Abstract
Structural neuroimaging studies in autism report atypical volume in deep brain structures which are related to symptomatology. Little is known about metabolic changes in these regions, and how they vary with age and sex, and/or relate to clinical behaviors. Using magnetic resonance spectroscopy we measured N-acetylaspartate, choline, creatine, myoinositol and glutamate in the caudate, putamen, and thalamus of 20 children with autism and 16 typically developing controls (7–18 years). Relative to controls, individuals with autism had elevated glutamate/creatine in the putamen. In addition, both groups showed age-related increases in glutamate in this region. Boys, relative to girls had increased choline/creatine in the thalamus. Lastly, there were correlations between glutamate, choline, and myoinositol in all three regions, and behavioral scores in the ASD group. These findings suggest changes in deep gray matter neurochemistry, which are sensitive to diagnosis, age and sex, and are associated with behavioral differences.
Keywords: Magnetic resonance spectroscopy, Autism spectrum disorders, Deep gray matter, Caudate nucleus, Putamen, Thalamus and social cognition
1. Introduction
Atypical structure and function in the caudate, putamen and thalamus have been reported in individuals with autism spectrum disorders (ASD). However to date, little is known about the relation between metabolite concentrations in these regions and ASD symptomatology. These structures link the cortex and basal ganglia in a functional loop (Alexander & Crutcher, 1990; Alexander, DeLong, & Strick, 1986). The caudate and putamen, otherwise known as the striatum, are the “input” structures of the basal ganglia and receive projections from the cortex (Alexander & Crutcher, 1990; Alexander et al., 1986). The striatum mediates behaviors such as voluntary motor control (Grillner, Hellgren, Ménard, Saitoh, & Wikström, 2005), learning (Doya, 2000) and cognition (Balleine, Delgado, & Hikosaka, 2007). The thalamus relays sensory and motor information from the basal ganglia and other regions of the brain to the cortex.
Structural magnetic resonance imaging studies have reported a significant increase in the volume of the caudate in individuals with ASD, relative to typically developing controls (Haznedar et al., 2006; Hollander et al., 2005; Rojas et al., 2006; Sears et al., 1999; Voelbel, Bates, Buckman, Pandina, & Hendren, 2006). Caudate volume in ASD has also been found to increase with age from childhood to adulthood, while caudate volume decreased with age in controls (Langen et al., 2009). Atypical caudate and total putamen volumes were found to correlate positively with repetitive behavior scores on the autism diagnostic interview-revised (ADI-R) (Hollander et al., 2005), and in addition, caudate volume abnormalities were significantly associated with an insistence on sameness also measured by the ADI-R (Langen et al., 2009). Additionally, lower glucose metabolic activity in the caudate, putamen and thalamus has been reported, bilaterally, in individuals with ASD (Haznedar et al., 2006). Despite these findings, our understanding of metabolite concentrations in these regions and how they relate to clinically significant ASD behaviors such as social-communication impairments and repetitive behaviors are largely unknown.
Magnetic resonance spectroscopy (MRS) is a non-invasive imaging technique that can provide metabolite and biochemical information about the brain. Specific metabolite concentrations can indicate neuronal and/or glial density, cell-membrane processes or energy metabolism within brain regions (Cecil & Jones, 2001; Soares & Law, 2009). N-acetylaspartate (NAA) forms the most robust spectral peak because of its abundance in the brain, second in quantity only to glutamate. NAA is often viewed as a marker of neuronal integrity and may be involved in synaptic maintenance, axonal myelination and cellular osmosis (Birken & Oldendorf, 1989; Cecil & Jones, 2001; Miller, 1991; Soares & Law, 2009). Peaks attributed to choline-containing compounds (Cho) are thought to indicate glial density and processes involved in membrane metabolism (Cecil & Jones, 2001; Miller, 1991; Soares & Law, 2009). The creatine + phosphocreatine (Cr) spectrum may also reflect neuronal and/or glial density, as well as energy metabolism (Cecil & Jones, 2001; Miller, 1991; Soares & Law, 2009). The myoinositol (Ins) concentration is thought to be a marker of glial cells, as well as to reflect processes associated with the breakdown of myelin (Berridge, 1984; Cecil & Jones, 2001; Soares & Law, 2009). Lastly, glutamate, glumatine and gamma-aminobutyric acid result in a complex set of peaks, which signal excitatory/inhibitory neuronal function (Berridge, 1984; Mark et al., 2001; Soares & Law, 2009). Glutamate is the main excitatory neurotransmitter and is the most abundant amino acid found in the brain; whereas gamma-aminobutyric acid is derived from glutamate and is the main inhibitory neurotransmitter in the brain (Berridge, 1984; Mark et al., 2001; Soares & Law, 2009).
