Abstract
Activated microglia and infiltrating lymphocytes are neuropathological hallmarks of amyotrophic lateral sclerosis (ALS), a fatal motoneuron disease. Although both cell types play pivotal roles in the ALS pathogenic process, the interactions between microglia and lymphocytes, specifically regulatory CD4+CD25High T lymphocytes (Tregs) and cytotoxic CD4+CD25− T lymphocytes (Teffs), have not been addressed. When co-cultured with mSOD1 adult microglia, mSOD1 Tregs suppressed the cytotoxic microglial factors NOX2 and iNOS through an IL-4-mediated mechanism, whereas Teffs were only minimally effective; IL-4 inhibitory antibodies blocked the suppressive function of mSOD1 Tregs, and conditioned media from mSOD1 Tregs or the addition of IL-4 reduced microglial NOX2 expression. During the stable disease phase, the total number of Tregs, specifically the numbers of CD4+CD25HighIL-4+, CD4+CD25HighIL-10+ and CD4+CD25HighTGF-β+ Tregs, were increased in ALS mice compared with WT mice; Tregs isolated during this phase reduced Teffs proliferation. In contrast, during the rapidly progressing phase, the number of mSOD1 Tregs decreased while the proliferation of mSOD1 Teffs increased. The combination of IL-4, IL-10, and TGF-β was required to inhibit the proliferation of mSOD1 Teffs by mSOD1 Tregs that were isolated during the slow phase, while inhibition of mSOD1 Teffs by mSOD1 Tregs during the rapid phase, as well as WT Teffs, was not dependent on these factors. Thus, mSOD1 Tregs at the slow phase suppressed microglial toxicity and SOD1 Teffs proliferation through different mechanisms; microglial activation was suppressed through IL-4 whereas mSOD1 Teffs were suppressed by IL-4, IL-10 and TGF-β. These data suggest that mSOD1 Tregs contribute to the slowly progressing phase in ALS mice and may offer a novel therapeutic option for ALS patients.
Keywords: ALS, T lymphocytes, microglia
Amyotrophic lateral sclerosis (ALS), known as Lou Gehrig's disease, is the most common, devastating, and invariably fatal adult motoneuron disease. About 10% of ALS patients have a family history of the disease and approximately 20% of these patients have mutations in the Cu2+/Zn2+ superoxide dismutase (SOD1) gene. Transgenic mice over-expressing several of these mutations (mSOD1) develop a disease replicating many of the clinical and pathological features of ALS (Appel et al., 2010; Henkel et al., 2009). Of critical importance is the fact that mSOD1 mice show similar central nervous system (CNS) inflammatory changes that are present in ALS patients and that these changes coincide with disease onset, and parallel disease progression rates (Alexianu et al., 2001; Almer et al., 2001, 1999).
Accumulating evidence suggests that through their distinctive temporal and spatial contributions, microglia and lymphocytes can have both neurotoxic and neuroprotective functions depending on their activation states and the physiologic conditions they encounter in the CNS (Appel et al., 2010; Schwartz et al., 2010). Microglial activation is a major component of the neuroinflammatory response following CNS injury and numerous studies have confirmed the presence of activated microglial in regions of motoneuron damage in both ALS patients and mSOD1 mice (Alexianu et al., 2001; Hall et al., 1998; Turner et al., 2004). On one hand, activated microglia exert their toxic effects on neurons through the release of substances such as free radicals (superoxide and nitric oxide) and neurotoxic pro-inflammatory cytokines (Alexianu et al., 2001; Henkel et al., 2004, 2006; Bruijin et al., 2004; Weydt et al., 2002). We recently demonstrated that through these factors, and through the decrease of IGF-1, classically activated microglia were cytotoxic to motoneurons; in vitro studies utilizing primary microglia/motoneuron co-cultures provided evidence that mSOD1 microglia were more neurotoxic than wild-type (WT) microglia due to their enhanced release of free radicals and proinflammatory cytokines, and attenuated release of neurotrophic factors (Xiao et al., 2007; Beers et al., 2006; Zhao et al., 2004). On the other hand, using a similar microglia/motoneuron in vitro system, we showed that IL-4 promoted motoneuron survival by suppressing classically activated microglia, attenuating the release of the ROS, and enhancing IGF-1 production (Zhao et al., 2006). Furthermore, Marden et al. (2007) determined that the dysregulated redox stress in ALS mice caused by NADPH oxidase subunits NOX1 and NOX2 (expressed predominantly in microglia) significantly influenced the progression of motoneuron disease; deletion of either Nox gene slowed disease progression and improved survival.
Several reports have presented evidence for the presence of infiltrating immune cells in ALS. These reports showed the infiltration of T lymphocytes, monocytes/macrophages, dendritic cells, and increased levels of CCL2/MCP-1 (the chemokine that attracts myeloid and dendritic cells to the CNS) in spinal cord tissues of ALS patients (Henkel et al., 2004; Engelhardt et al., 1993) and mSOD1 mice (Alexianu et al., 2001; Henkel et al., 2006; Beers et al., 2008); T lymphocytes and macrophages have also been described to roll along the walls of capillaries and venules, and extend into the parenchyma of affected areas (Ince et al., 1996; Corti et al., 2004). We recently demonstrated that only CD4+ T lymphocytes were observed in the lumbar spinal cord sections of ALS mice until the late phase of disease; CD8+ T lymphocytes were observed at near end-stage disease, but no consistent convincing evidence supported the presence of B lymphocytes. The absence of CD4+ T lymphocytes accelerated disease progression and shortened survival in ALS mice. Cytotoxic markers of microglial activation (NOX2 and TNF-α) were up-regulated in spinal cords of mSOD1/CD4−/− mice compared with their mSOD1/CD4+/− littermates (Beers et al., 2008). These data suggest that CD4+ T lymphocytes provide neuroprotection by suppressing cytotoxic activation of microglia.
CD4+ T lymphocytes can be sub-classified as CD4+CD25− T lymphocytes (Teffs) and CD4+CD25+ T lymphocytes. A subpopulation of the CD4+CD25+ T lymphocytes are CD4+CD25High regulatory T lymphocytes (Tregs), which also express Foxp3 as a functional marker. Tregs were originally identified by their capacity to suppress the proliferation and activation of other T lymphocytes, and they act as “master regulators” of immune homeostasis (Sakaguchi et al., 2008, 1995; Tang and Bluestone 2008); their suppressive effects on both the adaptive and innate immune systems have been well documented (Sakaguchi et al., 2008, 2005; Tiemessen et al., 2007; Avidan et al., 2004; Reynolds et al., 2007). Treg-mediated suppression involves multi-cellular clusters consisting of responder T lymphocytes, antigen-presenting cells (APC), and membrane-bound and/or soluble inhibitory molecules. Their suppressive effects involve the down-regulation of proinflammatory cytokine production (IFN-γ and TNF-α) and the inhibition of IL-2 mRNA transcription. Tregs secrete anti-inflammatory cytokines and neurotrophic factors, transform a pro-inflammatory Th1 response into an anti-inflammatory Th2 mediated response, and attenuate toxic microglial responses. Thus, Tregs have the potential to modify many aspects of an inflammatory response, including toxic microglial responses in the injured CNS. In addition to the suppressive effects of Tregs, CD4+ Th2 cells also produce anti-inflammatory cytokines, such as IL-4 and IL-10.
