Abstract
Despite advances in the treatment of acute tissue ischemia significant challenges remain in effective cytoprotection from ischemic cell death. It has been documented that injected stem cells, such as mesenchymal stem cells (MSCs), can confer protection to ischemic tissue through the release of paracrine factors. The study of these factors is essential for understanding tissue repair and the development of new therapeutic approaches for regenerative medicine. We have recently shown that a novel factor secreted by MSCs, which we called HASF (Hypoxia and Akt induced Stem cell Factor), promotes cardiomyocyte proliferation. In this study we show that HASF has a cytoprotective effect on ischemia induced cardiomyocyte death. We assessed whether HASF could potentially be used as a therapeutic agent to prevent the damage associated with myocardial infarction. In vitro treatment of cardiomyocytes with HASF protein resulted in decreased apoptosis; tunel positive nuclei were fewer in number, caspase activation and mitochondrial pore opening were inhibited. Purified HASF protein was injected into the heart immediately following myocardial infarction. Heart function was found to be comparable to sham operated animals one month following injury and fibrosis was significantly reduced. In vivo and in vitro HASF activated protein kinase C ε (PKCε). Inhibition of PKCε blocked the HASF effect on apoptosis. Furthermore, the beneficial effects of HASF were lost in mice lacking PKCε. Collectively these results identify HASF as a protein of significant therapeutic potential, acting in part through PKCε.
Keywords: cytoprotection, ischemia, paracrine, PKC epsilon, mesenchymal stem cell
1. Introduction
Acute ischemic injury, such as myocardial infarction and stroke, is the leading cause of morbidity and mortality in the world [1]. Prompt restoration of blood flow (reperfusion) through thrombolysis and/or vascular intervention has contributed significantly to improving clinical outcome. However, therapeutic agents which can prevent acute ischemic tissue damage remain unavailable. As a result, effective cell protection for tissue repair and regeneration after injury is still an unmet clinical need [2].
Recent studies have demonstrated that stem cells produce and secrete a broad variety of adhesive molecules, chemokines and growth factors, which acting in a paracrine fashion can influence the microenvironment of the injured tissue leading to cytoprotection, vascularization and/or activation of resident stem cells [3–7]. Consequently the characterization of proteins secreted by stem cells, and their cellular effects, are valuable not only for the understanding of tissue repair but also for the development of future therapeutic approaches in regenerative medicine.
We have recently shown that the protein product of the C3orf58 gene, which we named HASF for Hypoxia and Akt induced Stem cell Factor, stimulates cardiomyocyte proliferation via phosphatidylinositol-3 kinase (PI3K) and Akt [8]. In this study, we examined the effects of HASF on ischemia induced cardiomyocyte death and elucidated its cellular protective mechanism as mediated by PKCε. Importantly, our data demonstrated that HASF has significant cardioprotective effect in vivo. The results of this study provide validation of our strategy of discovering therapeutic molecules from stem cell derived paracrine factors.
2. Materials and Methods
2.1 Bioinformatics and Molecular Biology
GeneChip Mouse Genome 430A 2.0 Array (Affymetrix, Inc.) was used to discover differentially expressed uncharacterized transcripts in mouse Akt-MSCs. Putative secreted proteins encoded by these transcripts were discovered by querying for the presence of predicted N-signal peptide sequences (http://www.cbs.dtu.dk/services/SignalP/) and by the exclusion of transmembrane domains (http://www.cbs.dtu.dk/services/TMHMM-2.0/). Potential biological functions for proteins were predicted by using an Supplementary server (http://www.cbs.dtu.dk/services/ProtFun/).
A PCR fragment (626 bp) of mouse HASF was amplified from mouse Akt-MSCs with the forward primer, 5′-ggccatttgcaaaatatcttggagcttgtg-3′ and reverse primer, 5′-acttaactgtgccagatagccacgcagtt-3′. This PCR product was subsequently cloned into pGEM-TA vector (Promega) for sequencing. Human homologous cDNA of HASF, with gene name as chromosome 3 open reading frame 58, (C3orf58) was purchased from American Type Culture Collection (ATCC, clone MGC 33365 or IMAGE 5267770).