Changes in brain metabolite concentrations have been used clinically as a way to identify neural insult and understand disease progression in conditions such as tumoural disease (Daly & Cohen, 1989; Hagberg, 1998; Hollingworth et al., 2006; Preul et al., 1996), multiple sclerosis (Davie et al., 1994; De Stefano et al., 1998; Miller, Grossman, Reingold, & McFarland, 1998) and Alzheimer’s disease (Barber et al., 1999; Chui et al., 1992). There are increasing efforts to identity reliable biomarkers for ASD that may allow for early screening and treatment (Ipser et al., 2012; Pickett & London, 2005). To date only 3 studies, in children with ASD have examined metabolite concentration in deep gray matter (Friedman et al., 2003; Hardan et al., 2008; Levitt et al., 2003). Cr levels were reported as reduced in the left thalamus (Friedman et al., 2003; Hardan et al., 2008). Concentrations of Cho were found to be lower in the left thalamus (Hardan et al., 2008) and in the body of the left caudate nucleus (Hardan et al., 2008; Levitt et al., 2003), but higher in the head of the right caudate (Levitt et al., 2003). These studies did not examine age- and sex-related changes in metabolite concentrations and the relation between metabolic changes and the three core features of ASD, which are impaired social interaction, communication and stereotyped and repetitive behaviors (APA, 1994). The examination of these associations could provide insight into whether metabolic changes have behavioral significance, and potentially, clinical utility.
Thus, in the present study we used MRS to examine metabolite concentrations in the caudate, putamen and thalamus in individuals with ASD, aged 7–18 years relative to age- and IQ-matched typically developing controls, and examined how concentrations changed with age, sex and related to ASD symptomatology.
2. Materials and methods
2.1. Ethics and consent
This study was approved by institutional Research Ethics Board and conducted in accordance with its guidelines. All participants provided written informed assent and parents provided written informed consent in accordance with Research Ethics Board guidelines.
2.2. Participants
Twenty higher-functioning ASD participants between the ages of 7–18 years were recruited from the Seaver Autism Center at Mount Sinai School of Medicine (mean age = 11.5 ± 3.0; mean FSIQ = 99 ± 18). Sixteen controls were recruited from local newspaper advertisements and through word of mouth, and were group-matched on age and IQ (mean age = 12.5 ± 3.6, mean FSIQ = 101 ± 13) (Please see Table 1 for more details). ASD participants had a clinical diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (APA, 1994) and were not on psychotropic-medications or concomitant medications that would influence results. Diagnoses were confirmed at the time of testing using the Autism Diagnostic Observational Schedule-Generic (ADOS) (Lord, Risi, et al., 2000) and the ADI-R (Lord, Rutter, & Le Couteur, 1994). Any participants who had a primary psychiatric condition (other than ASD in the ASD group), a history of head injury, epilepsy, neuromotor impairment or a genetic disorders associated with ASD were excluded. Additionally, controls were excluded if they had a first-degree relative with ASD. All participants were right handed and had an IQ over 70 as estimated by either the Wechsler Intelligence Scale for Children – 4th edition (Wechsler, 2003) or the Wechsler Adult Intelligence Scale – 4th edition (Wechsler, 2008) depending on their age.
Table 1.