However, there is no direct evidence showing the effects of Tregs on microglia and cytotoxic T lymphocytes in ALS. Therefore, in this study, we determined potential mechanisms whereby these responses may occur in ALS by using primary cells isolated from mSOD1 and WT mice. Our data showed that mSOD1 Tregs suppressed microglial activation by secreting IL-4, but independent of CTLA-4 engagement or release of IL-10 and TGF-β. However, the combination of IL-4, IL-10 and TGF-β was required for inhibiting the proliferation of mSOD1 Teffs by mSOD1 Tregs. Furthermore, we also determined that mSOD1 Tregs and the proliferative capabilities of isolated mSOD1 Teffs were different depending upon the disease stage in ALS mice; increased numbers of mSOD1 Tregs, but decreased proliferation of mSOD1 Teffs, were observed during the slowly progressing phase of disease, whereas decreased numbers of mSOD1 Tregs and increased proliferation of mSOD1 Teffs were observed during the rapidly progressing phase.
Materials and Methods
Materials
Culture media, sera and antibiotics were purchased from Gibco BRL (Rockville, MD), and all other reagents were from Sigma (St. Louis, MO) unless otherwise noted.
Mice
mSOD1G93A mice, on a C57Bl/6 genetic background, were bred and maintained in an AAALAC accredited animal facility at The Methodist Hospital Research Institute. Foxp3(GFP)+ mice on same C57Bl/6 genetic background were purchased from The Jackson Laboratory. Foxp3(GFP)+/mSOD1G93A mice were obtained by crossing Foxp3(GFP)+ mice with mSOD1G93A mice. All animals were housed in microisolator cages with access to food and water ad libitum, and all animals were specific pathogen free (SPF); sentinel mice were tested quarterly. All experimental procedures involving animals were approved by The Methodist Hospital Research Institute's Institutional Animal Care and Use Committee in compliance with National Institutes of Health guidelines. Disease symptoms and course were assessed using the BASH scoring system (Beers et al., 2006; 2008).
In vitro cultures
Tregs and Teffs were obtained from lymph nodes and spleen of 100 or 160 day old mSOD1 mice as well as their WT littermates. Briefly, cell suspensions were made from spleen and lymph nodes (superficial cervical, axillary, inguinal lymph nodes) pressed through a 70 μm strainer, and then passed through a 30 μm pre-separation filter. Tregs and Teffs cells were isolated using mouse regulatory T cell isolation kits (Miltenyi Biotec) following the manufacturer's instructions. CD4+ T lymphocytes were first isolated through negative selection, and then the enriched CD4+ T lymphocyte fraction was further separated into CD4+CD25High Tregs and CD4+CD25− Teffs by incubating with anti-CD25 antibody. Flow cytometric analyses demonstrated that the purity of the resulting T lymphocyte populations was >95%, and more than 80% of CD4+CD25High T lymphocytes expressed forkhead-box transcription factor (Foxp3), the functional marker of Tregs.
Primary adult microglia were prepared from spinal cord tissues of 130 day old mSOD1 mice, as well as from their WT littermates, by modification of a non-enzymatic procedure (Aloisi et al., 2000; Frank et al., 2006). Briefly, mice were sacrificed with isoflurane (Baxter) by inhalation and perfused intracardially with 40–50 ml of ice cold GKN buffer (8 g/L NaCl, 0.4 g/L KCl, 3.56 g/L Na2HPO412H2O, 0.78 g/L NaH2PO42H2O, and 2 g/L D(+)-glucose, pH 7.4). Spinal cords were mechanically dissociated by passing through a 70 μm cell strainer, and then strained through a 40 μm filter. After centrifugation at 350 g for 10 min at 4°C, dissociated tissue were re-suspended in 2 ml of 70% isotonic Percoll and overlaid with 3 ml of 35% Percoll and 2 ml of PBS following a 30 min centrifugation at 800 g, room temperature (RT). Microglia were obtained from the 35%:70% interface, washed, and re-suspended in RPMI medium supplemented with 10% fetal bovine serum, 25 mM HEPES, 1 mM sodium pyruvate, 1×nonessential amino acids, 55 μM 2-mercaptoethanol, 100 units/ml penicillin and 100 μg/ml streptomycin (complete RPMI medium).
Primary adult microglia (1×104 cells/well) were co-cultured with Tregs or Teffs (1×104 cells/well) in 96-well U-bottom plates in complete RPMI medium with 0.5 μg/ml anti-CD3 antibody (eBioscience) and 100 U/ml IL-2 (BD Biosciences). In the inhibitory studies, blocking antibodies to IL-4 (150 ng/ml, R&D Systems), IL-10 (10 μg/ml, eBioscience; or 10 μg/ml, BD Biosciences), TGF-β (10 μg/ml, R&D Systems), CTLA-4 (3 μg/ml, BD Pharmingen) were added to the co-cultures after cells were plated. Conditioned-media were collected after mSOD1 Teffs (1×104 cells/well) or mSOD1 Tregs (1×104 cells/well) were incubated for 2 days. For the experiments using microglial conditioned-media, mSOD1 microglia (1×104 cells/well) were resuspended in 20μl fresh medium and incubated with 180μl of corresponding conditioned-media in 96-well plate. Two days later, cells were lysed and RNAs were isolated using RNeasy Micro kit (Qiagen). Supernatants were collected for detecting IL-4 protein (eBioscience).
Nitric oxide production
Nitrite and nitrate accumulation in the culture supernatant was used as an indicator of nitric oxide (NO) production and was determined by The DetectX Nitric Oxide Detection Kit (Arbor Assays), according to the manufacturer's instructions.
Western blotting
Microglial cells were lysed with RIPA buffer (Cell Signaling Technology) containing Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific). After mixing 5:1 with sample buffer and heating to 94°C for 5 min, samples were separated by electrophoresis on a 12% Tris-HCl gel (Bio-Rad Laboratories) and electroblotted onto a PVDF membrane (Millipore). After incubating in 6% milk in TBST (20mM Tris-HCl, 0.5 M NaCl, 0.05% Tween-20, pH 7.5) for 2 hours, blots were incubated overnight with anti-NOX2 antibody (1:300; BD Biosciences) at 4°C. The membrane was washed 3 times in TBST and then incubated for 2 hours in peroxidase-conjugated secondary antibody (1:2000, Vector Laboratories) at RT. The ECL plus detection system (Amersham Pharmacia Biotech) was used for protein detection, according to the manufacturer's instructions.
Flow cytometry
To detect intracellular cytokines, cells were treated with cell stimulation cocktail plus protein transport inhibitors (eBiosciences) for 5 hours to accumulate most cytokine proteins in the rough endoplasmic reticulum or Golgi complex; this procedure leads to an enhanced ability to detect cytokine-producing cells. Samples were then incubated with 10 μg/ml seroblock FcR (AbD Serotec) for 10 min to minimize non-specific binding of antibodies to Fc receptors. After washing, anti-mouse CD4-APC-Cy7 antibody and anti-mouse CD25-PerCP antibody (BD Biosciences) were added to each sample and incubate for 15 min at 4°C. Samples were then fixed and permeabilized, followed by incubation with anti-mouse Foxp3-PacBlue, anti-mouse IL-4-PE- Cy7, anti-mouse IL-4-FITC antibodies (BD Biosciences), anti-mouse IL-10-AF700 antibody (eBiosciences) and anti-human TGF-β-APC antibody (R&D Systems) for 30 min at 4°C. Isotype IgGs were used as controls. The cells were again washed and assayed using a BD™ LSRII-16 color flow cytometer configured with 488 nm, 633 nm, and 405 nm lasers (BD Biosciences). The results of the flow cytometry assay were analyzed using FACSDiva (BD Biosciences) and FlowJo (Tree Star) software.
To obtain pure Tregs, CD4+CD25HighFoxp3(GFP)+ Tregs were sorted from lymph nodes and spleens of Foxp3(GFP)+/mSOD1G93A mice by fluorescence-activated cell sorting (FACS). In brief, cells were stained with anti-mouse CD4-APC-Cy7 antibody and/or anti-mouse CD25-PerCP antibody (BD Biosciences) and sorted based on CD4-APC-Cy7, CD25-PerCP and Foxp3-GFP. After sorting, CD4+CD25HighFoxp3(GFP)+ Tregs were co-cultured with mSOD1 microglia for 2 days. IL-4 expression in CD4+CD25HighFoxp3(GFP)+ Tregs was analyzed by flow cytometry as described above.