2.2. Recombinant protein generation
The open reading frame of human HASF without the predicted N-signal sequence (1158 bp) was cloned in-frame in pMal-2C vector. The insert (1158 bp) was subsequently with the forward primer (underlined with Nde I restriction site), 5′-ggcggccatatggaccggcgcttcctgcag-3′ and the reverse primer (underlined with BamH I restriction site), 5′-ggcggcggatccctacctcacgttgttacttaattgtgctagg-3′, and cloned in-frame into pET 15b vector (EMD Biosciences) to generate 6×His tagged HASF construct. The protein was produced in E. coli and isolated using Ni+ column (Clontech). The protein sequence was confirmed by matrix-assisted laser desorption-ionization mass spectrometry (MALDI-MS) on an Applied Biosystems 4700 Proteomic Analyzer® time of flight (TOFTOF®) mass spectrometer (Duke core facility). Positive mode time of flight was used to identify peptides, and individual peptides were sequenced by MS/MS. All peptide fingerprint data was searched by SwissProt and Mascot search engine. The yield of protein production from E. coli for the 6×His-HASF recombinant protein was 0.2 μg/ml, with high purity. In addition, hpc4-tagged HASF Recombinant HASF Protein was produced from insect cells using Invitrogen’s Drosophila Expression System. Protein was purified from the media using Anti-Protein C Affinity Matrix from Roche (catalog number 11815024001). In vitro a dose response curve was conducted; for His-tagged HASF this concentration was 100nM.
2.3. In vivo model of ischemia/reperfusion injury
Sprague-Dawley rats were used for all in vivo experiments, according to experimental protocols approved by Duke Office of Animal Welfare Assurance of Duke University. A midsternal thoracotomy was performed to expose the anterior surface of the heart after anesthesia. The proximal left ascending coronary artery (LAD) was identified and a 6.0 suture (Ethicon) was placed around the artery and surrounding myocardium. Regional left ventricular ischemia was induced for 30 minutes by ligation of LAD, followed by immediate injection of 1 μg of recombinant protein HASF or PBS vehicle control in five spots, in a total volume of 250 μl, into the border zone adjacent to the infarcted myocardium,. The ligature was loosened and reperfusion was achieved after 30 min of the ischemia period and the incision was closed and the animals were allowed to recover.
Conditioned media experiments were performed as described previously [10].
2.4 HASF transgenic and PKCε knockout
For the generation of HASF Tg mice human HASF cDNA was placed under the control of the alpha myosin heavy chain promoter in p-Bluescript plasmid as described in Subramaniam et al., [9]. The DNA was linearized and introduced into FVB embryos and maintained on an FVB background for at least 10 generations. The HASF Tg Prkce−/− mice were generated by crossing HASF Tg mice with Prkce−/− mice from Jackson LAB (stock number 4189). The resulting mice were maintained in FVB/C57BL6 mixed background. Standard methods for the biochemical, electrophysiological analyses of Tg mice were employed. All experimental protocols using rat and mice were approved by the Duke Office of Animal Welfare Assurance of Duke University.
2.5. Calcein and TMRM
Mitochondrial viability was assessed with calcein and TMRP dyes. Calcein and TMRM were used according to manufacturer’s instructions. Images were taken by Zeiss fluorescent microscope using identical settings. Fluorescent intensity was determined by Image J.
2.6 Statistics
All the results are presented as the mean ± SE and were analyzed using unpaired Student’s t-test or ANOVA where appropriate.
