Abstract
Aims
To determine if endothelin-1 (ET-1) stimulates the phosphorylation of ERK1/2 in the mouse inner medullary collecting duct (IMCD), and if this in turn upregulates nitric oxide (NO) production.
Main methods
Confluent mouse IMCD segment-3 cells (mIMCD-3) were stimulated with 50 nM ET-1 for 24 hrs with and without various doses of ET receptor antagonists, BQ123 (ETA antagonist,) or BQ788 (ETB antagonist) and phosphorylation of ERK1/2 determined by immunoblots. As well, NOS isoform expression and nitrite production were assessed. Finally, increasing doses of the MEK inhibitors, PD98,059 or U0126, were incubated with mIMCD-3 cells and the ET-1 dependent nitrite production determined.
Key findings
ET-1 via the ETB receptor significantly increased ERK1/2 phosphorylation, and was prevented by MEK inhibition. ET-1 also stimulates nitrite production by mIMCD-3 cells (basal: 54.5 ± 26 pmol/mg pr/h vs ET-1: 221 ± 28 pmol/mg pr/h; N= 4) via the ETB receptor (BQ788 + ET-1: 83.7 ± 27 pmol/ mg pr/h); however, ET-1 does not regulate NOS1 or NOS3 expression. MEK inhibition did not prevent the ET-1 stimulated nitrite production contrary to our initial hypothesis (vehicle + ET-1: 157 ± 13 pmol/mg pr/ hr vs PD98,059 + ET-1: 305.7 ± 24 pmol/mg pr/h, N= 4, P>0.05).
Significance
Although the mouse IMCD-3 cells only express the NOS1β splice variant, ET-1 did regulate mouse IMCD nitrite production. ET-1 stimulates ERK1/2 phosphorylation in the mouse IMCD, but ERK1/2 signaling is not involved in the ET-1 dependent increase in NO production by IMCD cells. Thus, we propose that ET-1 regulates protein-protein interactions that are necessary for NO production, that are independent of MAPK signaling cascades.
Keywords: Collecting duct, nitric oxide, nitric oxide synthase, endothelin, ERK1/2, phosphorylation
Introduction
The endothelin (ET-1) and nitric oxide (NO) systems are highly expressed in the renal inner medullary collecting ducts (IMCD) [1–3], and pharmacological or genetic blockade of either of these systems results in an elevation in blood pressure [4–7]. It is well established that ET-1 via the ETB receptor can increase NO production in endothelial cells, and the thick ascending limb via NO synthase 3 (NOS3; eNOS) isoform [8]. In the rat IMCD, ET-1 via the ETB receptor increases NO synthase 1 (NOS1; nNOS) derived NO production [9]. Interestingly, rat IMCDs express the NOS1 splice variants, NOS1α and NOS1β [10, 11], while mouse IMCDs express NOS1β [10]. Purified NOS1β is reported to have 80% of the activity of NOS1α [12], yet little is known about the regulation of NOS1β. By studying mouse IMCDs, it gives the unique opportunity to determine whether ET-1 regulates NOS1β expression and/or NO production.
ET-1 has mitogenic properties and activates the mitogen activated protein kinase (MAPK) pathways. For example, in opossum kidney cells, proximal tubule epithelial cell line, ET-1 via ETB activates extracellular regulated kinases 1/2 (ERK1/2) indicating a role for the proliferative effects of growth factors on this epithelium [13]. Beyond mitogenic activities, activation of ERK1/2 has been to linked to constriction of vascular smooth muscle cells [14]; mineralocorticoid-dependent hypertension (through augmented vascular smooth muscle contraction) [15]; and endotoxemia (lipopolysaccharide-mediated) linked hypotension and vascular hyporeactivity [16]. Several studies highlight the interaction between ET-1, ERK1/2 activation, and NO production. There is evidence that ERK1/2 signaling regulates NOS1 expression in rat aortic smooth muscle [17] as well as in neurons and the spinal cord [18], NOS2 in the thoracic aorta [16] and astrocytes [19]. However, ERK1/2 regulation of NOS3 is controversial, with studies demonstrating that ERK1/2 regulates NOS3 [20] while others suggest these signaling cascades are not involved [21].
We hypothesized that ET-1 via the ETB receptor increases ERK1/2 phosphorylation resulting in an increased NO production and/or expression of NOS1β and/or NOS3 in the IMCD. The objectives of this study were to determine in mouse IMCD that, (1) ET-1 regulation of NOS1β and/or NOS3 expression via ETB signaling; (2) ET-1 regulation of ERK1/2 phosphorylation via ETB signaling; and (3) whether phosphorylation of ERK1/2 regulates NO production.
