Abstract
We examined factors that affect decision-making for families presented with a phase I clinical trial of hepatocyte transplant as a potential alternative to liver transplant for their children among two groups: 1) families who were actually offered enrollment in the hepatocyte trial and; 2) families whose children had liver transplants before the trial was available. We conducted semi-structured interviews about actual and hypothetical decision-making regarding trial participation and used grounded theory analysis to identify common themes. The most common motivator for participation was decline in the child's health. The most common deterrent was lack of data from prior hepatocyte transplants, particularly compared to data available about liver transplant. Interviewees' point of comparison for evaluating relative benefits and risks of hepatocyte transplant oscillated between the alternative of doing nothing while waiting for a liver (the relevant alternative) versus the alternative of getting a liver. These results suggest that families' reluctance to participate may result from misconceptions about severity of the child's disease, underestimating risks of liver transplant, or confusion about the role of hepatocyte transplant in the treatment pathway. Clarification of available treatment alternatives and associated risks as part of informed consent may improve the quality of decision-making regarding trial enrollment.
Keywords: parental consent, pediatric clinical trials, liver transplantation
Enrollment is a limiting factor in pediatric clinical trials, especially in Phase I studies (1). Challenges include limited patient populations, especially in trials targeting rare diseases, primary care physicians' hesitation to refer patients, and families' reluctance to participate (2). While prior studies have identified motivators for trial participation in pediatric populations, these involved either minimal risk trials (3–6) or oncology trials, in which participants choose between trial therapy and palliation (7–8).
In the current study, we explored families' decision-making processes with regard to an ongoing phase I trial of hepatocyte transplantation (9) for metabolic liver disease or acute hepatic failure. Hepatocyte transplantation involves infusion of hepatocytes through the portal vein via a catheter in the umbilical vein. For children with metabolic disorders, preliminary low-dose focal radiation therapy to the liver is used to promote donor cell engraftment. Subjects considering trial participation choose between receiving trial therapy while waiting for liver transplant versus doing nothing while waiting for liver transplant. Trial enrollment does not influence transplant waitlist position. Known incremental risks of the trial protocol include complications of portal vein cannulation and cancer risk from liver irradiation. The potential benefit is improved liver function that could obviate the need for liver transplant. Furthermore, in case of infection, immune suppression could be stopped in hepatocyte transplant patients without risk of organ loss. The known risks of the alternative, waiting for organ transplant (median time to transplant at the Children's Hospital of Pittsburgh, CHP, is 10.6 months (10)), include death or complications of continued hepatic dysfunction. This is particularly concerning for children with metabolic disorders, among whom neurological defects are related to the duration of hyperammonemia, rather than peak level (12), so even mild hyperammonemia, over months, puts children at risk for permanent neurologic damage (13).
Since the introduction of this trial in July, 2010, 19 patients at CHP were eligible for hepatocyte transplantation. Of these, only 8 consented to the procedure, while the rest chose to just wait for organ transplant. The purpose of the current study was to explore the decision-making process of families faced with the option of trial enrollment, determine factors that affect parental consent, and understand reasons for a lower-than-expected enrollment rate. A better understanding of motivating and deterring factors has the potential to improve counseling and increase trial participation.
MATERIALS AND METHODS
We conducted a qualitative analysis of semi-structured interviews, which focused on families' decision-making with regard to enrollment in a phase I hepatocyte transplantation trial.