Demographic table ASD (n = 20) TD (n = 16) p value.
| ASD (n = 20) (mean, SD, range) | TD (n = 16) (mean, SD, range) | p value | |
|---|---|---|---|
| Age distribution | |||
| Age 7–18 years | 11.5 ± 3.0, 7–18 | 12.9 ± 4.1, 7–18 | t(34) = 1.2, p = 0.25 |
| FSIQ | 99.0 ± 18.2, 70–130 | 100.1 ± 12.8, 78–128 | t(34) = 0.3, p = 0.76 |
| Gender (M:F) | 15:5 | 8:8v | |
| ADI-R total scores | |||
| Communication | 14.0 ± 4.0 | ||
| Social | 16.2 ± 4.7 | ||
| Behaviors | 5.7 ± 2.6 | ||
| ADOS total scores | |||
| Communication | 3.4 ± 1.6 | ||
| Social | 6.8 ± 2.3 | ||
| Stereotyped/Repetitive behaviors | 2.1 ± 2.4 | ||
Abbreviations: FSIQ: Full scale IQ; F: female; M: male; n: number of participants; SD: standard deviation; ADI-R: autism diagnostic interview – revised; ADOS: autism diagnostic observation schedule generic.
2.3. Data acquisition
Participants were scanned on a Siemens Allegra 3 Tesla MRI system. A low-resolution high-speed scout image was obtained, followed by a series of axial scans. A high-resolution T1-weighted MPRAGE sequence was acquired: isotropic resolution of 0.82 mm × 0.82 mm × 0.82 mm, matrix size = 256 × 256 × 208, field of view = 210 mm, 208 slices, repetition time = 2500 ms, echo time = 4.38 ms, inversion time = 1100 ms and an 8° flip angle fast low angle shot acquisition. A two-dimensional magnetic resonance spectroscopic imaging sequence in an axial plane through the striatum (echo time/repetition time = 30/2000 ms, 10 mm slice thickness, 16 cm field of view with 24 × 24 phase-encoded steps, zero-filled to 32 × 32) was obtained. Total scan time was ~45 min.
2.4. Data analyses
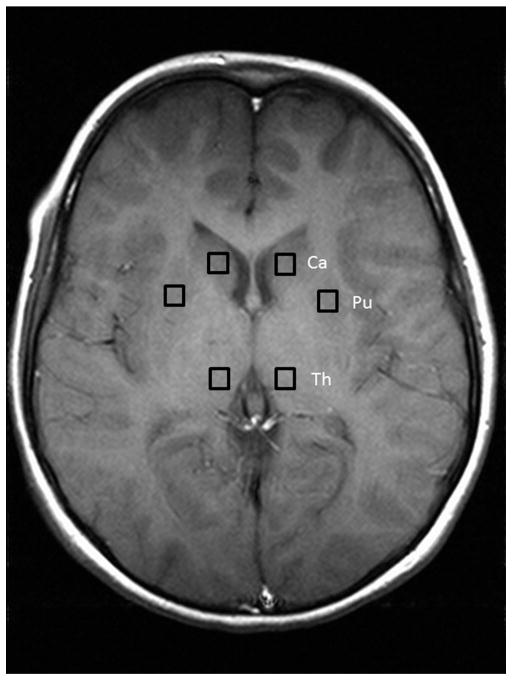
For each participant, points were visually selected in the caudate, putamen and thalamus bilaterally, and the magnetic resonance spectroscopic data was Fourier shifted to extract a spectrum for each of these locations (Fig. 1). The left and right spectra for each location were investigated separately and averaged. The results were processed with LCModel 6.2 (Provencher, 2001) to obtain concentration estimates for the following metabolites in each location in each subject: Cr, Cho, NAA, Ins and glutamate + glutamine (Glx). The error estimates from LCModel were used to exclude any concentration estimates with standard deviation <20% (Provencher, 2001). Ratios relative to Cr were calculated in order to control for the potential effects of differences in brain water content, T1 and T2 relaxation times of water, and the presence of CSF.
Fig. 1.
Location of regions of interest. A depiction of the locations of the voxels and the slice they were taken from in one participant.