3. Results
3.1 HASF is a MSC secreted protein
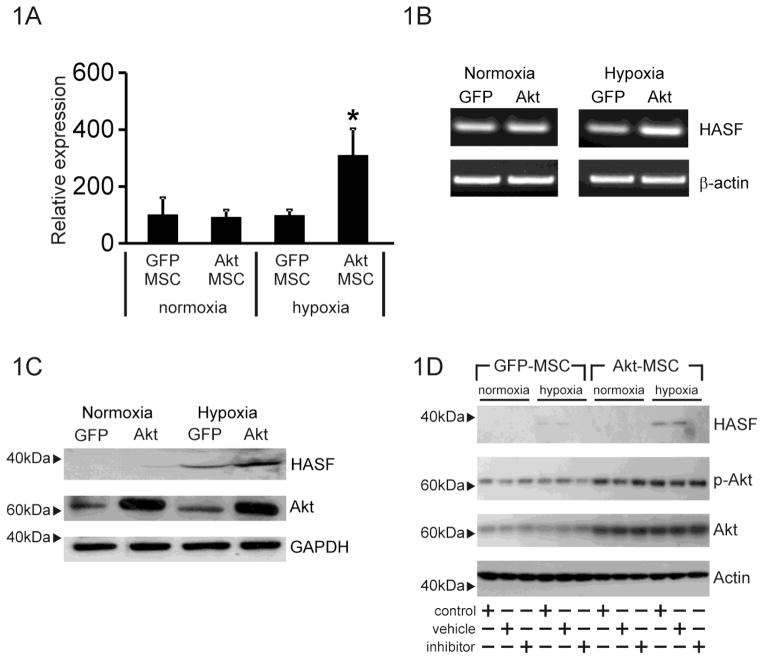
We have previously demonstrated that MSCs genetically modified to over-express the Akt1 gene (Akt-MSCs) show enhanced reparative properties predominantly due to the release of paracrine factors [10–12]. Using genomic and bioinformatic analyses we compared the responses of murine Akt-MSCs versus control GFP-MSCs to hypoxia and identified 169 transcripts encoding for genes with a previously unassigned function [10, 11, 13]. One transcript was dramatically up-regulated by both Akt and hypoxia (Figure 1A) in a semi quantitative assay and thus we named it Hypoxia and Akt induced Stem Cell Factor (HASF) (Figure 1A, B).
Figure 1. HASF is secreted by MSCs.
(A) Affymetrix microarray expression data of HASF in Akt-MSCs and control GFP-MSCs under normoxic or hypoxic conditions for 6hr. * P<0.001. Values are mean ± SE (n=3). (B) RT-PCR validation of mouse HASF expression in Akt-MSCs (Akt) and control GFP-MSCs (GFP) under normoxic or hypoxic conditions for 6hr. Beta-actin was used as internal control. (n=3). (C) Immunoblot analysis for HASF expression in conditioned media from MSCs using an anti-HASF polyclonal antibody. GFP: control GFP-MSCs, Akt: Akt-MSCs. Akt and GAPDH levels in the cell lysates served as controls. (D) Immunoblot analysis of conditioned media for HASF expression from MSCs cultured under normoxic or hypoxic conditions in the presence of the secretion inhibitor brefeldin-A (1μg/ml). Cell extracts (7.5μg) were probed with phospho-Akt, Akt and actin. Phospho-Akt and Akt to show that brefeldin-A had no effect on Akt activity or Akt expression, actin served as a loading control (n=3).
Alignment of mouse (gene name 1190002N15Rik) and human (gene name C3orf58) HASF protein sequences revealed a highly conserved homology of ~98%. In the mouse the HASF gene encodes a 430 amino acid protein with no similarity to known proteins. No functional domains were identified apart from the presence of a putative signal peptide in positions 1–35 suggesting that HASF is a secreted protein (Supplementary Figure 1A).