Sample
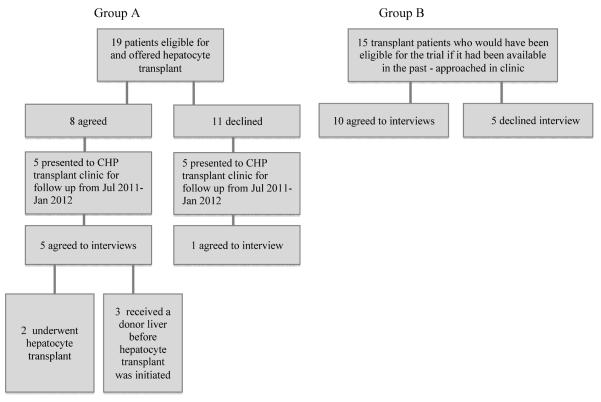
We used purposeful sampling to select information-rich cases, which would provide varied perspectives on participation in the hepatocyte trial. (14) We continued to accrue cases until reaching thematic saturation (15). A schematic representation of our sample can be found in Figure 1. We interviewed two groups: families whose children were offered hepatocyte transplantation (Group A - those who would discuss their actual decision making process) and families whose children had undergone liver transplantation before this trial was available (Group B - those who would discuss the decision hypothetically). The rationale for interviewing Group B was to include a wider range of informed viewpoints, given the small population exposed to the trial thus far.
Figure 1.
Sample
For Group A, we spoke with all six families who had considered hepatocyte trial participation and who presented to CHP transplant clinic for follow-up between July 2011 and January 2012.
For Group B, we spoke to 10 of 15 families of patients who had prior liver transplants (excluding those who underwent simultaneous liver-bowel transplant) who presented to CHP transplant clinic during the same time period.
A member of the clinical team first approached the families and asked for their permission to be approached by an investigator (AD). If they agreed to participate in the study, we scheduled the interview at a later date, 13 of which were held in-person and 3 of which (all Group B) were by telephone.
Hepatocyte Trial Information
Parents in Group A received no additional information before the interview. Parents in Group B received the trial consent form and were asked to decide, hypothetically, whether they would have participated had this trial been available to their child prior to receiving liver transplant. The consent form addresses the following: purpose of the hepatocyte study, eligibility criteria, procedures done for research purposes, possible risks, side effects and discomforts, possible benefits, treatments available for those who decline participation, insurance issues and study-related costs, payment for participation, payment for research-related injury, access to identifiable medical information, and withdrawal from the study. Unlike those in Group A, they did not discuss the trial with a physician.
Interview Structure
One investigator (AD) completed semi-structured interviews following an interview guide (see Table 1). We designed the questions to demographics, information about the child's illness, and decision-making (real or hypothetical) about the hepatocyte trial. We followed up participants' responses with open-ended probes, such as “tell me more about that.”
Table 1.
Standard Interview Questions
| Demographics |
| What is your age? |
| What is your child's age? |
| How old was your child when he/she had a liver transplant? |
| What is your occupation? |
| What is your last completed education level? |
| How would you describe your race or ethnic background? |
| Previous Experience |
| What was the illness that qualified your child for liver transplant? |
| How long have you been aware of this illness? |
| What treatments/procedures has he/she already undergone? |
| Where did they take place? |
| Experience with Clinical Trial |
| When did you first learn about the hepatocyte transplantation trial? |
| How was this information presented to you (written or verbal?) |
| What were your first thoughts in response to the trial? |
| How much time did you have to make your decision? |
| Did you consult anyone for advice? |
| To what extent was your child involved in the decision-making process? |
| To you, what were the most important risks associated with the trial? |
| To you, what were the most important benefits associated with the trial? |
| Which of these was your biggest concern? |
| Had you done any prior research about your child's illness? What were your sources of information? |
| Did you do any additional research after learning about the clinical trial? What were your sources of information? |
| Did any of the additional information (from research or from asking for advice) change your initial opinion? |
| Did anyone else's opinion alter yours? |
| Is there any additional information that you think would have been helpful as you were making your decision? |
| How do you think your decision-making process would have been different if you were the patient rather than your child? |
| Would you consider participation other clinical trials in the future? |
Data Analysis
Two investigators (AD, AB) independently read interview transcripts. Using grounded theory methodology (15), investigators produced a list of themes based on sequential paired comparison of interviews. We continued iterative close readings until both investigators agreed upon a comprehensive coding scheme, which included motivating factors for trial participation, deterring factors, and perspectives on available alternatives. One investigator (AD) applied this codebook to the data in order to produce a descriptive analysis of the factors influencing families' decisions.
Human Subjects
The University of Pittsburgh IRB reviewed and approved the study. Participants provided verbal consent and were not compensated for participation.