Determining the suppressive activity of Tregs
Teffs (1×105) were cultured for 3 days in U-bottom 96-well plates with or without Tregs (1×105) in the presence of soluble anti-mouse CD3 antibody (0.75 μg/ml, eBioscience) and soluble anti-mouse CD28 antibody (4 μg/ml, eBioscience). T lymphocyte proliferation was determined by measuring [3H]thymidine incorporation for the final 7 hours of culture. The percentage of inhibition was calculated as follows: [1-(cpm of Teffs + Tregs/cpm of Teffs)]×100%.
Quantitative RT-PCR
Cells were lysed and total RNAs were prepared by using RNeasy Micro kit (Qiagen) according to the manufacturer's instructions. Quantitative RT-PCR (qRT-PCR) was performed using the iScript one-step RT-PCR kit with SYBR Green (Bio-Rad Laboratories or Qiagen) and the iQ5 Multicolor Real-time PCR detection System (Bio-Rad Laboratories) according to the manufacturer's recommendations. The conditions of PCR were as follows: for NOX2, the primers: 5'-TGA ATG CCA GAG TCG GGA TTT-3' and 5'-CCC CCT TCA GGG TTC TTG ATT T-3', Tm=55.8°C; for iNOS, the primers: 5'-CAG CAC AGG AAA TGT TTC AGC-3' and 5'-TAG CCA GCG TAC CGG ATG A-3', Tm=55°C; for β-actin, the primers: 5'-AGT GTG ACG TTG ACA TCC GTA-3' and 5'-GCC AGA GCA GTA ATC TCC TTC T-3', Tm=61.9°C. Primer efficiency was assessed by analyzing serial dilutions of each RNA sample. The relative expression levels of each mRNA were calculated using the ΔΔCt method normalized to β-actin and relative to the control samples. The presence of one product of the correct size was verified by 2% agarose gel electrophoresis.
ELISA
Mouse IL-4 ELISA Ready-SET-Go kit (eBioscience) was used to determine the concentration of IL-4 protein in cell culture supernatants according to manufacturer's instructions.
Statistics
Comparisons were performed using ANOVA and Student's t-test, and data were expressed as mean ± SE and p value less than 0.05 was considered statistically significant.
Results
Mutant SOD1 Tregs suppress microglial activation
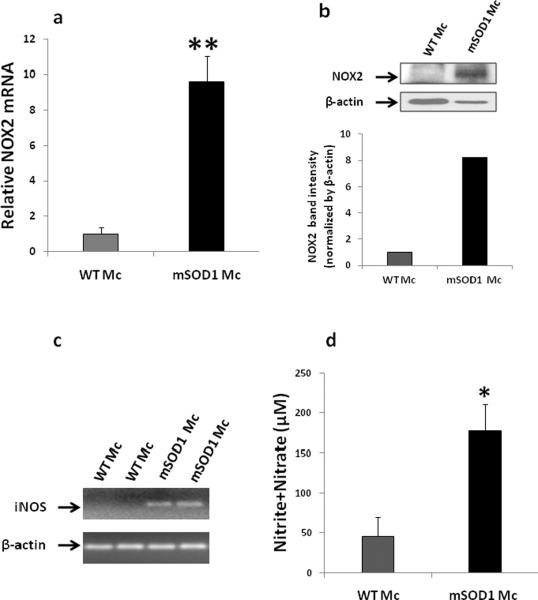
Since the symptoms of motoneuron degeneration in mSOD1 mice progress rapidly starting from 130 days (Beers et al., 2008, 2011), we isolated microglia from spinal cords of 130 day old mSOD1 mice and their WT littermates. After incubating microglia for 2 days, the expression levels of NOX2 and iNOS, indices of cytotoxic microglial activation, were assessed. As shown in Fig. 1a, mSOD1 microglia expressed more NOX2 mRNA than WT microglia (p<0.01). NOX2 protein was also up-regulated in mSOD1 microglia compared with WT microglia (Fig. 1b). NOX2 protein expression in WT microglia was quite low even when additional amount of WT microglial extract was loaded onto the gel (shown by β-actin in Fig. 1b). While iNOS mRNA was not detectable in WT microglia, it was up-regulated in mSOD1 microglia (Fig. 1c). Nitric oxide (NO) is the product of iNOS activation and is an important cytotoxic factor released by activated microglia. Since NO is not stable for detection, NO content is usually derived from the sum of nitrite and nitrate. The levels of nitrate and nitrite in the supernatants of mSOD1 microglial cultures were significantly higher than those in WT microglial supernatants (p<0.05, Fig. 1d).
Fig. 1.
Mutant SOD1 Tregs suppressed activation of adult mSOD1 microglia (Mc). (a–b) Mc were isolated from 130 day old mSOD1 mice or wild-type (WT) mice. After 2 days in culture, mSOD1 Mc expressed more NOX2 mRNA than WT Mc (a). NOX2 protein was also up-regulated in mSOD1 Mc compared with WT Mc; β-actin was used to control protein loading. Although more WT Mc protein was intentionally loaded onto the gel as indicated by the β-actin signal, NOX2 protein from WT Mc was minimally detectable. Semi-quantification of NOX2 protein was performed by analyzing band intensities after normalization to β-actin (b). (c) iNOS from WT Mc was not detectable; iNOS was upregulated in mSOD1 Mc when assayed by quantitative RT-PCR. β-actin was used as a internal control. (d) Nitrite+nitrate levels were used as an index of nitric oxide (NO) production and were measured in the supernatants of WT or mSOD1 Mc co-cultures at 2 days post-plating. mSOD1 Mc produced more NO than WT Mc. (e–g) Tregs and Teffs were purified from 100 day old mSOD1 mice, and then co-cultured with mSOD1 Mc for 2 days. mSOD1 Tregs inhibited NOX2 (e), iNOS (f) expression, and NO production (g) in mSOD1 Mc compared with mSOD1 Teffs and Mc co-cultures; Tregs and Teffs expressed minimal NOX2, iNOS and NO. Data shown as mean±SE of 4–5 independent experiments with duplicate or triplicate wells. *p<0.05, **p<0.01 vs. WT Mc; &&p<0.01 vs. WT Mc+WT Teffs; #p<0.05, ##p<0.01 vs. mSOD1 Mc+ mSOD1 Teffs. Mc = microglia; Tc = T cells; WT = wild-type.
Since our previous data suggested that the lack of CD4+ T lymphocytes enhanced the cytotoxic properties of microglia and attenuated the slowly progressing phase of mSOD1 mice (Beers et al., 2008), we determined the subpopulation of CD4+ T lymphocytes responsible for reducing microglial cytotoxicity in this study. Tregs or Teffs were isolated from 100 day old mSOD1 mice. Co-culturing the mSOD1 microglia with 100 day mSOD1 Tregs reduced NOX2 and iNOS mRNA levels as well as NO production (shown as nitrite+nitrate) compared with mSOD1 microglia and mSOD1 Teffs co-cultures (p<0.01, Figs. 1e, f, g). Since Tregs and Teffs expressed minimal NOX2, iNOS and NO (data not shown), almost all detected NOX2, iNOS and NO in the co-cultures were from microglia. These results indicate that mSOD1 Tregs are able to suppress activation of mSOD1 microglia.
mSOD1 Tregs suppresses microglial cytotoxic phenotypes through release of IL-4
IL-4 is an anti-inflammatory cytokine released by T lymphocytes. We had previously shown that IL-4 inhibited microglial activation and augment motoneuron survival in vitro (Zhao et al., 2006). In this study, IL-4 protein levels were measured in the supernatants of microglia and T cell co-cultures. IL-4 was not detectable in the supernatants of microglia alone cultures (data not shown), so the major sources of IL-4 in the co-cultures were from T cells. After co-culturing with microglia, mSOD1 Tregs produced more IL-4 than either WT Tregs or mSOD1 Teffs (p<0.01, Fig. 2).