2.5. Statistical analyses
Statistical analyses were done in R (R Foundation for Statistical Computing, Vienna, Austria).
To test for the possible influence of laterality and group on the individual MRS ratios from the left and right sides of the brain, we analyzed this hierarchically structured data using a multi-level mixed-effects model, with the MRS ratios from each hemisphere as the level-one covariate, nested within the individual participants (the level two covariate), where each participant was identified as belonging to the control or ASD groups.
This analysis was first run for all combinations of metabolite ratios (Cho/Cr, NAA/Cr, Ins/Cr, Glx/Cr) and locations (Caudate, Putamen, and Thalamus), using the relevant ratio as the dependent variable and laterality, group, and the laterality-group interaction as independent variables, to test for between level interactions, while adjusting for random effect. Where the three-way interaction term was not significant, that term was dropped and the analysis was re-run using only laterality and group as independent variables.
To maximize power, left and right spectra were also averaged. For each of the three locations, the four metabolite ratios were analyzed with respect to the impact of age, group, and sex. An ANCOVA analysis including age, group, sex and terms for the group by sex and group by sex by age interactions was used to verify that there were no significant differences in dependence on age in each case. Where the three-way interaction term was not significant, that term was dropped, and the analysis was rerun to check for a possible interaction between group and sex. Where the latter interaction was not significant, it too was dropped, and a final ANCOVA was run on group, sex and age, with no interactions, to evaluate possible differences in metabolite ratios between groups and/or sexes.
For the ASD group, an ANCOVA analysis was similarly used evaluate the relation between metabolite ratios and behavioral scores (ADI-R and ADOS algorithm total scores for all behavioral domains) accounting for sex. Where the interaction between behavioral scores and sex was not significant, the analysis was rerun without the interaction term. Where there was no significant difference between sexes in the resulting analysis, the data from both sexes were pooled, and the Pearson correlations were calculated between metabolite ratios and behavioral scores.
Results were considered significant at p < 0.05. Due to the exploratory nature of the analysis, results were not corrected for multiple comparisons.
3. Results
3.1. Group effects
No significant differences were found in age or in full-scale IQ between groups (Table 1).
3.1.1. Lateralization effects
No lateralization differences were found between groups for any metabolite (laterality-group interaction was p > 0.05 in all cases). When both groups were collapsed, Cho/Cr in the thalamus was significantly different between the left and right hemispheres (left > right; p = 0.0107), but not between groups.
3.2. Averaged data
3.2.1. Putamen
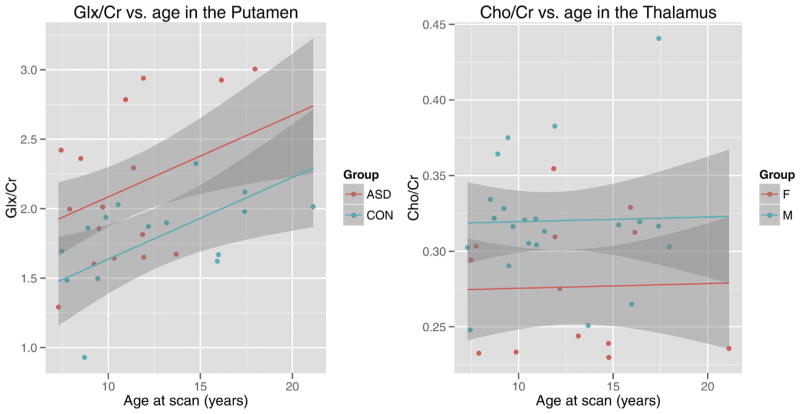
No interactions were found in the putamen involving age, group, and sex. Significant group (p = 0.008) and age (p = 0.044) effects were found for Glx/Cr in the putamen. Individuals with ASD had a higher Glx/Cr concentration than controls. Glx/Cr concentration, in both groups, increased with age (Fig. 2a).
Fig. 2.