RESULTS
Baseline Characteristics
6 families in Group A and 10 in Group B completed interviews. Both parents participated if available, resulting in 19 interviewees overall. The majority of interviewees in both groups were female (84% of Group A and 78% of Group B) and white (84% of Group A and 92% of Group B); 1 participant in each group identified as black. The median age of interviewees in group A was 29, while the median age for Group B was 37. Education levels varied widely from high school to masters degree in both groups. The median age of children was 2 years in Group A and in 10 in Group B, although their median age at transplant was 4.
The conditions currently eligible for hepatocyte transplant were represented, with 3 children who had acute liver failure, 2 from Group A and 1 from Group B, and 7 children with metabolic disorders, 4 from Group A and 3 from Group B. Additional diagnoses represented in Group B are outlined in Table 2.
Table 2.
Participant Demographics
| Parents | |
| Age (median) | 37 years |
| Gender | |
| Female | 15 (79%) |
| Male | 4 (21%) |
| Race | |
| White | 17 (89%) |
| Black | 2 (11%) |
| Occupation | |
| Home-maker | 6 (32%) |
| Educator | 2 (11%) |
| Construction worker | 2 (11%) |
| Other, including: | 1 (5%) each |
| Student, corrections officer, nurse, reporter, administrative assistant, paralegal, salesperson unemployed | |
| Education Level | |
| 8th grade | 3 (16%) |
| High School | 5 (26%) |
| Some College | 6 (32%) |
| College | 2 (11%) |
| Graduate school | 3 (16%) |
| Children | |
| Age (median) | 7.5 years |
| Age at Transplant (median) | 3 years |
| Diagnosis | |
| Acute Liver Failure | 3 (19%) |
| MSUD | 2 (13%) |
| CPS-1 Deficiency | 2(13%) |
| OTC Deficiency, Crigler-Najjar Syndrome), Alagille's Syndrome, Biliary Atresia, Polycystic Kidney and Liver Disease, PFIC, Alphal-antitrypsin deficiency, Infantile hepatitis | 1 (6%) each |
| Type of Transplant | |
| Liver Transplant (Hepatocytes not offered) | 10 (63%) |
| Liver Transplant (Hepatocytes offered) | 4 (25%) |
| Hepatocyte Transplant | 1 (6%) |
| Hepatocyte and Liver Transplant | 1 (6%) |
In Group A, 5 families consented to trial participation, and 1 declined. In Group B, 3 families said that they would have enrolled in the trial, 5 would have declined, and 2 were ambivalent. The majority of families had experience with clinical research in the past: 84% of Group A and 100% of Group B had participated in minimal risk research studies. However, in discussion of the hepatocyte transplant trial, 33% of Group A and 70 % of Group B qualified their responses with statements that expressed a lack of confidence in their own medical knowledge.
Several differences between interviews from Groups A and B reflected the actual versus hypothetical nature of their decisions. Four (40%) of the families in Group B described difficulty in considering hepatocyte transplant because organ transplant had already been successful for their child. In addition, 30% of Group B, but no one from Group A, suggested future applications for hepatocytes, for instance, use of hepatocytes for other relatives with liver disease.
Motivating Factors and Benefits
Despite differences between Groups A and B, the most commonly mentioned motivating and deterring factors were consistent.
In Group A, the most significant motivator, mentioned by 84% of participants, was an acute deterioration in their child's health. Of note, 2 families in Group A had children with acute liver failure, which presents within days, while the rest perceived a deterioration in health due to chronic illness. In contrast, the family who declined trial enrollment perceived their child's health as relatively stable: “She's never been acutely ill. Her ammonia's only…spiked three times, and then we've just been able to maintain her at home.” Interactions with physicians also affected trial participation. 66% of Group A described the physician's opinion as a motivator, specifying personal attention from physicians.
When asked to name benefits of hepatocyte transplant, 66% of Group A mentioned getting faster access to treatment, and 66% mentioned improved health until transplant. 50% also stated that the procedure was minimally invasive, allowing easy recovery and short hospitalization.