Fig. 2.
mSOD1 Tregs released IL-4 protein. Tregs or Teffs isolated from mSOD1 or wild-type (WT) mice were co-cultured with mSOD1 or WT microglia (Mc) for 2 days. IL-4 protein was measured in the supernatant of each co-culture. Increased IL-4 protein levels were observed in mSOD1 Tregs and microglia co-cultures. IL-4 was not detectable in microglia alone cultures. Data shown as mean±SE of 4–5 independent experiments with duplicate or triplicate wells. **p<0.01 vs. mSOD1 Mc+ mSOD1 Teffs; ##p<0.01 vs. WT Mc+ WT Tregs. Mc = microglia; Tc = T cells; WT = wild-type.
To demonstrate that IL-4 was involved in modulating the cytotoxic aspects of microglia, a blocking antibody to IL-4 was added to the co-cultures of mSOD1 microglia/mSOD1 Tregs or mSOD1 microglia/mSOD1 Teffs co-cultures. The addition of IL-4 neutralizing antibody completely reversed the NOX2 mRNA levels in mSOD1 microglia/mSOD1 Treg co-cultures, but had little effect in the mSOD1 microglia/mSOD1 Teff co-cultures (p<0.01, Fig. 3a). Similarly, IL-4 blocking antibody completely reversed the suppressive effect of mSOD1 Tregs on microglial iNOS mRNA expression (p<0.01, Fig. 3b). The NOX2 and iNOS message levels in the mSOD1 microglia/mSOD1 Treg co-cultures plus IL-4 neutralizing antibodies were not significantly different from the levels produced by the mSOD1 microglia/mSOD1 Teff co-cultures (p>0.05). In accordance with iNOS mRNA expression levels (Fig 3b), IL-4 neutralizing antibody reversed the mSOD1 Tregs suppression on microglial NO production, which was increased and similar to the NO production in the mSOD1 microglial/mSOD1 Teff co-cultures; again, NO production was measures as the sum of nitrite and nitrate (Fig. 3c).
Fig. 3.
IL-4 released by mSOD1 Tregs suppressed microglial (Mc) activation. (a–b) A IL-4 blocking antibody (150 ng/ml) was added to mSOD1 Mc+Teffs or mSOD1 Mc+Tregs co-cultures. After 2 days of incubation, RNAs were extracted for quantitative RT-PCR analyses and supernatants were collected for detecting nitrite and nitrate levels as an indicator of nitric oxide (NO) production. IL-4 blocking antibody reversed NOX2 mRNA (a), iNOS mRNA (b), and NO production (c) in mSOD1 Mc+Tregs co-cultures. (d) Conditioned-media (CM) of mSOD1 Teffs or Tregs were used to culture mSOD1 Mc for 2 days. CM of mSOD1 Tregs suppressed microglial NOX2 mRNA, and IL-4 neutralizing antibody blocked the suppression of Tregs CM. (e) Different doses of IL-4 protein (0.01–10ng/ml) were added to mSOD1 Mc cultures. IL-4 protein inhibited NOX2 of mSOD1 Mc in a dose-dependent manner. Data shown as mean±SE of 3–5 independent experiments with duplicate or triplicate wells. #p<0.05, ##p<0.01 vs. mSOD1 Mc+ mSOD1 Teffs; &&p<0.01 vs. Mc+Tregs; **p<0.01 vs. mSOD1 Mc; ‡‡p<0.01 vs. mSOD1 Mc in CM of mSOD1 Teffs; §p<0.05 vs. mSOD1 Mc in CM of mSOD1 Tregs without IL-4 Ab. Ab = antibody; CM: Conditioned-media; Mc = microglia; Tc = T cells.
To further confirm that the cytotoxic mRNAs expressed in microglia are indeed reduced due to the IL-4 released from the mSOD1 Tregs and not due to cell-to-cell contact between the microglia and T lymphocytes, mSOD1 microglia were incubated in the conditioned media of mSOD1 Teffs or Tregs. While conditioned media of mSOD1 Teffs did not alter the expression of NOX2 in mSOD1 microglia, conditioned media of mSOD1 Tregs reduced mSOD1 microglial NOX2 compared with untreated mSOD1 microglia and mSOD1 microglia incubated with conditioned media from Teffs (p<0.01 and p<0.01, Fig. 3d). Furthermore, similar to the earlier results, following the addition of IL-4 neutralizing antibody to the cultures, mSOD1 microglial NOX2 levels were completely reversed when cultured in mSOD1 Tregs conditioned media compared with mSOD1 microglia in the same conditioned media without IL-4 antibodies (p<0.05, Fig. 3c). In addition, we performed an IL-4 dose-response study using cultured mSOD1 microglia and assessed the levels of NOX2 mRNA expression. These results demonstrated that with increasing amounts of IL-4 protein, there was a concomitant dose-dependent decrease in microglial NOX2 (Fig. 3e). Thus, the cumulative data suggest that mSOD1 Tregs modulate the cytotoxic profiles of mSOD1 microglia by releasing IL-4.
Our flow cytometry data demonstrated that more than 80% of the isolated mSOD1 CD4+CD25High T lymphocyte population express Foxp3 (Fig. 4a). Foxp3 is currently the accepted marker for functional Tregs. Therefore, most of mSOD1 CD4+CD25High T lymphocytes are Tregs. To examine if pure mSOD1 Tregs produce IL-4, Foxp3(GFP)+/mSOD1G93A mice were used. Foxp3 is expressed intracellularly, which makes it unsuitable for viable cell isolation. Foxp3(GFP)+ mice have a bicistronic enhanced GFP reporter cloned into the endogenous Foxp3 locus and GFP is co-expressed only in the Foxp3+ cells. Using Foxp3(GFP)+/mSOD1G93A mice, we were able to isolate highly purified mSOD1 CD4+CD25HighFoxp3(GFP)+ Tregs during slowly progressing phase (100 day old) by FACS. mSOD1 CD4+CD25HighFoxp3(GFP)+ Tregs were subjected to flow cytometric analyses following 1 day in culture with either mSOD1 microglia or without microglia. Our results demonstrated that Foxp3 and IL-4 were co-expressed in the same cell; when cultured alone, 6.1% of mSOD1 CD4+CD25HighFoxp3+ Tregs expressed IL-4 (Fig. 4b); when cultured in the presence of mSOD1 microglia, 12% of mSOD1 CD4+CD25HighFoxp3+ Tregs expressed IL-4 (Fig. 4c). Therefore, these results suggest that mSOD1 Tregs can produce IL-4, and mSOD1 microglia stimulated more Tregs to express higher levels of IL-4. Because it is widely known that the CD4+ Th2 lymphocytes also produce IL-4, we assayed the mSOD1 CD4+CD25High Tregs population for possible contamination of Th2 lymphocytes and observed 1.1% of the CD4+Foxp3− lymphocytes stained positively for IL-4. Therefore, it is possible that Th2 lymphocytes in mSOD1 CD4+CD25High cell population also contributed to the IL-4 production in the co-cultures and were partially responsible for suppressing the microglial activation in this study.
Fig. 4.