Material and methods
All chemicals were cell culture grade and purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Mouse inner medullary collecting duct segment-3 cells (mIMCD-3) were purchased from ATCC (Manassas, VA). Passages 3–5 were grown in 12-well plates and used in all studies. Cells were grown in Dulbecco's Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA) plus 1% penicillin-streptomycin (Invitrogen) and 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) to confluency, and serum/antibiotic starved 3 h prior to experimentation.
Experiment 1: ET-1 stimulation of nitrite production
mIMCD-3 cells were washed twice with 10 mM Hank’s Buffered Saline Solution (HBSS, with calcium, magnesium, no phenol red (Cellgro, Mediatech, Manassas, VA) and incubated for 1h (37°C, 5% CO2) in HBSS + 250 µM L-arginine + 20 U/ml superoxide dismutase with one of the following treatments: 0 (water), 1, 10, 50, 100, or 500 nM ET-1 (dissolved in water, American Peptide, Sunnyvale, CA). Additionally, mMCD-3 cells were pre-incubated with the ETA antagonist, BQ123, (dissolved in DMSO) or the ETB antagonist, BQ788, (dissolved in DMSO) at 0 (DMSO only), 1, 10, 100, or 1000 nM for 3 h in DMEM, followed by the addition of 50 nM ET-1 for 24 h. Next, the cells were washed twice with 10 mM HBSS and incubated in HBSS + 250 µM L-arginine + 20U/ml superoxide dismutase for 1 h. The HBSS supernatant was removed, snap frozen and stored until nitrite analysis (see below). The adherent cells were scraped, pelleted and snap frozen for Western blot analysis of NOS isoforms (see below) or dissolved with 0.1N sodium hydroxide for 20 min at 25°C. Protein concentrations were determined by Bradford assay following manufacturer’s instructions (Biorad Quickstart, Hercules, CA).
Experiment 2: ET-1 activation of ERK1/2
mIMCD-3 cells were washed and incubated in HBSS as stated above, with the addition of 50 nM ET-1 for 0, 5, 15, 30 and 60 min. Cells were immediately washed with 10 mM PBS with PhosSTOP™ phosphatase inhibitor (Roche Life Sciences, Indianapolis, IN), scraped, pelleted and snap frozen for Western blot analysis of ERK1/2 phosphorylation status. mIMCD- 3 cells were preincubated for 3 h with 0 (DMSO), 1, 10, 100, or 1000 nM of BQ123 or BQ788 and treated with 50 nM ET-1 for 15 min. Cells were washed, scraped, pelleted and frozen for Western blot analysis of ERK1/2 phosphorylation status.
Experiment 3: MEK inhibition of ET-1 activation of ERK 1/2 and/or nitrite production
mIMCD-3 cells were preincubated with either, PD98,059 or U0126 (0, 1, 10 100 µM; dissolved in DMSO) for 1 h. Following this, cells were incubated with 50 nM ET-1 for 15 min, 1 h or 24 h in HBSS + 250 µM L-arginine + 20U/ml superoxide dismutase. The HBSS supernatants were removed and snap frozen for nitrite analysis. In addition, cells from the 15 min incubation were scraped, pelleted and snap frozen for Western blot analysis. Cells were dissolved in 0.1N NaOH for 20 min, and protein concentration determined.
Western Blot Analyses
Western blots (5 –10 µg of protein loaded/lane) were performed as previously described [10]. Rabbit monoclonal, anti-phosphorylated ERK1/2 (Thr202/Tyr204; 1:1000, Cell Signaling Technology, Danvers, MA), mouse monoclonal, anti-ERK 1/2 (1:1000, Cell Signaling Technology) antibodies were used to analyze ERK1/2 phosphorylation activation status. NOS isoform specific expression was normalized to β-actin expression and analyzed as previously described (10).
Nitrite Measurements
Nitrite production was measured as an index of NO by high-performance liquid chromatography (ENO20, Eicom, Japan). All values were recorded as pmol of nitrite/ mg protein/h.
Statistics
All experiments were replicated on different plates of cells 4 times (N= 4). ANOVA and Two- Factor ANOVA (Drug and ET-1 treatment) were performed where appropriate. Dunnett’s post hoc tests were performed when ANOVA found significant differences, and Bonferroni post hoc test was performed when a Two-Factor ANOVA determined significance. P < 0.05 was considered significant.