Several benefits were recognized only by Group A. Maintaining native liver function “for what it was worth,” rather than risking loss of all function in case of rejection, was a benefit for 50% of Group A. This was the only benefit mentioned by the family who declined trial participation. Also, 50% of Group A named potentially avoiding liver transplant surgery as a benefit.
In Group B, the most significant motivator was also acute deterioration of the child's health, mentioned by 70% of participants. Only 1 of these families had a child with acute liver failure. 30% of Group B also described the interactions with physicians as a potential motivator. As one interviewee explained, “To try to convince us, what would work is to come out to the house and to sit down in the children's environment.”
When asked to name benefits of hepatocyte transplant, 70% of Group B named the minimally invasive nature of the procedure. 40% mentioned faster access to treatment, and 20% mentioned improved health until transplant. While no one in Group B considered avoiding liver transplant beneficial for their own child, 30% discussed broader benefits of avoiding organ transplants, stating that it could save organs for others or alleviate living donors. One family in Group B could not think of any benefits of the hepatocyte transplant. We provide illustrative quotations about motivators and benefits in Table 3.
Table 3.
Motivators and Benefits
| Motivator | Group A | Group B | Example Quote |
|---|---|---|---|
| Child's declining health | 5 (84%) | 7 (70%) | “I probably would have went ahead and gave my permission to do it. Just because she was so, just so sick and so close to dying.” |
| Physician's opinion | 4 (66%) | 3 (30%) | “I would probably go to Dr. -- He's usually given us the best advice. I would probably, yeah see what he says and then go with that decision.” |
| Personal attention from researchers | 1 (16%) | 2 (20%) | “But to make, to try to convince us, what would work, is actually, to be honest with you, is to come out to the house, and to sit down in the children's environment and to present it to them.” |
| Perceived Benefits | |||
| Less invasive | 3 (50%) | 7 (70%) | “If it works – it would be less invasive, less physical stress, less physical damage, less physical stress on the body." "Hopefully this will help some kids not be in the hospital for too long." |
| Shorter wait | 5 (84%) | 7 (70%) | “Not having to wait for an organ, if there was a shortage at the time, not having to wait for an organ.” “to not have to wait for an organ and not have to wait for your child to get that sick that they rank high on the scale to get the organ” |
| Improved health until Tx | 4 (66%) | 2 (20%) | “Because if she could have got better this whole time and been home waiting for her liver…she might be at school, she might have a few bad days, but mostly she'll be good, then I would be like, `Well ok.' ” |
| Keeping native liver | 3 (50%) | 2 (20%) | “…but if she rejected the hepatocytes, she would still have her liver. She would still have her liver function…still, for what it was worth.” |
| Avoid liver Tx (for child) | 3 (50%) | 0 | “Ok, the pros are…I know I keep saying it, but he won't have to actually get the liver.” |
| Avoid liver Tx (for others) | 0 | 3 (30%) | “It would, you know, it would save organs actually for somebody else.” “He had talked a little about getting worked up [as a living donor], but you would hate to put someone through that, so this is an option where you wouldn't, you know, even have to ask somebody to consider something like that.” |
Deterring Factors and Risks
The most significant deterring factor for families in Group A was insufficient prior data on hepatocyte transplant, which was mentioned by 50% of interviewees. While 84% expressed general support for research, and 2 families expressed gratitude to those families that participated in early liver transplants, only 1 family, who consented to the trial, expressed a desire to personally contribute to research. Another significant deterrent was the perception that hepatocytes were an unnecessary procedure for the child if they served only as a bridge to liver transplant.
When asked to name risks of hepatocyte transplant, 66% of Group A described the fear of uncertainty associated with a new therapy, rather than any specific part of the procedure. There were, however, some procedural risks mentioned, including radiation therapy, infection, and rejection. In Group A, 66% perceived hepatocyte transplant as lower risk than liver transplant. On the other hand, 66% also discussed the risk that the hepatocytes might not correct liver function.