To verify that HASF is a secreted protein HEK cells were transfected with a plasmid construct containing human HASF with a C-terminal V5-polyhistidine (6xHis) epitope tag. Immunoblotting with a rabbit anti-V5 antibody confirmed the presence of V5-epitope tagged HASF in the culture media of HEK293 cells at 24 h and 48 h after transfection, but not in the medium of the vehicle control lipofectamine transfected HEK293 cells (Supplementary Figure 1B), indicating that HASF when over-expressed can be secreted. In-order to verify that native MSCs can secrete HASF endogenously several experiments were performed. Immunoblot analysis, using a rabbit polyclonal antibody raised against HASF, confirmed the presence of HASF protein in the conditioned media from mouse MSCs. Levels of the HASF protein were higher in the media from Akt-MSCs under hypoxia conditions (Figure 1C). Furthermore brefeldin-A, a secretion inhibitor, prevented the appearance of HASF in media (Figure 1D).
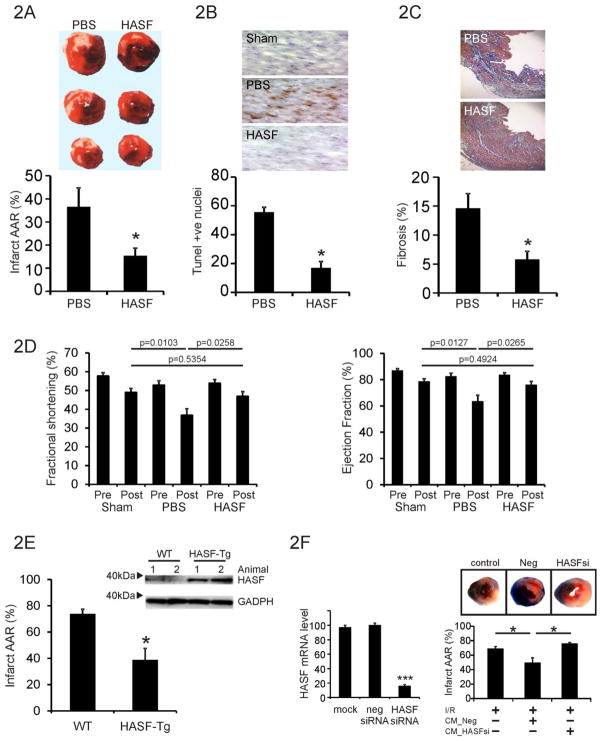
3.2 HASF protects against Myocardial damage in vivo
Considering that Akt-MSCs secrete factors that facilitate tissue repair we wished to ascertain whether HASF had protective effects. We first employed a rat model of ischemia reperfusion tissue damage. Animals were subjected to 30 minutes of myocardial ischemia by LAD ligation followed by 24 hr reperfusion. Recombinant HASF protein (1 μg) was injected in to the myocardium immediately following LAD ligation.
Infarct size was then measured by triphenyl tetrazolium chloride (TTC) and Evan’s Blue stained heart cross sections. As shown in Figure 2A, intra-myocardial injection of 1 μg of HASF recombinant protein, immediately after LAD ligation, led to a dramatic ~58% reduction in infarct size as compared to control PBS treated animals (similar results were obtained from different batches of HASF protein produced in either bacterial or insect cells). Moreover, TUNEL staining in tissue sections from HASF treated animals revealed that apoptosis within the peri-infarct region was reduced significantly (Figure 2B). Analysis of myocardial fibrosis in rat heart sections 4 weeks post- injury showed that HASF treated hearts had a significant reduction of scar area (Figure 2C).
Figure 2. HASF protects the heart against myocardial injury.