Results
ET-1 regulation of nitrite production in mIMCD-3 cells is ETB receptor dependent
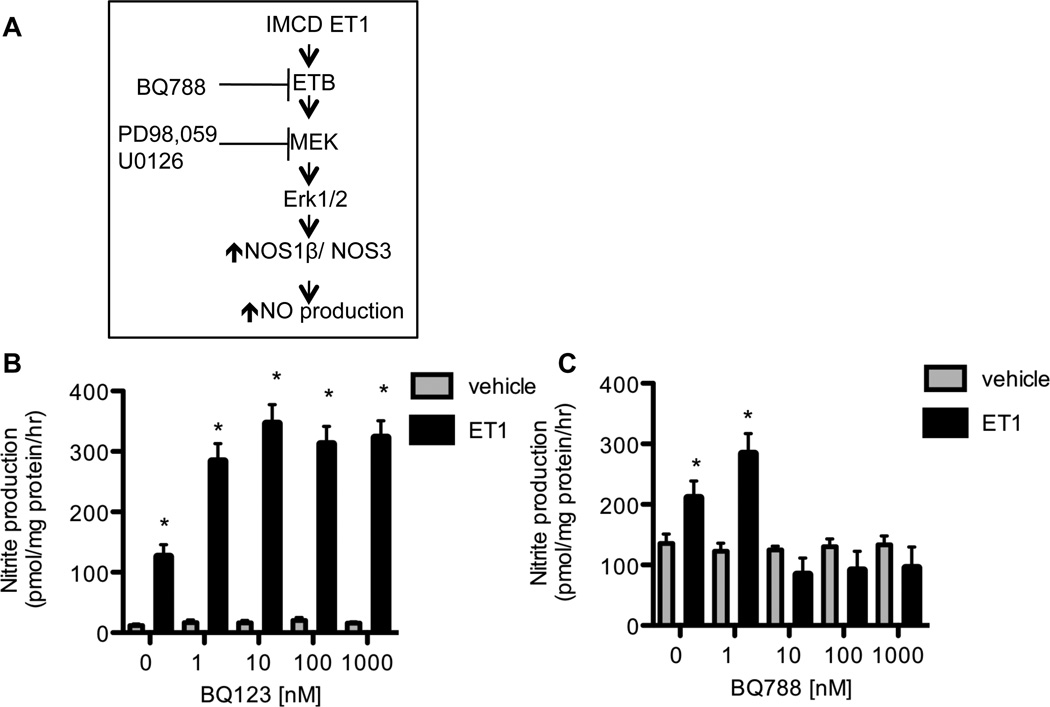
We found a significant increase in nitrite production with 50 nM and 100 nM ET-1 (221.1 ± 28.4 pmol/mg pr/h and 222.5 ± 33.7 pmol/mg pr/h, respectively), while 1 nM and 10 nM did not significantly increase nitrite production (51.1 ± 21.3 and 71.4 ± 30.3 pmol/mg pr/h, respectively) over basal nitrite production (54.5 ± 26.3 pmol/mg pr/h). All remaining experiments utilized 50 nM ET-1. Figure 1A shows the hypothetical scheme to be tested in this study.
Figure 1. ET-1 regulation of mIMCD-3 nitrite production.
A, Predicted scheme of inner medullary colleting duct ET-1, acting via the ETB receptor to regulate NO production through a MAPK dependent pathway. B) ETA receptor antagonism with BQ123 does not prevent the ET- 1 (50 nM for 24 h) dependent increase in mIMCD-3 nitrite production (Two-Factor ANOVA: antagonist p = 0.0025, ET-1 treatment P < 0.0001, interaction p = 0.125). C) ETB receptor antagonism with BQ788 blunts the ET-1 (50 nM for 24 h) dependent increase in mIMCD-3 nitrite production (Two-Factor ANOVA: Drug p = 0.007, ET-1 Treatment p < 0.001, interaction p = 0.002). N= 4. * P <0.05 vehicle vs. ET1.
Increasing concentrations of the ETA antagonist, BQ123, or the ETB antagonist, BQ788, were incubated with cells for 3 h prior to a 24 h ET-1 treatment. As seen in figure 1B, BQ123 did not blunt the ET-1 dependent increase in nitrite production (N= 4). However, BQ788 at 10–1000 nM significantly attenuated the ET-1 dependent increase in mIMCD-3 nitrite production (Figure 1C) (N= 4).