FACS followed by flow cytometric analyses revealed mSOD1 CD4+CD25HighFoxp3+ Tregs produce IL-4. (a) CD4+CD25High Tregs were isolated from 100 day old mSOD1 mice using mouse regulatory T cell isolation kits from Miltenyi Biotec. Flow cytometry data showed that 80% of CD4+CD25High Tregs express Foxp3. (b–c) Highly purified CD4+CD25HighFoxp3(GFP)+ Tregs were sorted from 100 day old Foxp3(GFP)+/mSOD1G93A mice by FACS. After 1 day incubation, CD4+CD25HighFoxp3(GFP)+ Tregs were assayed for IL-4 detection by flow cytometry. CD4+CD25HighFoxp3+IL-4+ Tregs accounted for 6.1% in Tregs alone cultures (b). When co-cultured with microglia (Mc) for 1 day, more CD4+CD25HighFoxp3+IL-4+ Tregs (12.0%) were observed (c) than mSOD1 Tregs alone cultures (b). Mc = microglia.
The suppressive effect of mSOD1 Tregs on microglia is independent of IL-10, TGF-β, or CTLA-4
IL-10 and TGF-β are two other anti-inflammatory cytokines released by Tregs. To assess whether IL-10 was also participating in mSOD1 Treg modulation of the cytotoxic microglial activation, a blocking antibody to IL-10 was added to co-cultures of mSOD1 microglia with mSOD1 Tregs or Teffs. Unlike IL-4 blocking antibody, IL-10 neutralizing antibody did not alter NOX2 mRNA levels in similarly prepared co-cultures of mSOD1 microglia/mSOD1 Tregs (p>0.05, Fig. 5). Comparable results were obtained when another IL-10 blocking antibody from a different company were used (data not shown). Similarly, when a neutralizing antibody to TGF-β was added to the cultures, just like IL-10 neutralizing antibodies, the TGF-β blocking antibody did not increase NOX2 message levels in mSOD1 microglia/mSOD1 Treg co-cultures (p>0.05, Fig. 5).
Fig. 5.
The suppressive effects of mSOD1 Tregs on microglial (Mc) activation was not dependent on IL-10, TGF-β, or CTLA-4. mSOD1 Mc were co-cultured with mSOD1 Teffs or mSOD1 Tregs for 2 days with or without blocking antibodies to IL-4, IL-10, TGF-β, or CTLA-4. IL-4 neutralizing antibody reversed microglial NOX2 mRNA expression in mSOD1 Mc and Tregs co-cultures. However, the addition of IL-10, TGF-β, or CTLA-4 neutralizing antibodies did not change mRNA levels of NOX2 in mSOD1 Mc and Tregs co-cultures. Data shown as mean±SE of three independent experiments with duplicate or triplicate wells. ##p<0.01 vs. Mc+Teffs without blocking antibody; &&p<0.01 vs. Mc+Tregs without blocking antibody. Ab = antibody; Mc = microglia.
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) represents an important negative switch, or checkpoint, that controls the duration and intensity of an immune response (Paterson and Sharpe, 2010). CTLA-4 binds both CD80 and CD86 on antigen presenting cells, such as microglia. To assess whether CTLA-4 also participates in the Treg effect of modulating mSOD1 microglia by cell-to-cell contact, a blocking antibody to CTLA-4 was used. Our results indicated that in the presence of CTLA-4 antibody, NOX2 mRNA levels in mSOD1 microglia/mSOD1 Tregs co-cultures were identical to the same co-cultures without CTLA-4 antibody (p>0.05, Fig. 5).
Tregs from 160 day old mSOD1 mice also suppress mSOD1 microglial activation
We have shown that disease rates in ALS mice are dependent on the age of the mice; 100 day old mSOD1 mice progress slowly whereas 160 day old mSOD1 mice have a rapidly progressing disease (Beers et al., 2008; 2011). Furthermore, since we recently demonstrated that NOX2 mRNA levels are increased in older rapidly progressing ALS mice compared with younger slowly progressing ALS mice (Beers et al., 2011), we asked whether Tregs in rapidly progressing mice are dysfunctional with respect to their abilities to suppress microglial activation. Co-culturing 160 day mSOD1 Tregs with microglia were just as effective in suppressing NOX2 mRNA levels as co-culturing an equivalent number of 100 day mSOD1 Tregs with microglia; neither 100 or 160 day Teffs suppressed microglial NOX2 (Fig. 6). Thus, the abilities of mSOD1 Tregs to suppress microglial activation are same in mSOD1 mice during the 100 day old stable phase as those present during the 160 day old rapidly progressing phase. However, these data did not rule out the possibility that the numbers of Tregs had declined during the rapidly progressing disease phase in ALS mice.
Fig. 6.
Tregs isolated from mSOD1 mice during the rapidly progressing phase suppressed mSOD1 microglial (Mc) activation. Tregs from slowly progressing phase (100 day old) or from rapidly progressing phase (160 day old) mSOD1 mice were co-cultured with mSOD1 Mc for 2 days. Both 100 day Tregs and 160 day Tregs equally suppressed microglial NOX2 mRNA levels. Data shown as mean±SE of at least three independent experiments with duplicate or triplicate wells. **p<0.01 vs. WT Mc + WT Teffs; ##p<0.01 vs. Mc +Teffs in each group. WT = wild-type; Mc = microglia.
Number and cytokine expression of mSOD1 Tregs are altered at different disease stages
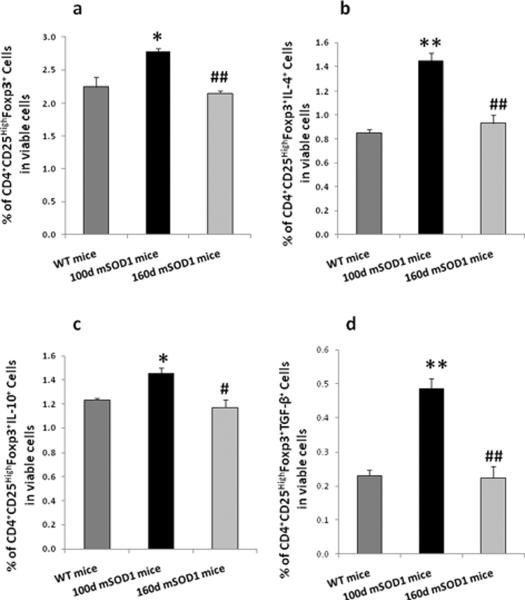
The numbers of Tregs in the lymph nodes of WT mice, and 100 day old (slow stage), and 160 day old (rapid stage) mSOD1 mice, were assessed by flow cytometry. Our analyses determined that 100 day old mSOD1 mice had more CD4+CD25HighFoxp3+ Tregs than WT mice (p<0.05, Fig 7a). However, 160 day old mSOD1 mice had fewer CD4+CD25HighFoxp3+ Tregs than 100 day old mSOD1 mice (p<0.01, Fig 7a). Furthermore, we determined whether the expression of the anti-inflammatory cytokines IL-4, IL-10 and TGF-β in mSOD1 CD4+CD25HighFoxp3+ Tregs was also altered between the slowly and rapidly progressing phases. Our data showed that the number of IL-4 expressing CD4+CD25HighFoxp3+ Tregs was increased in 100 day old mSOD1 mice compared with WT mice (p<0.01), whereas the number of these cells in 160 day old mSOD1 mice were decreased compared with 100 day old mSOD1 mice (p<0.01, Fig. 7b). Similarly, CD4+CD25HighFoxp3+IL-10+ Tregs increased in slowly progressing mSOD1 mice compared with WT mice (p<0.05), and decreased in rapidly progressing mSOD1 mice compared with slow phase mSOD1 mice (p<0.05, p<0.05, Fig. 7c). TGF-β expressing CD4+CD25HighFoxp3+ Tregs were increased in 100 day old mSOD1 mice compared with WT mice and were decreased in 160 day old mSOD1 mice (p<0.01, p<0.01, Fig. 7d). Thus, these data suggest that not only are the numbers of CD4+CD25HighFoxp3+ Tregs increased during the slowly progressing phase and then subsequently declining during the rapidly progressing phase, but also that the expression of three anti-inflammatory cytokines waxes and wanes with disease progression.