(A) Quantification of infarct size (% of Infract area/AAR) in the rat hearts undergone I/R and randomly divided into PBS control and HASF injected groups. Data are presented as means ± SE of replicates, n= 10. Representative photos from each group are also shown. * P<0.001. (B) TUNEL staining in control or HASF injected animals from parallel experiments as above. The means ± SE of replicate heart samples per group are presented, n= 10, * P<0.001. Sections were counterstained with hematoxylin. Representative photos from each group are also shown. (C) Analysis of fibrosis 4 weeks after the initial ischemia/reperfusion injury. Percentage (%) of fibrosis was calculated as collagen positive area/total area. Data represent mean ± SE, n = 6–8 mice per group, * P<0.001. Representative photos from each group are also shown. (D) Echocardiograph data of rats following HASF injection, fractional shortening and ejection fraction are shown. P-values as indicated. Sham n=8, PBS n=9, HASF n=10. (E) Immunoblot assay of left ventricle protein lysates in Tg and WT mice. Antibodies against HASF were used. GAPDH served as loading control. Hearts from wild type (WT) and alpha-MHC driven human HASF transgenic mice (α-MHC HASF-Tg) were analyzed by TTC staining after Ischemia/reperfusion injury. AAR was equal on all groups tested (Supplementary Figure 2B). *, P < 0.05, Data are presented as means ± SE of replicates, n= 4–5. (F) Left panel: Akt-MSCs were transfected with lipid reagent alone (mock), negative control siRNA (neg siRNA), and HASF siRNA (HASF siRNA). qPCR was used to determine HASF RNA expression. Conditioned media was collected from cells transfected with either negative control or HASF siRNA. Right panel: TTC staining showing damage by Ischemia/Reperfusion (I/R) in mice hearts treated with conditioned media from Akt-MSCs. (% Infract area/Area at risk (AAR). CM_Neg = conditioned media from MSCs treated with scrambled negative control siRNA; CM_HASF-si = conditioned media from MSCs treated with SiRNA targeting HASF; AAR was equal on all groups tested (Supplementary Figure 2D).* P < 0.05, Data are presented as means ± SE of replicates, n= 10.
Echocardiographic analysis was performed prior to and 1 month following surgery. HASF had a significant protective effect on the heart following ischemia; injection of the recombinant protein completely prevented the deterioration in cardiac function observed in the control PBS animals (Figure 2D, Supplementary Table 1). Further underlining the protective effect of HASF, Fractional shortening and Ejection fraction values were not significantly different between the sham-operated and HASF treated groups (Figure 2D, Supplementary Table 1).
To corroborate these results, transgenic mice were created in which the α-myosin heavy chain promoter drove the expression of HASF in cardiomyocytes following birth (HASF-Tg) [9]. As expected, ectopic expression of HASF resulted in increased levels of HASF RNA and protein in the heart of the transgenic mice as compared with non-transgenic littermate control mice (Figure 2E and Supplementary Figure 2A). Gross morphometric, histologic, and noninvasive echocardiographic analysis in two different transgenic lines showed that moderate levels (20–30 fold increase) of HASF overexpression in cardiomyocytes did not have any apparent negative consequences on cardiac development, structure, or function at baseline (Supplementary Table 2). Importantly, when HASF transgenic mice were subjected to cardiac ischemia reperfusion (I/R) as described before, the HASF transgenic mice showed significantly reduced infarct size compared to wild type littermate control mice (Figure 2E). This was not due to differences in the initial injury in the two groups (Supplementary Figure 2B).
To further validate the importance of HASF in mediating the paracrine effects of Akt-MSCs, we compared the in vivo effects of conditioned medium collected from murine Akt-MSCs treated with siRNA against HASF (CM_Hasf-si) with those of control (transfected with scrambled control siRNA, CM_Neg) cells. Akt-MSCs treated with siRNA against HASF had a 70–90% decrease in HASF mRNA expression after 48 hr of exposure to siRNA (Supplementary Figure 2C). The conditioned media either from control or HASF siRNA treated cells was collected, concentrated, and then injected after coronary artery ligation (LAD) into the infarct border zone of mice hearts [10]. Hearts were isolated 24 hr later, and infarct size was estimated by triphenyl tetrazolium chloride (TTC) and Evan’s Blue staining. As expected from our previous studies, injection of control CM_Neg conditioned medium in infarcted hearts resulted in a 28% reduction in the infarct size after myocardial infarction at 24 hr (Figure 2F) [10–12]. However, injection of conditioned medium from HASF siRNA-treated Akt-MSCs (CM_Has-si) did not show any significant protection suggesting that HASF is important for the paracrine protective effects of transplanted Akt-MSCs after myocardial infarction. This was not due to differences in the initial injury (Supplemental Figure 2D).