For Group B, the most significant deterrent was also insufficient prior data, mentioned by 70% of the group. Gratitude to others who take part in research was also expressed by 2 families, but no one mentioned a personal desire to contribute to research. For one family from Group B, disappointment with prior research studies discouraged further trial participation. 20% of Group B also mentioned that hepatocytes may be unnecessary as a bridge to transplant. Moreover, 2 families in Group B questioned the utility of hepatocytes because the trial protocol gave preference to a donor organ, should it become available before hepatocyte transplant.
When asked about risks, the most common response in Group B was also a fear of uncertainty, mentioned by 80% of interviewees. Only 20% of Group B perceived hepatocyte transplant as lower risk than liver transplant and only 10% considered the risk that the hepatocytes may not correct liver function. In addition, a perceived risk among 30% of Group B was the misconception that trial participation would interfere with receiving a liver.
We provide illustrative quotations about deterrents and risks in Table 4.
Table 4.
Deterrents and Risks
| Deterrent | Group A | Group B | Example Quote |
|---|---|---|---|
| Insufficient prior data | 3 (50%) | 5 (50%) | “When you're in the middle of this, you just want someone to tell you they've got something that can fix it, and it's going to make things better. And the numbers were a huge thing for us.” |
| Unnecessary procedure | 2 (33%) | 2 (20%) | “She's just gone through, I feel like, so much, that if you don't know it's going to work, and if you know you're going to get a transplant anyway, then I would rather kind of just…” |
| Protocol preference for organ Tx | 0 | 2 (20%) | “Is that a last ditch effort to save a life or? …because it sounds like they would rather have the solid organ.” |
| Disillusioned with prior research | 0 | 1 (10%) | “There was some other things that they thought they could do to take care of our kids, and they'd be like, `yup, we'll have it in a month or two, a year from now we're going to be ready to roll, and we got kind of excited, but we were always let down. That was always what happened.” |
| Risks | |||
| Unknown procedure | 4 (66%) | 8 (80%) | “I don't think the procedure itself sounds that scary, given what we've been through, it's just the unknown of it.” |
| Radiation | 2 (33%) | 6 (60%) | “Also, the radiation part of it…I have more questions about why that's needed and how that affects the cells.” |
| Hepatocytes not working | 4 (66%) | 1 (10%) | “And the cons with the…cons would be of course, if it doesn't work, then we still have to do the liver.” |
| Surgical Risk | 3 (50%) | 0 | “It seemed like the …you know, the procedure itself was the biggest risk.” |
| Infection | 2 (33%) | 0 | “I mean I wasn't really worried about anything…you know, just possible infections or stuff like that.” |
| Unknown Source of Hepatocytes | 0 | 4 (40%) | “And also, they said they get cells from more than one donor, so I wonder how that plays, like how that works, because I worry about having more than one cell.” |
| Immunosuppression | 0 | 1 (10%) | “Maybe the anti-rejection and other illnesses that they would pick up that would cause death.” |
Views of Alternatives to Hepatocyte Transplant
Perceptions of the role of hepatocyte transplant in the treatment pathway varied among families. In Group A, 33% perceived hepatocytes as a replacement for organ transplant, 50% considered them a temporary treatment, and one family (16%) viewed hepatocyte transplant as temporary but acknowledged that it could obviate liver transplant. In Group B, 70% viewed hepatocytes as a replacement for organ transplant, while 30% considered them a temporary treatment
We analyzed direct comparative statements throughout interviews from both groups to determine whether interviewees were evaluating hepatocyte transplant as an alternative to liver transplant or to waiting for a donor organ. Direct comparison to liver transplant was more frequent, occurring in 24 statements, while comparison to waiting occurred in 15 statements. Surprisingly, although hepatocyte transplant was conceived as a safer alternative to liver transplant, 79% of comparisons to liver transplant occurred during discussion of risks or deterring factors. In contrast, 80% of comparisons to waiting occurred during discussion of benefits or motivating factors, suggesting that families perceived hepatocyte transplant as more beneficial than waiting, but riskier than liver transplant.