Fig. 7.
Numbers of CD4+CD25HighFoxp3+ Tregs, IL-4-, TGF-β-, and IL-10-expressing CD4+CD25HighFoxp3+ Tregs were different in wild-type (WT) mice (100 day old), slowly progressing mSOD1 mice (100 day old), and rapidly progressing mSOD1 mice (160 day old). The Tregs were isolated from the lymph nodes (axillary, inguinal and superficial cervical) of WT or mSOD1 mice, and analyzed by flow cytometry. (a) More CD4+CD25HighFoxp3+ Tregs were present in 100 day mSOD1 mice than WT mice, while less CD4+CD25HighFoxp3+ Tregs were found in 160 day mSOD1 mice compared with 100 day mSOD1 mice. (b–d) Expression of IL-4, TGF-β, and IL-10 were also analyzed in CD4+CD25HighFoxp3+ Tregs populations. The numbers of CD4+CD25HighFoxp3+IL-4+ Tregs (b), CD4+CD25HighFoxp3+TGF-β+ Tregs (c), CD4+CD25HighFoxp3+IL-10+ Tregs (d) were increased in 100 day old mSOD1 mice, whereas these cytokines were decreased in 160 day old mSOD1 mice. Data shown as mean±SE of three independent experiments. *p<0.05, **p<0.01 vs. WT mice; #p<0.05, ##p<0.01 vs. 100 day mSOD1 mice. WT = wild-type.
Proliferation and cytotoxicity of Teffs are different in WT, 100 day old, and 160 day old ALS mice
Although Teffs from slowly progressing (100 days) mSOD1 mice proliferated slower than WT mice Teffs (p<0.01, Fig. 8a), Teffs isolated from rapidly progressing (160 days) mSOD1 mice proliferated to a greater extent (190%) than Teffs from slowly progressing (100 days) mSOD1 mice (p<0.01, Fig. 8a). IFN-γ is the prototypic cytotoxic cytokine expressed by the CD4+ Th1 sub-population of T lymphocytes and in accord with the previously described Teff proliferation capacity, relative IFN-γ message levels increased in 160 day mSOD1 Teffs compared with 100 day mSOD1 Teffs (p<0.05, Fig. 8b); 100 day mSOD1 Teffs expressed less IFN-γ than WT Teffs (p<0.05, Fig. 8b). Thus, mSOD1 Teffs isolated during the rapidly progressing phase are more toxic and proliferate to a greater extent than mSOD1 Teffs isolated from mSOD1 mice during the slowly progressing phase.
Fig. 8.
Proliferation and cytotoxicity of Teffs were different among wild-type (WT) mice (100 day old), slowly progressing mSOD1 mice (100 day old), and rapidly progressing mSOD1 mice (160 day old). (a) Purified Tregs and Teffs were cultured for 3 days and cell proliferation was measured. Teffs from 100 day old mSOD1 mice proliferated slower than WT Teffs, and Teffs from 160 day old mSOD1 mice (160 day old) proliferated faster than Teffs from 100 day old mSOD1 mice. (b) IFN-γ mRNA was measured as an index of Teffs cytotoxicity. One hundred day mSOD1 Teffs expressed less IFN-γ than WT Teffs; 160 day mSOD1 Teffs expressed more IFN-γ than 100 day mSOD1 Teffs. Tregs did not proliferate. Data shown as mean±SE of three independent experiments with duplicate or triplicate wells. *p<0.05, **p<0.01 vs. WT Teffs; &p<0.05, &&p<0.01 vs. 100 day mSOD1 Teffs. WT = wild-type.
Inhibition of mSOD1 Teffs by 100 day mSOD1 Tregs depends on TGF-β, IL-4, and IL-10
After co-culturing Tregs with Teffs, both 100 day and 160 day mSOD1 Tregs inhibited proliferation and IFN-γ expression of Teffs as effectively as WT Tregs (Fig. 9a, b). To explore the mechanisms of this proliferative inhibition, neutralizing antibodies were added to the co-cultures. The addition of IL-4, IL-10, or TGF-β neutralizing antibodies, no matter whether they were added individually or in combination, did not reduce the inhibiting effect of WT Tregs on WT Teff proliferation. However, the combination of IL-4, IL-10, and TGF-β antibodies did reduce the inhibitory activity of 100 day mSOD1 Tregs co-cultured with mSOD1 Teffs (p<0.01, Fig. 9a); the blocking effects were not observed when using each neutralizing antibody alone or in combination with either two antibodies in 100 day mSOD1 Treg and Teff co-cultures. Interestingly, the inhibitory effect of 160 day Tregs on Teff proliferation was not blocked by IL-4, IL-10, or TGF-β neutralizing antibodies, either individually or in combination (p>0.05, Fig. 9a). Similarly, in the presence of IL-4, IL-10, and TGF-β neutralizing antibodies, IFN-γ message was increased in 100 day mSOD1 Treg and Teff co-cultures (p<0.05, Fig. 9b). However, the combination of IL-4, IL-10, and TGF-β neutralizing antibodies did not change IFN-γ expression in either WT or 160 day mSOD1 Treg and Teff co-cultures (p>0.05, Fig. 9b). These data suggest that the Tregs isolated from WT and 160 day old mSOD1 mice suppress Teff function through different mechanisms from that used by 100 day mSOD1 Tregs, possibly through a cell-contact or other cytokine driven mechanisms.
Fig. 9.
Inhibition of Teffs by 100 day mSOD1 Tregs is dependent on IL-4, IL-10, and TGF-β. Teffs were co-cultured with corresponding Tregs for 3 days with or without blocking antibodies to IL-4, IL-10, and TGF-β. Teffs proliferation was measured and suppressive activity of Tregs on Teffs was calculated. (a) The inhibition rate of 100 day or 160 day mSOD1 Tregs on mSOD1 Teffs was similar as the rate of wild-type (WT) Tregs on WT Teffs. The combination of neutralizing antibodies to IL-4, IL-10, and TGF-β decreased the inhibition rate of 100 day mSOD1 Tregs, but these 3 antibodies together did not change the inhibition rate of WT Tregs on WT Teffs or 160 day old Tregs on 160 day Teffs. Neither two of these 3 antibodies blocked the suppressive effect of 100 day, 160 day mSOD1 Tregs or WT Tregs. (b) One hundred day mSOD1 Tregs or 160 day mSOD1 Tregs inhibited IFN-γ mRNA in Teffs to the similar levels as those in WT Teffs and Tregs co-cultures. The addition of neutralizing antibodies to IL-4, IL-10, and TGF-β increased IFN-γ expression in 100 day mSOD1 Teffs and Tregs co-cultures, but did not change the levels of IFN-γ in either WT Teffs + Tregs or 160 day mSOD1 Teffs + Tregs co-cultures. Data shown as mean±SE of three independent experiments with duplicate or triplicate wells. *p<0.05, vs. WT Teffs; &p<0.05, vs. 100 day mSOD1 Teffs; ##p<0.01 vs. Teffs in each group; ‡p<0.05, ‡‡p<0.01 vs. 100 day mSOD1 Teffs + Tregs without blocking antibodies; Δp<0.05, ΔΔp<0.01 vs. WT Teffs + Tregs + 3 blocking antibodies. Ab = antibody; Tc = T cells; WT = wild-type.