3.3 Recombinant HASF protects cardiomyocyte against cell death in vitro
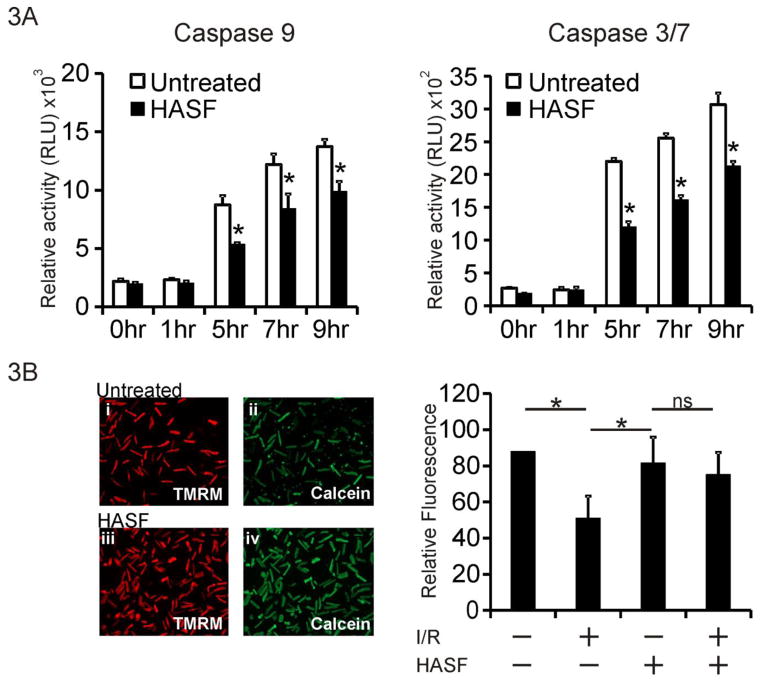
To further characterize the function of the protein we produced purified human HASF recombinant protein and tested its effect on cardiomyocytes under conditions of stress. Initial tests in H9C2 cardiac myoblasts showed that 10 nM HASF significantly decreased (~50%) H2O2 induced cell death as evidenced by Annexin V/PI staining (Supplemental Figure 3A). This effect was comparable to treatment with human IGF recombinant protein at the same concentration. To investigate this observation further we investigated whether HASF affects proteins in the apoptotic pathway using primary adult rat cardiomyocytes [14, 15]. When adult rat cardiomyocytes were pre-incubated with 10 nmol/L of HASF for 30 minutes and then subjected to treatment with 100 μmol/L of H2O2, the levels of both the initiator caspase 9 and effector caspase 3/7 were substantially reduced compared to control samples (~38% reduction of caspase 9 and ~45% reduction of caspase 3/7 at 5 hr of H2O2 respectively, Figure 3A). The reduction in caspase activities was accompanied by increased levels of mitochondrial anti-apoptotic Bcl-2 protein, as well as reduction of DNA fragmentation (Supplementary Figure 3B and 3C). Furthermore, when adult rat cardiomyocytes were treated with HASF prior to hypoxia/reoxygenation (H/R) in vitro, mitochondrial channel (mPTP) opening was reduced compared to control untreated samples, indicating increased survival (Figure 3B). Taken together, the above data indicate that HASF protects cells in vitro from stress and/or hypoxia induced death by inhibition of caspases and mPTP channel opening.
Figure 3. HASF protects cardiomyocytes against cell death in vitro.