Age-related changes in metabolic concentrations in individuals with and without autism spectrum disorders. On the left: A scatter plot depicting glutamate (Glx/Cr) in the putamen for individuals with ASD and typically developing controls. On the right: a scatter plot depicting choline (Cho/Cr) in the thalamus for boys and girls. 95% confidence intervals are shown in the shaded regions. Abbreviations: ASD: autism spectrum disorders; CON: typically developing controls, F: female and M: male.
3.2.2. Caudate
No differences in metabolite concentrations were found between groups, across age or between sexes, nor were there any interactions found among these factors in the caudate.
3.2.3. Thalamus
No group by age interactions were found in the thalamus involving age, group and sex. A significant effect of sex (p = 0.007) was found in Cho/Cr concentration in the thalamus (Fig. 2b). Males, compared to females had a higher concentration of Cho/Cr in the thalamus, irrespective of diagnosis.
3.3. Associations between metabolite concentrations and ASD symptoms
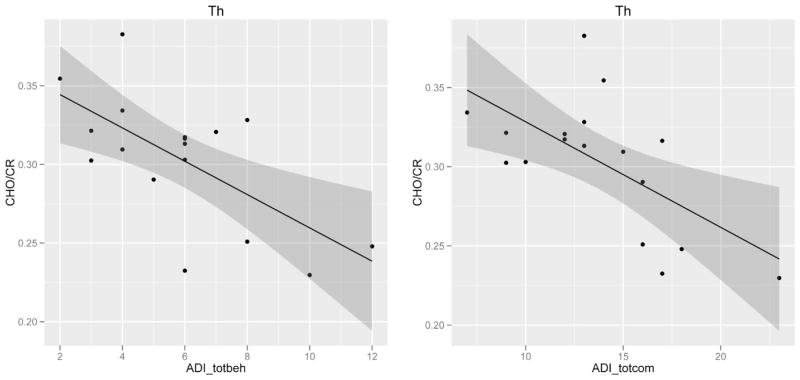
In the ASD group, Ins/Cr in the putamen and caudate was positively associated with ADI-R total communication scores (p = 0.049, r = 0.605) and ADOS total stereotyped and repetitive behavior scores (p = 0.046, r = 0.610), respectively. Cho/Cr in the thalamus was negatively associated with ADI-R total communication score (p = 0.007, r = −0.628), and total restricted and repetitive behaviors score (p = 0.004, r = −0.656) (Fig. 3). Lastly, Glx/Cr in the thalamus was positively related to ADI-R total social interaction score (p = 0.018, r = 0.599).
Fig. 3.
Example of correlation of metabolite ratios with core symptom domain severity. Scatter plots of choline (Cho/Cr) concentration in the thalamus against total behavior, compulsions, and stereotyped behaviors scores, and total communication scores from the autism diagnostic interview-revised, in individuals with autism spectrum disorders. 95% confidence intervals are shown in the shaded regions. Abbreviations: Th: thalamus, ADI_totbeh: autism diagnostic interview revised – total behavior, compulsions, and stereotyped behaviors score, and ADI_totcom: utism diagnostic interview revised – total communication scores.
4. Discussion
This study found an elevated glutamate concentration, relative to creatine, in the putamen in the ASD group relative to controls. Glutamate/creatine in the putamen was also found to increase with age in both groups. Males, relative to females were observed to have increased levels of choline/creatine in the thalamus. In the ASD group, brain-behavior associations were found between Ins/Cr in the putamen and caudate, as well as Cho/Cr and Glx/Cr in the thalamus, and social interaction, communication and repetitive behavior scores on diagnostic measures.
The significantly elevated Glx/Cr concentration in the putamen found in the present study is consistent with the hyperglutamatergic hypothesis in ASD (Fatemi, 2008). Serum glutamate levels have been found to be substantially increased in individuals with ASD (Shinohe et al., 2006) and this elevated level is hypothesized to be related to findings of increased gliosis in ASD (Laurence & Fatemi, 2005). In addition, ASD has been associated with an increase in the ratio of cortical excitation to inhibition (Rubenstein & Merzenich, 2003) which may be related to reduced ‘inhibitory surround’ in cortical minicolums (Casanova, Buxhoeveden, & Brown, 2002). Increased excitation is also believed to contribute to sensory hyper-reactivity in auditory, visual and tactile domains in ASD (Sansa et al., 2011). Thus, the elevated Glx/Cr in the putamen that we observed may suggest that glutametergic atypicalities also extend to deep brain structures at least in a subset of individuals with ASD.