Discussion
The interactions between microglia and T lymphocytes, and the mechanisms of intercellular communication between these two cell populations, have not been well-delineated in ALS. We previously reported that the lack of functional CD4+ T lymphocytes enhanced the functional toxic attributes of microglia and accelerated disease progression in ALS mice (Beers et al., 2008). In the present study, the activation state of mSOD1 microglia was documented by the increased expression levels of NOX2, iNOS and nitric oxide production. When activated mSOD1 microglia were co-cultured with mSOD1 Tregs, the expression of these microglial toxic factors was attenuated by the release of IL-4 from mSOD1 Tregs. In contrast, mSOD1 Teffs were unable to inhibit the free radical mRNA expression in mSOD1 microglia, possibly due to their limited production and release of IL-4. These results are consistent with our earlier reports demonstrating that the neuroprotective effects of IL-4 in microglia/motoneuron co-cultures were mediated through the inhibition of microglial activation, and that passive transfer of mSOD1 CD4+ T lymphocytes restored survival, and suppressed microglial cytotoxicity in ALS mice (Zhao et al., 2006; Beers et al., 2011). We also demonstrated that the Tregs in the transferred cells are the population of cells responsible for mediating the neuroprotective effects in the ALS mice (Beers et al., 2011). Thus, these data suggest that Tregs, and more specifically CD4+CD25HighFoxp3+IL-4+ Tregs, can suppress neurotoxic microglial activation and augment survival of mSOD1 mice.
Over the past several years, many possible mechanisms for Tregs mediating suppression on immune cells have been proposed (Ohkura and Sakaguchi, 2010; Yamaguchi et al., 2011). One potential mechanism whereby Tregs may mediate their suppressive effects is through cell-to-cell contact. The contribution of cell contact dependent mechanisms was suggested by inability of Tregs to suppress proliferative response of responder T cells when the two populations are separated by a semi-permeable membrane in vitro (Takahashi et al., 1998; Thornton and Shevach, 1998). Another possibility is that Tregs mediate suppression through the secretion of cytokines, such as IL-10 and TGF-β, two additional key anti-inflammatory factors produced by the immune system (Tang and Bluestone, 2008; Ohkura and Sakaguchi, 2010; Shevach, 2009). In this study, mSOD1 Tregs suppression of toxic mSOD1 microglia may not depend on a cell-to-cell contact mechanism. Firstly, our data showed that CTLA4 was not involved, which suggests that inhibiting contact through CTLA4 on Tregs and B7 molecules on microglia does not play a role on the Tregs suppression of microglial activation. Secondly, the application of conditioned media from mSOD1 Tregs reduced mSOD1 microglial NOX2. Furthermore, the addition of IL-4 neutralizing antibody to the mSOD1 microglial cultures containing conditioned media of mSOD1 Tregs reversed the expression of microglial NOX2 compared with mSOD1 microglia in the same conditioned media without IL-4 antibody. It is known that CD4+ Th2 cells release IL-4. Although most of mSOD1 Tregs in our study were Foxp3 expressing Tregs, the remaining population may contain Th2 cells. We did observe 1.1% CD4+Foxp3−IL-4+ T-cells in mSOD1 Tregs population, suggesting Th2 cells may have partially contributed to the IL-4 production and suppressive effects on microglial activation. However, flow data from highly purified mSOD1 CD4+CD25HighFoxp3(GFP)+ Tregs demonstrate that 6.1% of Tregs also expressed IL-4. In addition, more IL-4+ mSOD1 Tregs were observed after incubating with mSOD1 microglia, indicating that mSOD1 microglia may promote IL-4 expression in mSOD1 Tregs. Similarly, other reports have also shown that specific subpopulations of Tregs are able to produce IL-4 (Tiemessen et al., 2007; Baecher-Allan et al., 2006). Although we did not observe the involvement of IL-10 and TGF-β in Tregs suppression on microglial NOX2 expression in this ALS model, we do not exclude that these anti-inflammatory cytokines affect other microglial phenotypes. It has been shown that IL-10 and TGF-β modulated CD40 and class II MHC gene expression in microglia (Qin et al., 2006; O'Keefe et al., 1999).
WT Tregs, 100 day and 160 day mSOD1 Tregs, can equivalently inhibit proliferation and cytotoxicity of their respective Teffs, but their mechanisms of inhibition are different. The inhibitory capacity of 100 day mSOD1 Tregs on 100 day mSOD1 Teffs is mediated by the combination of IL-4, IL-10, and TGF-β. However, combination of blocking antibodies to IL-4, IL-10, and TGF-β did not change the Tregs inhibition on Teffs isolated from WT or 160 days old mSOD1 mice, suggesting WT or 160 day Tregs may suppress Teffs function through a cell-cell contact or other cytokine driven mechanisms. As noted earlier, while Tregs-mediated inhibition of Teffs was shown to be dependent on cell-to-cell contact (Takahashi et al., 1998; Thornton and Shevach, 1998; Read et al., 2000), soluble anti-inflammatory factors contribute to the suppressive effects of Tregs under pathological conditions (Lloyd and Hawrylowicz, 2009; Suri-Payer and Cantor, 2001; Asseman et al., 1999). Therefore, it is possible that multiple mechanisms may operate in Tregs-mediated suppression and that various molecules may contribute to their suppressive functions; a particular mechanism may play a dominant role under a particular condition, with different mechanisms operating in various situations (Sakaguchi et al., 2009). In terms of ALS, our data indicate that the inhibition of Tregs at the slowly progressing phase is dependent on soluble cytokines, but this feature is lost when the ALS mice progress into the rapid phase.
Our observations revealed that mSOD1 Tregs isolated from ALS mice during the rapidly progressing phase of disease (160 days) have similar suppressive effects on microglial toxicity and Teffs proliferation as an equal number of mSOD1 Tregs isolated during the slowly progressing disease phase (100 days). However, the total number of Tregs at 100 days was increased, while the number of Tregs at 160 days was decreased. Similar changes were observed on the numbers of CD4+CD25HighFoxp3+IL-4+, CD4+CD25HighFoxp3+IL-10+, and CD4+CD25HighFoxp3+TGF-β+ Tregs during slow and rapid progressing phases. These data suggest that greater numbers of Tregs with expression of three anti-inflammatory cytokines have enhanced suppressive effects on microglia and Teffs in vivo, and thus may contribute to the slower progression of ALS disease during this period of time. This is in accordance with our recent report that Tregs isolated from slow stage mice, and passively transferred repeatedly into mSOD1 mice prior to onset, lengthened this slowly progressing phase and prolonged survival (Beers et al., 2011). Furthermore, the proliferative capacity of mSOD1 Teffs at 160 days was enhanced compared with mSOD1 Teffs at 100 days, suggesting a transformation from a protective Tregs response to an increased Teffs response during the rapidly progressing phase of ALS disease.
NOX2, a subunit of NADPH oxidase predominantly expressed in macrophages/microglia that generates superoxide, and iNOS, the enzyme that catalyzes the production nitric oxide, are major sources of microglial free radicals production. Our present and previous studies demonstrated that adult microglia isolated from spinal cords of mSOD1 mice during the rapidly progressing phase (130 day old) are activated and up-regulated their expression of NOX2, suggesting that adult mSOD1 microglia are capable of producing augmented levels of superoxide. In addition, the current study also demonstrates that iNOS expression and nitric oxide production are increased in mSOD1 microglia. Our earlier reports documented that activated microglia were toxic to motoneurons by releasing superoxide and nitric oxide (Zhao et al., 2004, 2006). Therefore, these cumulative data indicate that adult mSOD1 microglia are neurotoxic and may contribute to the rapid rate of disease progression in ALS mice.