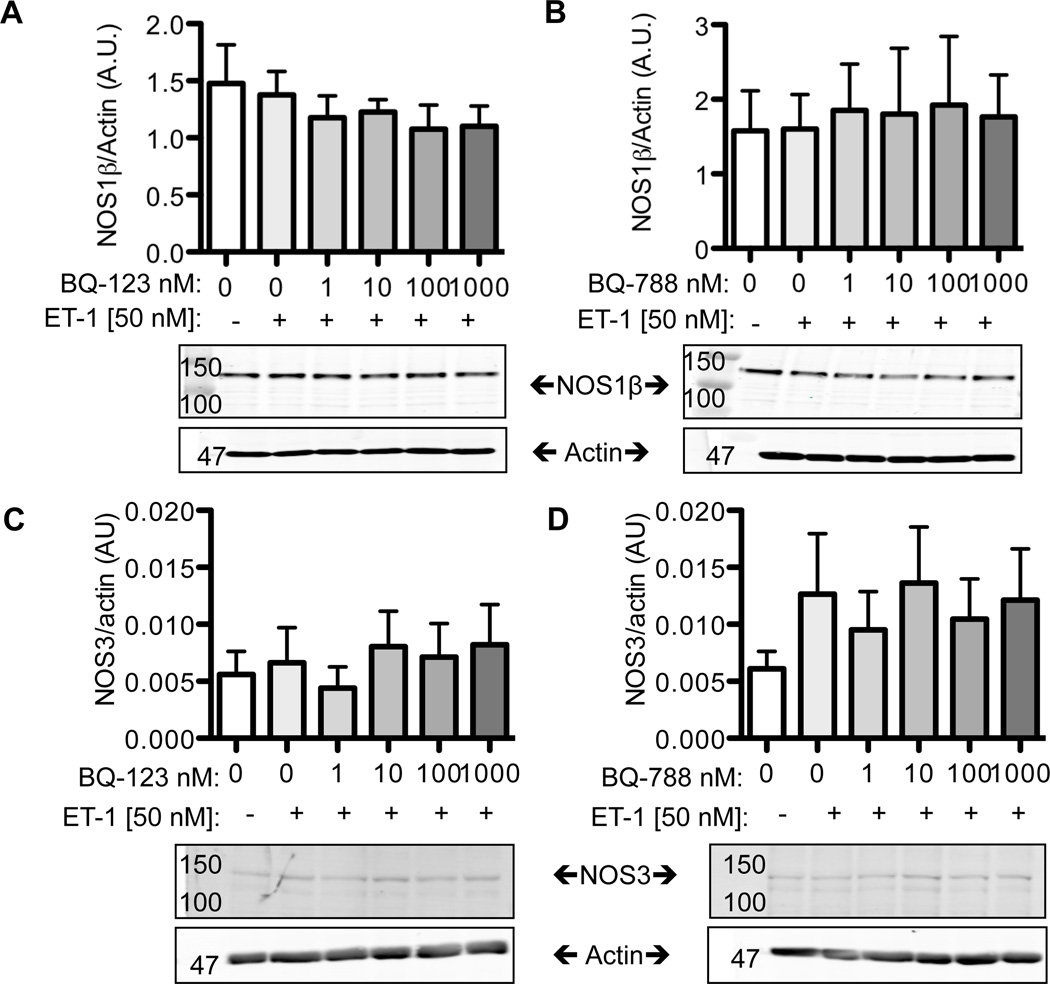
ET-1 does not regulate NOS1 or NOS3 expression in mIMCD-3 cells
To determine if the increases in nitrite production induced by 24 h ET-1 treatment in mIMCD-3 cells was dependent on changes in NOS1 and/or NOS3 expression, Western blots were performed. We found that mIMCD-3 cells express the NOS1β splice variant similar to freshly isolated mouse IMCD tubules, while freshly isolated rat IMCD tubules express both NOS1α and NOS1β (data not shown). Pretreatment of BQ123 (Fig 2A) or BQ788 (Fig 2B) prior to 24 h incubation with ET-1 did not alter the NOS1β expression in mIMCD-3 cells. Likewise, BQ123 (Fig 2C) or BQ788 (Fig 2D) did not alter mIMCD-3 NOS3 expression.
Figure 2. ET-1 does not regulate NOS expression in the mIMCD-3.
A, B) 50 nM ET-1 given for 24 h did not affect NOS1β expression in the mIMCD-3. Pretreatment for 3 h with various doses of the ETA antagonist (A), BQ123, or the ETB antagonist (B), BQ788 also did not affect NOS1β expression. C,D) 24 h, 50 nM ET-1 did not affect NOS3 expression in the mIMCD-3. Likewise, ETA blockade (C) or ETB (D) blockade did not significant affect NOS3 expression. N= 4.