Previous studies have reported group differences, in particular in Cr and Cho in the thalamus and Cho in the caudate (Friedman et al., 2003; Hardan et al., 2008; Levitt et al., 2003), which we did not find. Detailed reports of symptomatology were not outlined in these prior studies; therefore clinical differences between samples could not be considered. Furthermore, previous studies reported absolute concentration levels, whereas the present study reported metabolite levels as ratios against Cr. Properly reporting absolute metabolite concentrations requires accounting for additional factors related to tissue-type, and relaxation times. Unfortunately, reference scans without water suppression were not acquired in this study. As a result, absolute concentrations for individual metabolites cannot be accurately estimated for each subject. The use of ratios does not necessarily assume one of the metabolites to be constant (Miller, 1991; Soares & Law, 2009), but rather treats the ratio as a single measurement. The use of ratios accounts for all non-metabolite-specific differences, and these ratios therefore can be reasonably compared across all participants scanned at the same institution with the same protocol.
Some brain-behavior associations that we found were for metabolites where concentrations were not significantly different between groups. Although this may reflect a lack of power and a limitation of this study, our results were consistent with subtle brain-behavior relations and with results previously reported in ASD and other clinical populations.
Lower concentrations of Cho/Cr in the thalamus were associated with more severe stereotyped behaviors and communication impairments. Prior studies have reported significantly lower levels of Cho in the thalamus in ASD (Hardan et al., 2008; Levitt et al., 2003). The Cho peak is thought to be a measure of glial density and processes involved in membrane metabolism (Miller, 1991; Soares & Law, 2009). For example, elevated Cho concentrations are observed in the presence of tumors and inflammation (Cecil & Jones, 2001; Miller, 1991). In contrast, higher levels of Ins/Cr in the caudate and putamen were associated with more stereotypies and communication impairments, respectively. Ins is also found in glial cells (Berridge, 1984; Cecil & Jones, 2001; Soares & Law, 2009). In pathological conditions such as Alzheimer’s dementia, significantly higher levels are thought to reflect gliosis and astrocytosis (Chen et al., 2009; Miller et al., 1993; Shonk et al., 1995; Zhu et al., 2006). Thus similar behavioral impairments were associated with differences in opposite directions for these metabolites. These findings suggest that although choline and myoinositol are both linked to glial cells and its membrane, we may not fully understand the mechanisms of these metabolites. Further research is needed to elucidate this issue.
It should be noted that metabolite concentrations are an indirect measure of cellular properties, therefore we can only infer potential cellular atypicalities in the regions of interest. Neuropathological studies have been limited in their reports of cellular atypicalities in the striatum and thalamus, and few studies have commented on molecular properties in the striatum and thalamus in ASD, and no neuropathological findings were reported (Bailey et al., 1998; Bauman & Kemper, 2005; Kemper and Bauman, 1998; Lord, Risi, et al., 2000). However, only a few cases were examined in these investigations and therefore more neuropathology studies are needed to examine neural cell density, organization and synaptic properties and their relation to changes in metabolite concentrations in ASD.
While the present study provides insight into metabolite concentrations in deep brain structures and their relation to ASD symptomatology, this study should be replicated in a larger sample as well as with ASD individuals with a wider range of functional abilities. A larger sample will increase statistical power to substantiate results, and measurements in lower functioning ASD children may reveal more significant changes in metabolic levels and associations with symptomatology. In addition, longitudinal measurement may reveal abnormalities in the developmental trajectory of these metabolites, since studies in adults with ASD with typical intelligence showed involvement of the same deep brain structures in the pathophysiology of ASD (Manouilenko, Pagani, Stone-Elander, Odh, & Brolin, 2013). Our investigation was limited to deep brain structures captured in the axial plane where measurements were acquired. Imaging in other planes may reveal other metabolic changes in ASD across the brain, which may be further related to symptomatology.
