hussain MB, Mayr M, Xu Q, Singer DR. Role of oxidative stress in angiotensin-II mediated contraction of human conduit arteries in patients with cardiovascular disease. Vascul Pharmacol. 2005;43:277–82. doi: 10.1016/j.vph.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J Appl Physiol. 2005;98:203–10. doi: 10.1152/japplphysiol.00463.2004. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–34. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–51. doi: 10.1161/01.atv.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Angiotensin II-induced vascular dysfunction is mediated by the AT1A receptor in mice. Hypertension. 2004;43:1074–9. doi: 10.1161/01.HYP.0000123074.89717.3d. [DOI] [PubMed] [Google Scholar]
- Sauzeau V, Sevilla MA, Montero MJ, Bustelo XR. The Rho/Rac exchange factor Vav2 controls nitric oxide-dependent responses in mouse vascular smooth muscle cells. J Clin Invest. 2010;120:315–30. doi: 10.1172/JCI38356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri S, Gopalakrishnan V, Poduri R, Di Wang H. Tempol selectively attenuates angiotensin II evoked vasoconstrictor responses in spontaneously hypertensive rats. J Hypertens. 2002;20:1381–91. doi: 10.1097/00004872-200207000-00025. [DOI] [PubMed] [Google Scholar]
- Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–85. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradley FT, Ho DH, Kang KT, Pollock DM, Pollock JS. Changing standard chow diet promotes vascular NOS dysfunction in Dahl S rats. Am J Physiol Regul Integr Comp Physiol. 2011 doi: 10.1152/ajpregu.00482.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Eguchi K, Ohtsu H, Higuchi S, Dhobale S, Frank GD, Motley ED, Eguchi S. Activation of endothelial nitric oxide synthase by the angiotensin II type 1 receptor. Endocrinology. 2006;147:5914–20. doi: 10.1210/en.2006-0834. [DOI] [PubMed] [Google Scholar]
- Tesanovic S, Vinh A, Gaspari TA, Casley D, Widdop RE. Vasoprotective and atheroprotective effects of angiotensin (1–7) in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:1606–13. doi: 10.1161/ATVBAHA.110.204453. [DOI] [PubMed] [Google Scholar]
- Torrecillas G, Boyano-Adanez MC, Medina J, Parra T, Griera M, Lopez-Ongil S, Arilla E, Rodriguez-Puyol M, Rodriguez-Puyol D. The role of hydrogen peroxide in the contractile response to angiotensin II. Mol Pharmacol. 2001;59:104–12. doi: 10.1124/mol.59.1.104. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, Miyazaki M, Nozawa Y, Ozono R, Nakagawa K, Miwa T, Kawada N, Mori Y, Shibasaki Y, Tanaka Y, Fujiyama S, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–35. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaartjes I, Hendrix A, Hertogh EM, Grobbee DE, Doevendans PA, Mosterd A, Bots ML. Sudden death in persons younger than 40 years of age: incidence and causes. Eur J Cardiovasc Prev Rehabil. 2009;16:592–6. doi: 10.1097/HJR.0b013e32832d555b. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Yang Q, Yan JT, Zhao C, Cianflone K, Wang DW. Effects of bezafibrate on the expression of endothelial nitric oxide synthase gene and its mechanisms in cultured bovine endothelial cells. Atherosclerosis. 2006;187:265–73. doi: 10.1016/j.atherosclerosis.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Czech T, van Eickels M, Fleming I, Bohm M, Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–7. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- Wendt N, Schulz A, Qadri F, Bolbrinker J, Kossmehl P, Winkler K, Stoll M, Vetter R, Kreutz R. Genetic analysis of salt-sensitive hypertension in Dahl rats reveals a link between cardiac fibrosis and high cholesterol. Cardiovasc Res. 2009;81:618–26. doi: 10.1093/cvr/cvn263. [DOI] [PubMed] [Google Scholar]
- Wever RM, Luscher TF, Cosentino F, Rabelink TJ. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation. 1998;97:108–12. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]
- Yang BC, Phillips MI, Mohuczy D, Meng H, Shen L, Mehta P, Mehta JL. Increased angiotensin II type 1 receptor expression in hypercholesterolemic atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1998;18:1433–9. doi: 10.1161/01.atv.18.9.1433. [DOI] [PubMed] [Google Scholar]
- Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–95. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92:322–9. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
- Zhang C, Knudson JD, Setty S, Araiza A, Dincer UD, Kuo L, Tune JD. Coronary arteriolar vasoconstriction to angiotensin II is augmented in prediabetic metabolic syndrome via activation of AT1 receptors. Am J Physiol Heart Circ Physiol. 2005;288:H2154–62. doi: 10.1152/ajpheart.00987.2004. [DOI] [PubMed] [Google Scholar]
- Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003a;42:945–51. doi: 10.1161/01.HYP.0000094220.06020.C8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Chen Y, Dirksen WP, Morris M, Periasamy M. AT1b receptor predominantly mediates contractions in major mouse blood vessels. Circ Res. 2003b;93:1089–94. doi: 10.1161/01.RES.0000101912.01071.FF. [DOI] [PubMed] [Google Scholar]