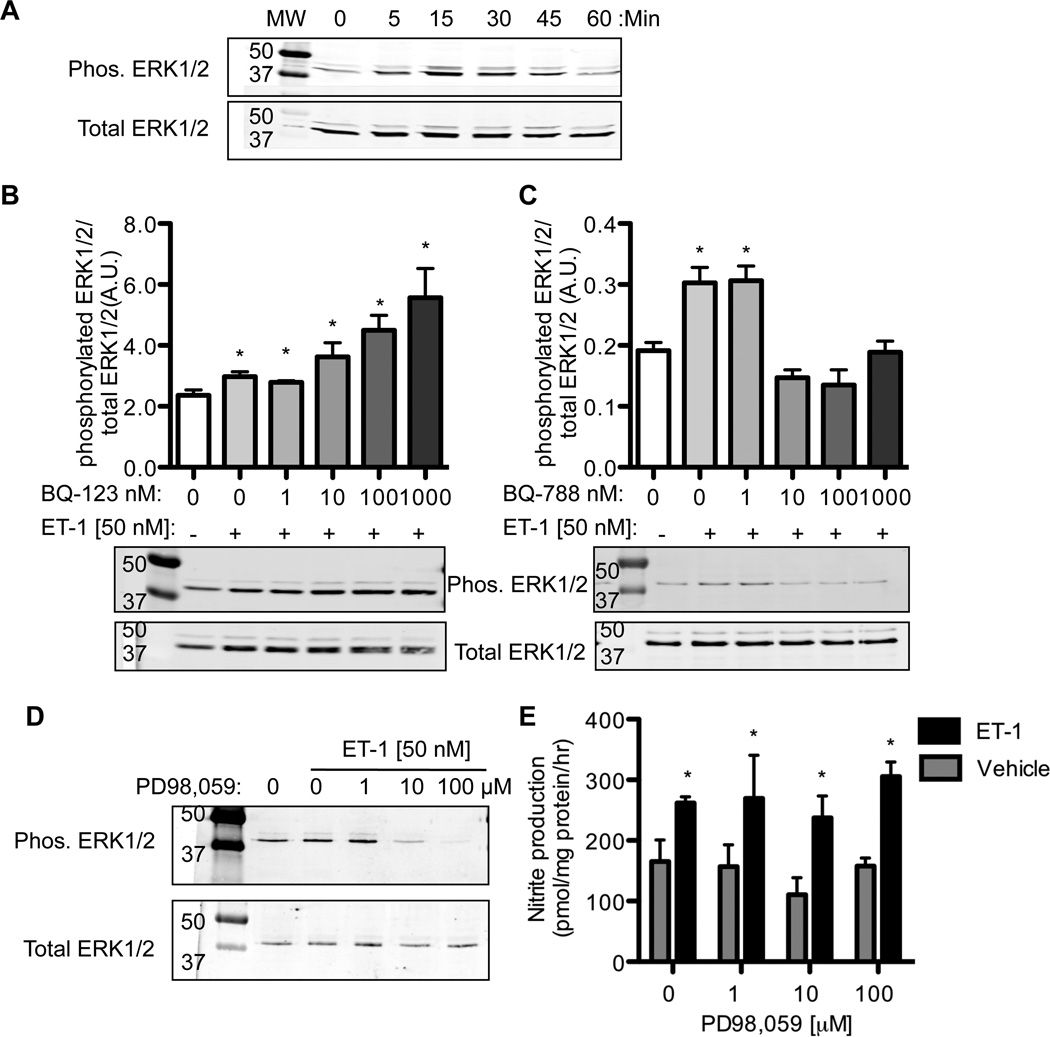
ET-1 dependent phosphorylation of ERK1/2
mIMCD-3 cells have a low level of basal phosphorylation of ERK1/2 (Fig 3A) and p38 MAPK (data not shown). ET-1 (50 nM) caused an increase in ERK1/2 phosphorylation after 15 and 30 min returning to basal levels after 60 min. ET-1 treatment did not affect p38 MAPK phosphorylation (data not shown). mIMCD-3 cells were pretreated with various concentrations of BQ123 or BQ788 and incubated with ET-1 for 15 min. As shown in Fig 3B, BQ123 treatment did not prevent the ET-1 induced phosphorylation of ERK1/2. As well, there was a concentration-dependent increase in phosphorylated ERK1/2 with increasing concentrations of BQ123 (N=4, ANOVA p =0.0008) suggesting ETB-mediated activation of ERK1/2. Consequently, 10–1000 nM BQ788 attenuated the ET-1 dependent increased phosphorylation of ERK1/2 (N= 4, ANOVA p < 0.0001).
Figure 3. mIMCD-3 cell phosphorylation of ERK1/2 by ET-1.