In sum, we found differences in glutamate/creatine in individuals with ASD compared to typically developing controls. Glutamate/creatine was found to increase with age in both groups. Also differences in choline/creatine were found between males and females in the thalamus. Within the ASD group, concentrations of Glx/Cr, Ins/Cr and Cho/Cr in the caudate, putamen and thalamus were associated with clinically significant behaviors. These findings show neurochemical changes in deep gray matter in ASD, and suggest that some atypicalities may be associated with clinically meaningful behavioral differences.
Acknowledgments
This research was supported in part by National Alliance for Research on Schizophrenia and Depression (NARSAD); Autism Speaks; and the Seaver Foundation. The data for this study were collected at Mount Sinai School of Medicine in New York, New York. The analyses were carried out at Holland Bloorview Kids Rehabilitation Hospital and the Hospital for Sick Children.
Footnotes
Conflicts of interest
Dr. Anagnostou has received consultation fees from Novartis and Seaside Therapeutics and an unrestricted grant by Sanofi Canada. None of the other authors have any conflicts of interest to disclose.
Contributor Information
Krissy A.R. Doyle-Thomas, Email: kdoylethomas@hollandbloorview.ca.
Dallas Card, Email: dallas.card@gmail.com.
Latha V. Soorya, Email: latha.soorya@mssm.edu.
A. Ting Wang, Email: ting.wang@mssm.edu.
Jin Fan, Email: jin.fan@mssm.edu.
Evdokia Anagnostou, Email: eanagnostou@hollandbloorview.ca.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends in Neuroscience. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Publishing; 1994. [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, et al. White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. Journal of Neurology, Neurosurgery & Psychiatry. 1999;67:66–72. doi: 10.1136/jnnp.67.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. International Journal of Developmental Neuroscience. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and diacylglycerol as second messengers. Biochemical Journal. 1984;220:345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H NMR spectroscopic studies of brain. Neuroscience & Biobehavioural Reviews. 1989;13:23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. Journal of Child Neurology. 2002;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Jones BV. Magnetic resonance spectroscopy of the pediatric brain. Topics in Magnetic Resonance Imaging. 2001;12:435–452. doi: 10.1097/00002142-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Chen SQ, Wang PJ, Ten GJ, Zhan W, Li MH, Zang FC. Role of myo-inositol by magnetic resonance spectroscopy in early diagnosis of Alzheimer’s disease in APP/PS1 transgenic mice. Dementia & Geriatric Cognitive Disorders. 2009;28:558–566. doi: 10.1159/000261646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- Daly PF, Cohen JS. Magnetic resonance spectroscopy of tumors and potential in vivo clinical applications: a review. Cancer Research. 1989;49:770–779. [PubMed] [Google Scholar]
- Davie CA, Hawkins CP, Barker GJ, Brennan A, Tofts PS, Miller DH, et al. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994;117:49–58. doi: 10.1093/brain/117.1.49. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, et al. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis, results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121:1469–1477. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Current Opinion in Neurobiology. 2000;10:732–739. doi: 10.1016/s0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. The hyperglutamatergic hypothesis of autism. Progress in Neuropsychopharmacology & Biological Psychiatry. 2008;32:911. doi: 10.1016/j.pnpbp.2007.11.004. author reply 912–913. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Richards TL, Gardner J, Dawson G, et al. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology. 2003;60:100–107. doi: 10.1212/wnl.60.1.100. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs – Roles for the striatum and pallidum. Trends in Neuroscience. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hagberg G. From magnetic resonance spectroscopy to classification of tumors. A review of pattern recognition methods. NMR in Biomedicine. 1998;11:148–156. doi: 10.1002/(sici)1099-1492(199806/08)11:4/5<148::aid-nbm511>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Melhem NM, Srihari S, Jo B, Bansal R, et al. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Research. 2008;163:97–105. doi: 10.1016/j.pscychresns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. American Journal of Psychiatry. 