DISCUSSION
In this single-center qualitative study of parents' decision-making processes when faced with a real or hypothetical option of phase I hepatocyte transplant trial enrollment, we identified three key misperceptions that could potentially explain the lower than expected trial enrollment. First, families of children with metabolic liver disease, rather than acute liver failure, may underestimate the severity of their child's illness. Second, families were more averse to unknown risks than to liver transplant with known risks. Third, families were unclear about what point of comparison to use when evaluating hepatocyte transplant.
Deterioration of the child's health was the most frequently mentioned motivating factor. Acute hepatic failure appeared to motivate parents to act immediately. Metabolic liver disease, however, did not induce the same motivation, suggesting a hedonic adaptation to the child's chronic health state. Many of these families named deterioration of child's health as a motivator, saying they would have enrolled had their child been in a “life-threating situation,” but would hesitate to do so now because their child seemed to be “doing amazing” while others were “getting brain damage from the same disease.” Given the unpredictable course of metabolic disorders (16), this is an example of optimism bias – perception that negative events are less likely to happen to oneself than to others (17). A combination of hedonic adaptation and optimism bias may lead families to underestimate the risk of waiting and decline a trial therapy with potential for improving liver function.
The most significant deterring factor – lack of prior data – corresponded with the most commonly mentioned risk – unknown outcomes. Parents had concerns about lack of information on both the probabilities of expected risks and benefits and about unanticipated risks. Unknown risk is a key dimension of risk perception and results in a systematic bias towards technologies already known to science, even if they have large, known, and immediately observable risks (e.g., transplant surgery). Unknown technologies, particularly those for which the risk is not immediately observable or unknown to science, carry significant weight in decision-making (18).
The known surgical risks of the hepatocyte transplant procedure include cancer due to pre-transplant radiation exposure and complications from portal vein cannulation. A study of liver irradiation during Wilms' tumor treatment found 4 instances of hepatocellular carcinoma in 5,278 patients (19). Affected patients received 35–46 Gy of radiation, while the proposed therapy for hepatocyte transplantation would only involve a 5–10 Gy dose. Hepatocyte cell infusion to the portal vein is comparable to the 3% risk of partial portal vein thrombosis reported for islet cell infusions (20). The likelihood of benefit is unknown. Interviewees who were unwilling to participate in the trial said they would only allow a procedure if they were “100% sure that it would work.” Ironically, these same parents are awaiting a treatment that does not have a 100% chance of success: post-transplant survival is 90% at one year (21), and at 10 years, there is a 12% retransplantation rate due to hepatic artery thrombosis, chronic rejection, or primary graft dysfunction (22). Significant surgical complications include biliary duct complications (11.8% incidence), portal vein thrombosis (5.2% incidence), and hepatic artery thrombosis (7.3% incidence) (21–23). Opportunistic infections associated with immune suppression (40% incidence) are also a risk to survival (21). The apparent underestimation of liver transplant risk may be due to connotations of the treatment's status as “standard,” and hence a misperception of risk tolerance due to the “known” nature of these risks.
Significant inconsistencies in decision-making process may also stem from families' perceptions of the alternative to trial participation. Some viewed hepatocytes as a bridge to liver transplant, while others viewed them as a direct alternative. All interviewees oscillated between liver transplant and waiting as points of comparison when discussing risks and benefits. This variation shows that the relevant point of comparison may not be clear to families considering trial enrollment. Although successful hepatocyte transplant could obviate liver transplant, it is only offered to families already on the waiting list for an organ. Thus, the choice is between just waiting or trying hepatocyte transplant during this wait. The relevant comparison is whether hepatocyte transplant offers a benefit over waiting and whether the risks incurred outweigh the combined risk of the child's disease and the expected liver transplant.
To clarify this to families, risks could be outlined to include those of the child's disease as well as available treatment plans. The discussion of benefits, if focused on waiting as an alternative, already favors trial enrollment: statements in which interviewees compared hepatocyte transplant to waiting occurred almost exclusively in the context of benefits or motivators. Additionally, our center is considering changing the timing of hepatocyte trial presentation to families. Previously, it was introduced simultaneously with liver transplant, but based upon the current study, we believe that separating trial presentation from the introduction of liver transplant could emphasize the trial as an alternative to just staying on the waiting list, rather than an alternative to getting a liver transplant. It would also allow for reiteration of the risks and benefits of both procedures, using appropriate comparisons. This change in presentation may help clarify the role of trial therapy and make families more amenable to participation.