A) ET-1 (50 nM) was given for various times to mIMCD-3 and phosphorylation of ERK1/2 measured by Western blot. Peak phosphorylation occurred after 15 min of ET-1 exposure. B) ETA receptor antagonism (BQ123) lead to an increase in 15 min ET-1 stimulated ERK1/2 phosphorylation. C) ETB receptor antagonism (BQ788) lead to a decrease in 15 min ET-1 dependent ERK1/2 phosphorylation. D) The MEK1 inhibitor, PD98,059, prevented the phosphorylation of ERK1/2; however, PD98,059 did not prevent the ET-1 dependent increase in mIMCD-3 nitrite 15 production (E) (Two-Factor ANOVA: Drug p = 0.46, ET-1 treatment p < 0.0002, interaction p = 0.91). Note nitrite production was calculated as pmol nitrite/ mg protein/ h. * P<0.05 compared to time 0 or vehicle. N = 4.
MEK inhibition blocks ERK1/2 phosphorylation but does not affect nitrite production
MEK, the upstream kinase that phosphorylates ERK1/2 [22, 23], inhibitors were utilized to validate the ET-1 dependent activation of ERK1/2 phosphorylation in mIMCD-3 cells. The MEK inhibitors, PD98,059 or U0126, were preincubated with mIMCD-3 cells for 1 h before ET- 1 stimulation. PD98,059 treatment prevented the ET-1 induced phosphorylation of ERK1/2 (Fig 3D). Similar results were observed with U0126 (data not shown). PD98,059 (Fig 3E) and U0126 (data not shown) did not prevent the ET-1 stimulation of nitrite production (N = 4).
Discussion
Here we demonstrate that ET-1 stimulates NO production by the mouse IMCD via the ETB receptor in agreement with findings in the rat IMCD [9]. We hypothesized that ET-1 regulates IMCD NO production via the MEK/ERK1/2 pathway. ET-1 activates the MEK/ERK1/2 pathway in the rat nephron [24] and our data confirms that ET-1 activates the MEK/ERK1/2 pathway in the mouse IMCD via ETB receptor activation. Although ET-1 stimulates ERK1/2, inhibition of MEK did not prevent the ET-1 dependent increase in nitrite production in mIMCD-3 cells. This was unexpected because in various cell types ERK1/2 signaling was determined to regulate NO production [19] as well as NOS expression [15–18]. Thus, although ET-1 via ETB receptor increases phosphorylation of ERK1/2, and increases NO production, these pathways appear to be independent.
ET-1 regulation of NOS expression is complex, with the nephron segment (or cell type) and specific NOS isoforms and/or splice variants all being contributing factors. We did not find an increase in expression of NOS1 or NOS3 (the NOS isoforms expressed in the IMCD) with ET-1 activation of NO production. In the thick ascending limb (TAL), ET-1 stimulation (for 24 h) via the ETB receptor significantly increased NOS3 expression (the major NOS isoform expressed in the TAL).
Contrary to our findings presented here, we previously reported that mIMCD-3 express NOS1α and NOS3 and that exogenous ET-1 via the ETA receptor upregulates NOS1α, while NOS3 was unaffected [25]. Interestingly, in those experiments, NOS1α (155 kD) and not NOS1β was expressed in the mIMCD-3 cells. With our current lot of mIMCD-3 from ATCC (purchased in 2010), we found only NOS1β (130 kD) expression. Freshly isolated mouse IMCD express NOS1β, exclusively, while the rat IMCD expresses NOS1α and NOS1β [10, 11], thus the current mIMCD-3 cells appear to be reflective of primary mouse IMCD. Supporting the current finding that ET-1 does not regulate NOS1β or NOS3 expression in the IMCD, we previously reported that collecting-duct specific ET-1 knockout mice have similar levels of NOS1β and NOS3 in homogenates of the inner medulla compared to flox control mice [26]. Yet, we also reported that transgenic ETB deficient rats (sl/sl), with chronic increased circulating ET-1, demonstrate ETA-dependent increased inner medullary NOS1α and NOS1β expression when compared to wild-type rats [25]. Thus, further studies are needed to elucidate whether these differences are species-specific or model-specific but why the mIMCD-3 cells have different NOS1 splice variant expression, we can only speculate. It is plausible that between the ten years these two experiments were completed, that due to issues of subculturing and passaging of cell lines [e.g. 27, 28, 29], the mIMCD-3 have changed. mIMCD- 3 were isolated in 1991 [30], and the previous work was completed in 2000 and passages 6–9 were used [25]. In this study, cells were purchased in 2010, and only passages 3–5 were used. Thus, we speculate that protein expression profiles between these two cell cultures, separated by a decade, have changed.