The evidence that mSOD1 Tregs were able to attenuate microglial oxidative stress in spinal cord of ALS suggests mSOD1 Tregs may protect motoneurons from microglial cytotoxicity mediated by toxic free radicals. Our in vivo data have shown that adoptive transfer of mSOD1 Tregs into ALS mice prolonged the slowly progressing phase (Beers et al., 2011). These observations suggest that Tregs-mediated suppression of microglial activation may be a neuroprotective pathway in ALS. In accordance with these findings, Tregs have been reported to modulate redox-active enzymes and suppress reactive oxygen species from nitrated α-synuclein-activated microglia (Reynolds et al., 2009). It has also been shown that Tregs inhibited microglial oxidative stress and inflammation with concomitant neuroprotection in animal models of Parkinson's disease and HIV-1 associated neurodegenerative disorders (Reynolds et al., 2007; Liu et al., 2009). Therefore, Tregs-mediated modulation of microglial cytotoxicity provides a new insight for therapeutic strategies on neurodegenerative diseases.
Our data demonstrated that mSOD1 Tregs suppress immune toxicity by inhibiting microglial activation, Teffs proliferation, and the accompanying cytotoxicity, thus providing motoneuron protection in ALS. The ability of Tregs to modulate microglial inflammation, cell function, and specific enzymatic activities provides novel tools to manipulate ongoing microglial inflammatory responses; it is clear that there is cross-talk between the immune system and brain, and this dialogue merits our full attention. The ability to utilize Tregs to decrease cytotoxicity may offer a novel therapeutic option for ALS.
Highlights
Tregs from ALS mice suppress microglia through an IL-4 mediated mechanism.
Tregs from slow phase ALS mice have increased anti-inflammatory cytokines levels.
Tregs from slow phase ALS mice inhibit ALS Teffs through IL-4, IL-10 and TGF-β.
ALS mouse microglia induce ALS mouse Tregs to co-express higher levels of IL-4.
There is an active dialogue between innate and adaptive immune systems in ALS.
Acknowledgements
This study was supported by Muscular Dystrophy Association, NIH Grants (NS070050 and NS067153), and the Texas Methodist Foundation. We thank Ailing Huang, Jinghong Wang, Xiaoli Wang and Shixiang Wen for their technical assistance.
Abbreviations
- AD
Alzheimer's disease;
- ALS
amyotrophic lateral sclerosis;
- CTLA-4
Cytotoxic T-Lymphocyte Antigen 4;
- FACS
Fluorescence-activated cell sorting;
- Foxp3
forkhead-box transcription factor;
- GFP
green fluorescent protein;
- IL-4
interleukin 4;
- IL-10
interleukin 10;
- iNOS
inducible nitric oxide synthase;
- Mc
microglia;
- NOX2
a subunit of NADPH oxidase;
- PD
Parkinson's disease;
- SOD1
Cu2+/Zn2+ superoxide dismutase 1;
- mSOD1
mutant SOD1;
- mSOD1G93A
G93A mutant form of SOD1;
- NO
nitric oxide;
- TGF-β
Transforming growth factor beta;
- Teffs
effector T lymphcytes;
- Tregs
regulatory T lymphcytes;
- WT
wild-type.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures The authors have no financial conflicts of interest.
References
- Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- Almer G, Guegan C, Teismann P, Naini A, Rosoklija G, Hays AP, Chen C, Przedborski S. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann. Neurol. 2001;49:176–185. [PubMed] [Google Scholar]
- Almer G, Vukosavic S, Romero N, Przedborski S. Inducible nitric oxide synthase up-regulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 1999;72:2415–2425. doi: 10.1046/j.1471-4159.1999.0722415.x. [DOI] [PubMed] [Google Scholar]
- Aloisi F, De Simone R, Columba-Cabezas S, Penna G, Adorini L. Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J. Immunol. 2000;164:1705–1712. doi: 10.4049/jimmunol.164.4.1705. [DOI] [PubMed] [Google Scholar]
- Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson's disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 2010;31:7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan H, Kipnis J, Butovsky O, Caspi RR, Schwartz M. Vaccination with autoantigen protects against aggregated beta-amyloid and glutamate toxicity by controlling microglia: effect of CD4+CD25+ T cells. Eur. J. Immunol. 2004;34:3434–3445. doi: 10.1002/eji.200424883. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J. Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklós L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Wen S, Liao B, Appel SH. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134:1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Donadoni C, Guglieri M, Papadimitriou D, Strazzer S, Del Bo R, Comi GP. Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain. 2004;127:2518–2532. doi: 10.1093/brain/awh273. [DOI] [PubMed] [Google Scholar]
- Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch. Neurol. 1993;50:30–36. doi: 10.1001/archneur.1993.00540010026013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J. Neurosci. Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Henkel JH, Beers DR, Siklós L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol. Cell. Neurosci. 2006;31:427–437. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Zhao W, Appel SH. Microglia in ALS: The good, the bad, and the resting. J. Neuroimmune Pharmacol. 2009;4:389–98. doi: 10.1007/s11481-009-9171-5. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Engelhardt JI, Siklós L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann. Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- Ince PG, Shaw PJ, Slade JY, Jones C, Hudgson P. Familial amyotrophic lateral sclerosis with a mutation in exon 4 of Cu/Zn superoxide dismutase gene: pathological and immunocytochemical changes. Acta Neuropathol. 1996;92:395–403. doi: 10.1007/s004010050535. [DOI] [PubMed] [Google Scholar]
- Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, Gendelman HE. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J. Immunol. 2009;182:3855–3865. doi: 10.4049/jimmunol.0803330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–49. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J. Clin. Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura N, Sakaguchi S. Regulatory T cells: roles of T cell receptor for their development and function. Semin. Immunopathol. 2010;32:95–106. doi: 10.1007/s00281-010-0200-5. [DOI] [PubMed] [Google Scholar]
- O'Keefe GM, Nguyen VT, Benveniste EN. Class II transactivator and class II MHC gene expression in microglia: modulation by the cytokines TGF-beta, IL-4, IL-13 and IL-10. Eur. J. Immunol. 1999;29:1275–1285. doi: 10.1002/(SICI)1521-4141(199904)29:04<1275::AID-IMMU1275>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Paterson AM, Sharpe AH. Taming tissue-specific T cells: CTLA-4 reins in self-reactive T cells. Nat. Immunol. 2010;11:109–111. doi: 10.1038/ni0210-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Wilson CA, Roberts KL, Baker BJ, Zhao X, Benveniste EN. IL-10 inhibits lipopolysaccharide-induced CD40 gene expression through induction of suppressor of cytokine signaling-3. J. Immunol. 2006;177:7761–7771. doi: 10.4049/jimmunol.177.11.7761. [DOI] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson's disease. J. Leukoc. Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells. J. Proteome Res. 2009;8:3497–3511. doi: 10.1021/pr9001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int. Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Schwartz M, Kipnis J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav. Immun. 2011;25:817–819. doi: 10.1016/j.bbi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+CD25+ T cells. J. Autoimmun. 2001;16:115–123. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemessen MM, Jagger ASL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, Leigh PN, Banati RB. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 position emission tomography study. Neurobiol. Dis. 2004;15:601–609. doi: 10.1016/j.nbd.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Weydt P, Weiss MD, Moller T, Carter GT. Neuro-inflammation as a therapeutic target in amyotrophic lateral sclerosis. Curr. Opin. Investig. Drugs. 2002;3:1720–1724. [PubMed] [Google Scholar]
- Xiao Q, Zhao W, Beers DR, Yen AA, Xie W, Henkel JS, Appel SH. Mutant SOD1G93A microglia are more neurotoxic relative to wild-type microglia. J. Neurochem. 2007;102:2008–2019. doi: 10.1111/j.1471-4159.2007.04677.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Le W, Beers DR, He Y, Henkel JS, Simpson EP, Yen AA, Xiao Q, Appel SH. Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J. Neuropathol. Exp. Neurol. 2004;63:964–977. doi: 10.1093/jnen/63.9.964. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J. Neurochem. 2006;99:1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]