Limitations of this study included a small study population, variation in the timing of interviews with respect to the child's transplant, and systematic bias introduced by limiting the sample to parents of survivors. Although the number of interviews was limited by the rarity of pediatric liver transplants and recent introduction of the hepatocyte transplant trial, we achieved thematic saturation in discussions of motivators and deterrents as well as risk/benefit balances. Since families were in different stages of transplant planning or recovery, the child's health at the time of interview may have created variation in responses. Notably, we did not sample parents of children who died after liver transplant (as they did not present to CHP liver transplant clinic for follow-up), and these parents may have had a unique perspective on risks and benefits of treatment alternatives. Finally, interview questions did not directly address perceived risks and benefits of liver transplant, which emerged as a relevant factor in decision making.
CONCLUSION
We identified cognitive biases in families' decision-making processes that have potential to adversely affect trial participation. Absent an acute clinical deterioration, families may underestimate the severity of the illness and the risks of waiting for transplant. Also, families may inadvertently compare hepatocyte transplant to certain liver transplant, rather than to waiting for possible future liver transplant. In so doing, they weigh unknown risks of trial therapy more heavily than known risks associated with liver transplant.
ACKNOWLDEGMENTS
The project described was supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005 and the Clinical and Translational Science Institute START UP Program, awarded through the Clinical and Translational Science Institute and the Institute for Clinical Research Education at the University of Pittsburgh (grant 5TL1RR024155-05).
Footnotes
Author Contributions Alexandra Dreyzin contributed to conception and design of the study, acquisition of data, analysis of data, as well as drafting and revision of the article.
Amber Barnato contributed to conception and design of the study, analysis of data, as well as drafting and revision of the article.
Kyle Soltys and Kimberly Haberman contributed to the identification and recruitment of patients for the study as well as revision of the article.
Coreen Farris contributed to conception and design of the study, as well as drafting and revision of the article.
Rachel Sada contributed to the design of this study, acquisition of data, as well as revision of the article.
Ira Fox contributed to conception and design of the study, as well as drafting and revision of the article.
All authors had final approval of the version to be published.
REFERENCES
- 1.BARFIELD RC, CHURCH C. Informed Consent in Pediatric Trials. Curr Opin Pediatr. 2005;17:20–24. doi: 10.1097/01.mop.0000145718.77939.b1. [DOI] [PubMed] [Google Scholar]
- 2.CALDWELL PHY, MURPHY SB, BUTOW PN, CRAIG JC. Clinical Trials in Children. Lancet. 2004;364:803–811. doi: 10.1016/S0140-6736(04)16942-0. [DOI] [PubMed] [Google Scholar]
- 3.ROTHMIER JD, LASLEY MV, SHAPIRO GG. Factors influencing parental consent in pediatric clinical research. Pediatrics. 2003;111:1037–1041. doi: 10.1542/peds.111.5.1037. [DOI] [PubMed] [Google Scholar]
- 4.GAMMELGAARD A, KNUDSEN LE, BISGAARD H. Perceptions of parents on the participation of their infants in clinical research. Arch Dis Child. 2006;91:977–980. doi: 10.1136/adc.2006.096073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HOEHN KS, et al. What factors are important to parents making decisions about neonatal research? Arch Dis Child. 2005;90:F267–F269. doi: 10.1136/adc.2004.065078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MAAYAN-METZGER A, KEDEM-FRIEDRICH P, KUINT J. Motivations of mothers to enroll their newborn infants in general clinical research on well-infant care and development. Pediatrics. 2008;121:e590–e596. doi: 10.1542/peds.2007-1571. [DOI] [PubMed] [Google Scholar]