Thus the question remains, how does ET-1 via the ETB receptor regulate NO production? ET-1 could potentially regulate NO production through numerous posttranslational modifications of NOS1 and/or NOS3, which is independent of the MAPK pathways. Phosphorylation of NOS3 is well documented [e.g. 31] and there is evidence for phosphorylation of NOS1 [e.g. 32]. Indeed, within the thick ascending limb of the kidney, ET-1 has been shown to increase NOS3 phosphorylation [33]; however whether this is true for the IMCD remains to be determined.
Protein-protein interactions are also potent stimulators of NOS activity [e.g. 32]. We hypothesize that ET-1 activates a protein-protein interaction to stimulate NOS1 and/or NOS3 activity and an increase in NO production. We recently identified that dynamin-2 interacts with NOS1 in the rat and mouse IMCD [10]. Dynamin-2 interacts with NOS1α as well as NOS1β leading to a significant increase in NO production (10). Our future experiments will be focused on determining whether ET-1 via ETB receptor increases the interaction between dynamin-2 and NOS1 and ultimately regulates NO production in IMCD.
Conclusion
The mIMCD-3 in the current study expresses NOS1β, unlike the rat IMCD, which expresses NOS1α and NOS1β [10, 11], allowing the unique opportunity to study ET-1 regulation of NOS1β in the collecting duct. ET-1 stimulates NO production, but it does not regulate NOS1β or NOS3 expression in this system. As well, ET-1 regulates phosphorylation of ERK1/2, but this signaling cascade is independent of ET-1 effects on mIMCD-3 NO production. Thus, the signaling cascade involved remains unknown, but likely involves protein-protein interactions integral in NOS activation and NO production.
Acknowledgements
This work was supported by NIH HL60653 and HL95499 (to J. S. Pollock), National Kidney Foundation Post Doctoral Fellowship (to K. A. Hyndman), and Diabetes and Obesity Discovery Institute summer medical student fellowship (to A. H. MacDonell).
Literature Cited
- 1.Kitamura K, Tanaka T, Kato J, Ogawa T, Eto T, Tanaka K. Immunoreactive endothelin in rat kidney inner medulla: marked decrease in spontaneously hypertensive rats. Biochem Biophys Res Commun. 1989;162(1):38–44. doi: 10.1016/0006-291x(89)91958-x. [DOI] [PubMed] [Google Scholar]
- 2.Ujiie K, Terada Y, Nonoguchi H, Shinohara M, Tomita K, Marumo F. Messenger RNA expression and synthesis of endothelin-1 along rat nephron segments. J Clin Invest. 1992;90(3):1043–1048. doi: 10.1172/JCI115918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol. 1999;276(6 Pt 2):F874–F881. doi: 10.1152/ajprenal.1999.276.6.F874. [DOI] [PubMed] [Google Scholar]
- 4.Mattson DL, Bellehumeur TG. Neural nitric oxide synthase in the renal medulla and blood pressure regulation. Hypertension. 1996;28(2):297–303. doi: 10.1161/01.hyp.28.2.297. [DOI] [PubMed] [Google Scholar]
- 5.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114(4):504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291(6):F1274–F1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 7.Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2008;295(6):F1635–F1640. doi: 10.1152/ajprenal.90279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera M, Garvin JL. Endothelin stimulates endothelial nitric oxide synthase expression in the thick ascending limb. Am J Physiol Renal Physiol. 2004;287(2):F231–F235. doi: 10.1152/ajprenal.00413.2003. [DOI] [PubMed] [Google Scholar]
- 9.Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol Renal Physiol. 2006;290(6):F1315–F1319. doi: 10.1152/ajprenal.00450.2005. [DOI] [PubMed] [Google Scholar]
- 10.Hyndman KA, Musall JB, Xue J, Pollock JS. Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1. Am J Physiol Renal Physiol. 2011;301(1):F118–F124. doi: 10.1152/ajprenal.00534.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith C, Merchant M, Fekete A, Nyugen HL, Oh P, Tain YL, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant. 2009;24(5):1422–1428. doi: 10.1093/ndt/gfn676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenman JE, Xia H, Chao DS, Black SM, Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci. 1997;19(3):224–231. doi: 10.1159/000111211. [DOI] [PubMed] [Google Scholar]
- 13.Chu TS, Wu MS, Wu KD, Hsieh BS. Endothelin-1 activates MAPKs and modulates cell cycle proteins in OKP cells. J Formos Med Assoc. 2007;106(4):273–280. doi: 10.1016/S0929-6646(09)60252-7. [DOI] [PubMed] [Google Scholar]