2006;163:1252–1263. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Hollingworth W, Medina LS, Lenkinski RE, Shibata DK, Bernal B, Zurakowski D, et al. A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors. American Journal of Neuroradiology. 2006;27:1404–1411. [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Syal S, Bentley J, Adnams CM, Steyn B, Stein DJ. 1H-MRS in autism spectrum disorders: A systematic meta-analysis. Metabolic Brain Disease. 2012;27:275–287. doi: 10.1007/s11011-012-9293-y. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. Journal of Neuropathology & Experimental Neurology. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, de Jonge MV, et al. Changes in the developmental trajectories of striatum in autism. Biological Psychiatry. 2009;66:327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Laurence JA, Fatemi SH. Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum. 2005;4:206–210. doi: 10.1080/14734220500208846. [DOI] [PubMed] [Google Scholar]
- Levitt JG, O’Neill J, Blanton RE, Smalley S, Fadale D, McCracken JT, et al. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biological Psychiatry. 2003;54:1355–1366. doi: 10.1016/s0006-3223(03)00688-7. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism & Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Manouilenko I, Pagani M, Stone-Elander S, Odh R, Brolin R, et al. Autistic traits, ADHD symptoms, neurological soft signs and regional cerebral blood clow in adults with autism spectrum disorders. Research in Autism Spectrum Disorders. 2013;7:566–578. [Google Scholar]
- Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM, et al. Pictorial review of glutamate excitotoxicity: Fundamental concepts for neuroimaging. American Journal of Neuroradiology. 2001;22:1813–1824. [PMC free article] [PubMed] [Google Scholar]
- Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR in Biomedicine. 1991;4:47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: Depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187:433–437. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain. 1998;121:3–24. doi: 10.1093/brain/121.1.3. [DOI] [PubMed] [Google Scholar]
- Pickett J, London E. The neuropathology of autism: a review. Journal of Neuropathology & Experimental Neurology. 2005;64:925–935. doi: 10.1097/01.jnen.0000186921.42592.6c. [DOI] [PubMed] [Google Scholar]
- Preul MC, Caramanos Z, Collins DL, Villemure JG, Leblanc R, Olivier A, et al. Accurate, noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nature Medicine. 1996;2:323–325. doi: 10.1038/nm0396-323. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LC model. NMR in Biomedicine. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56–69. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain & Behaviour. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1999;23:613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Sansa G, Carlson C, Doyle W, Weiner HL, Bluvstein J, Barr W, et al. Medically refractory epilepsy in autism. Epilepsia. 2011;52:1071–1075. doi: 10.1111/j.1528-1167.2011.03069.x. [DOI] [PubMed] [Google Scholar]
- Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, et al. Increased serum levels of glutamate in adult patients with autism. Progress in Neuropsychopharmacology & Biological Psychiatry. 2006;30:1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Shonk TK, Moats RA, Gifford P, Michaelis T, Mandigo JC, Izumi J, et al. Probable Alzheimer disease: Diagnosis with proton MR spectroscopy. Radiology. 1995;195:65–72. doi: 10.1148/radiology.195.1.7892497. [DOI] [PubMed] [Google Scholar]
- Soares DP, Law M. Magnetic resonance spectroscopy of the brain: Review of metabolites and clinical applications. Clinical Radiology. 2009;64:12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Voelbel GT, Bates ME, Buckman JF, Pandina G, Hendren RL. Caudate nucleus volume and cognitive performance: Are they related in childhood psychopathology. Biological Psychiatry. 2006;60:942–950. doi: 10.1016/j.biopsych.2006.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children (WISC-IV) 4. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 4. San Antonio, TX: Pearson; 2008. [Google Scholar]
- Zhu X, Schuff N, Kornak J, Soher B, Yaffe K, Kramer JH, et al. Effects of Alzheimer disease on fronto-parietal brain N-acetyl aspartate and myo-inositol using magnetic resonance spectroscopic imaging. Alzheimer Disease & Associated Disorders. 2006;20:77–85. doi: 10.1097/01.wad.0000213809.12553.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]