- 7.READ K, et al. Decision-making by Adolescents and Parents of Children with Cancer Regarding Health Research Participation. Pediatrics. 2009;124(3):959–965. doi: 10.1542/peds.2008-2878. [DOI] [PubMed] [Google Scholar]
- 8.KIM A, FOX E, WARREN K, BLANEY SM, BERG SI, ADAMSON PC, LIBUCHA M, BYRLEY E, BALIS FM, WIDEMANN BC. Characteristics and outcomes of pediatric patients enrolled in phase I oncology trials. Oncologist. 2008;13(6):679–89. doi: 10.1634/theoncologist.2008-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SOLTYS KA, et al. Barriers to the successful treatment of liver disease by hepatocyte transplantation. J Hepatol. 2010;53:769–774. doi: 10.1016/j.jhep.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center-Specific Report (CSR) for Children's Hospital of Pittsbrugh of UPMC; Scientific Registry of Transplant Recipients (SRTR) Released 7-13-2012. [Google Scholar]
- 11.MCDIARMID SV, ANAND R, MARTZ K, MILLIS MJ, MAZARIEGOS G. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann Surg. 2011;254(1):145–154. doi: 10.1097/SLA.0b013e31821ad86a. [DOI] [PubMed] [Google Scholar]
- 12.GROPMAN AL, SUMMAR M, LEONARD JV. Neurological Implications of urea cycle disorders. J Inherit Metab Dis. 2007;30:865–869. doi: 10.1007/s10545-007-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHITINGTON PF, ALONSO EM, BOYLE JT, MOLLESTON JP, ROSENTHAL P, EMOND JC, MILLIS JM. Liver transplantation for the treatment of urea cycle disorders. J Inherit Metab Dis. 1998;21(Suppl 1):112–118. doi: 10.1023/a:1005317909946. [DOI] [PubMed] [Google Scholar]
- 14.EISENHARDT KM. Building theories from case study research. The Academy of Management Review. 1989;14(4):532–550. [Google Scholar]
- 15.PATTON M. Qualitative evaluation and research methods. Sage; Beverly Hills, CA: 1990. pp. 169–186. [Google Scholar]
- 16.WRAITH JE. Diagnosis and management of inborn errors of metabolism. Arch Dis Child. 1989;64(10 Spec No):1410–1415. doi: 10.1136/adc.64.10_spec_no.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SHAROT T. The Optimism Bias. Curr Biol. 2011;21(23):R941–R945. doi: 10.1016/j.cub.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 18.FISCHOFF B, WATSON SR, HOPE C. Defining Risk. Policy Sci. 1984;17(2):123–139. [Google Scholar]
- 19.KOVALIC JJ, THOMAS PR, BECKWITH JB, FEUSNER JH, NORKOOL PA. A National Wilms' Tumor Study report. Cancer. 1991;67:342–344. doi: 10.1002/1097-0142(19910115)67:2<342::aid-cncr2820670204>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.RYAN E, PATY BW, SENIOR PA, SHAPIRO AMJ. Risks and side effects of islet transplantation. Curr Diabetes Rep. 2004;4:304–309. doi: 10.1007/s11892-004-0083-8. [DOI] [PubMed] [Google Scholar]
- 21.EMRE A, UMMAN V, CISMIT B, ROSENCRANTZ R. Current Concepts in Pediatric Liver Transplantation. Mt Sinai J Med. 2012;79:199–213. doi: 10.1002/msj.21305. [DOI] [PubMed] [Google Scholar]
- 22.NG V, ALONSO E, BUCUVALAS JC, COHEN G, LIMBERS C, VARNI JW, MAZARIEGOS G, MAGEE J, MCDIARMID SV, ANAND R. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplant experience. J Pediatr. 2012;160:820–6. doi: 10.1016/j.jpeds.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ARNON R, KERKAR N, DAVIS MK, ANAND R, YIN W, GONZALEZ-PERALTA RP. Liver Transplantation in children with metabolic diseases: the studies of pediatric liver transplantation experience. Pediatr Transplant. 2010;14:796, 8–5. doi: 10.1111/j.1399-3046.2010.01339.x. [DOI] [PubMed] [Google Scholar]