(A) Caspase elisa assay in adult rat cardiomyocytes treated with 10 nM HASF, or PBS control for 30 minutes, and then challenged with 100 μM of H2O2 for various time lengths. Data are presented as means ± SE of replicates, * P<0.05, n=4–6. (B) Left panel: Representative images of cardiomyocytes subjected to 3hr hypoxia, 60 minutes reoxygenation (I/R) showing cells pretreated with HASF and control PBS treated cells. Calcein (Green); Mitochondrial dye TMRM (red). Increased Green fluorescence acts a surrogate marker for increased inhibition of mPTP channel opening. Right panel: Quantitative fluorescence analysis of images shown in left. Inhibition of mPTP channel opening was defined as the percentage of Calcein intensity (green fluorescence) normalized to viable cell number. Data are presented as means ± SE of values estimated from 8 images per condition.
3.4 HASF activates PKCε in cardiomyocytes
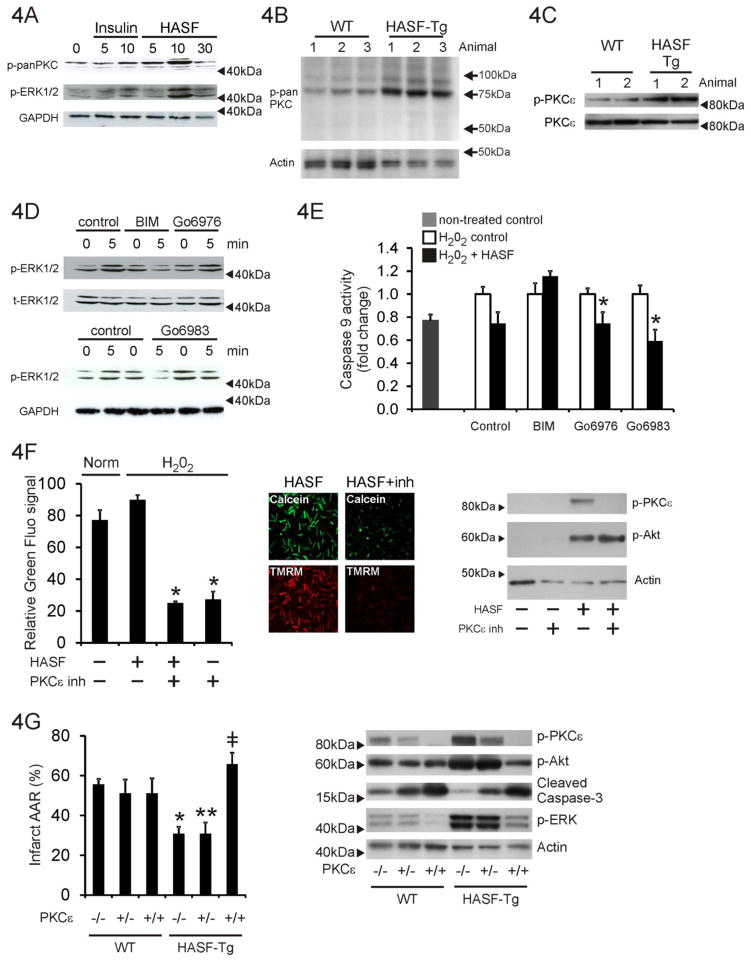
To explore the intracellular mechanisms underlying the cytoprotective effects of HASF we examined PKC activity as this pathway has been shown to play several pivotal roles in tissue preconditioning and cytoprotection [16–19]. Primary adult rat cardiomyocytes were stimulated with 100nmol/L of HASF for 0–30 minutes and extracts immunoblotted with a pan-phospho-PKC antibody. This antibody recognizes phosphorylation solely at the PDK1 consensus site within PKCs but has no cross-reactivity to other PDK1 substrates (i.e. Akt, SGK) nor to other phosphorylation sites within PKC. PDK1 phosphorylation of PKCs is important for their maturation and activation. As shown in Figure 4A, HASF increased dramatically PDK1 phosphorylation of PKC (an established measurement of PKC activity) and PKC downstream targets, such as ERK1/2, at levels similar to the activation induced by insulin which was used as positive control. Similarly in-vivo HASF over-expression in the transgenic animal correlated with increased phosphorylation of PKC at the PDK1 site (Figure 4B). Two bands were observed at ~75kDa and ~85kDa. On the basis of the known molecular weights of PKCs the ~75kDa band is likely PKCζ, the band at ~85kDa PKCε (Figure 4B). Further analysis with a specific antibody verified that in vivo HASF overexpression induced PKCε phosphorylation through PDK1 mediated autophosphorylation (Figure 4C). We wished to further investigate the whether PKCε was important for mediating the effects of HASF as this PKC isoform has been shown previously to be important for cytoprotection. Further analysis using PKC family specific inhibitors and specific antibodies confirmed that HASF selectively enhanced activation of PKCε isoform (Figure 4C). Rat cardiomyocytes were pretreated with several PKC inhibitors, BIM, Gö6976, and Gö6983 which had specificity towards different PKC isoforms (Supplementary Table 3). Of the three inhibitors used in this