- 14.Watts SW. 5-Hydroxytryptamine-induced potentiation of endothelin-1- and norepinephrine-induced contraction is mitogen-activated protein kinase pathway dependent. Hypertension. 2000;35(1 Pt 2):244–248. doi: 10.1161/01.hyp.35.1.244. [DOI] [PubMed] [Google Scholar]
- 15.Giachini FR, Sullivan JC, Lima VV, Carneiro FS, Fortes ZB, Pollock DM, Carvalho MH, Webb RC, Tostes RC. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in desoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension. 2011;55(1):172–179. doi: 10.1161/HYPERTENSIONAHA.109.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korkmaz B, Buharalioglu K, Sahan-Firat S, Cuez T, Tuncay Demiryurek A, Tunctan B. Activation of MEK1/ERK1/2/iNOS/sGC/PKG pathway associated with peroxynitrite formation contributes to hypotension and vascular hyporeactivity in endotoxemic rats. Nitric Oxide. 2011;24(3):160–172. doi: 10.1016/j.niox.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Nakata S, Tsutsui M, Shimokawa H, Tamura M, Tasaki H, Morishita T, Suda O, Ueno S, Toyohira Y, Nakashima Y, Yanagihara N. Vascular neuronal NO synthase is selectively upregulated by platelet-derived growth factor: involvement of the MEK/ERK pathway. Arterioscler Thromb Vasc Biol. 2005;25(12):2502–2508. doi: 10.1161/01.ATV.0000190663.88143.97. [DOI] [PubMed] [Google Scholar]
- 18.Cao JL, Liu HL, Wang JK, Zeng YM. Cross talk between nitric oxide and ERK1/2 signaling pathway in the spinal cord mediates naloxone-precipitated withdrawal in morphine-dependent rats. Neuropharmacology. 2006;51(2):315–326. doi: 10.1016/j.neuropharm.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Wang HH, Hsieh HL, Yang CM. Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. J Cell Physiol. 2011;226(9):2244–2256. doi: 10.1002/jcp.22560. [DOI] [PubMed] [Google Scholar]
- 20.Cale JM, Bird IM. Inhibition of MEK/ERK1/2 signalling alters endothelial nitric oxide synthase activity in an agonist-dependent manner. Biochem J. 2006;398(2):279–288. doi: 10.1042/BJ20060371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt K, Gibraeil HD, Mayer B. Lack of involvement of extracellular signal-regulated kinase (ERK) in the agonist-induced endothelial nitric oxide synthesis. Biochem Pharmacol. 2002;63(6):1137–1142. doi: 10.1016/s0006-2952(01)00936-4. [DOI] [PubMed] [Google Scholar]
- 22.Brott BK, Alessandrini A, Largaespada DA, Copeland NG, Jenkins NA, Crews CM, Erikson RL. MEK2 is a kinase related to MEK1 and is differentially expressed in murine tissues. Cell Growth Differ. 1993;4(11):921–929. [PubMed] [Google Scholar]
- 23.Crews CM, Alessandrini A, Erikson RL. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- 24.Terada Y, Yamada T, Takayama M, Nonoguchi H, Sasaki S, Tomita K, Marumo F. Presence and regulation of Raf-1-K (Kinase), MAPK-K, MAP-K, and S6-K in rat nephron segments. J Am Soc Nephrol. 1995;6(6):1565–1577. doi: 10.1681/ASN.V661565. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan JC, Goodchild TT, Cai Z, Pollock DM, Pollock JS. Endothelin(A) (ET(A)) and ET(B) receptor-mediated regulation of nitric oxide synthase 1 (NOS1) and NOS3 isoforms in the renal inner medulla. Acta Physiol (Oxf) 2007;191(4):329–336. doi: 10.1111/j.1748-1716.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 26.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension. 2008;51(6):1605–1610. doi: 10.1161/HYPERTENSIONAHA.107.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esquenet M, Swinnen JV, Heyns W, Verhoeven G. LNCaP prostatic adenocarcinoma cells derived from low and high passage numbers display divergent responses not only to androgens but also to retinoids. J Steroid Biochem Mol Biol. 1997;62(5–6):391–399. doi: 10.1016/s0960-0760(97)00054-x. [DOI] [PubMed] [Google Scholar]
- 28.Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21(1):1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 29.Neumann E, Riepl B, Knedla A, Lefevre S, Tarner IH, Grifka J, Steinmeyer J, Scholmerich J, Gay S, Muller-Ladner U. Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2011;12(3):R83. doi: 10.1186/ar3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol. 1993;265(3 Pt 2):F416–F424. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- 31.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42(2):271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20(4):223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. J Biol Chem. 2009;284(3):1454–1460. doi: 10.1074/jbc.M804322200. [DOI] [PMC free article] [PubMed] [Google Scholar]