study only BIM inhibits PKCε (Supplementary Table 3). HASF mediated ERK activation was abolished by BIM, but not Gö6976 or Gö6983 (Figure 4D). Pre-incubation with BIM also attenuated the protective effects of HASF in cardiomyocytes under stress; caspase-9 activity was higher when compared to cells cultured with HASF alone or in combination with Gö6976 or Gö6983 (Figure 4E). Taken together, these data support the notion that HASF selectively activates PKCε.
Figure 4. The cytoprotective effects of HASF are mediated by PKCε.
(A) Immunoblot analysis for PKC and ERK protein phosphorylation levels in serum-restricted adult rat cardiomyocytes stimulated with HASF recombinant protein for various times. Insulin was used as a positive control. min: minutes of treatment (B) Immunoblot analysis of left ventricle protein lysates in wild-type and HASF-Tg mice for pan-phospho-PKC (PDK1 phosphorylation motif). Actin was used as a loading control (C) Immunoblot assay of left ventricle protein lysates in Tg and WT mice. Antibodies against PKCε phosphorylation (S729) and total PKCε were used. GAPDH was used as a loading control. (D) Adult rat cardiomyocytes were pre-treated with 3 μmol/L PKC inhibitors BIM, Gö6976, or Gö6983 for 30 minutes, stimulated with 100nmol/L HASF recombinant protein for 5–10 minutes and harvested. Total cell lysates were used to evaluate the levels of ERK1/2 phosphorylation by immunoblot blot analysis. GAPDH was used as a loading control. (E) Serum-restricted adult rat cardiomyocytes were treated with 3 μmol/L PKC inhibitors or vehicle DMSO for 30 minutes, followed by HASF recombinant protein or PBS control treatment for 30 minutes and treatment with 200μmol/L H2O2 for 3 h. Apoptosis was determined by detection of caspase-9 activity using a luminescent assay. All samples were measured in triplicates and the data were normalized to the corresponding non HASF- control cells. *P < 0.05 vs. corresponding PBS treated control cells. Note that PKCε is the only isoform differentially affected by BIM (see also Supplementary Table 3). (F) Quantitative image analysis of mPTP channel opening during hypoxia/reoxygenation as described above in the presence of a peptide PKCε inhibitor. Fluorescence levels are shown in the graph. Data are shown as means ± SE of values estimated from 8 images per condition. * P<0.01. Representative images are shown on the left. Protein extracts (20μg) were immunoblotted for phospho-PKCε, phospho-Akt and actin. (G) HASF mediated cardioprotection is abrogated in mice that lack PKCε. HASF Tg mice were crossed with PKCε knock-out mice. Groups of mice from all the resulting genotypes were then subjected to ischemia/reperfusion injury and TTC staining as described before. The calculations of % of Infract/AAR are presented for each group. AAR was equal on all groups tested (Supplementary Figure 4B). Only statistical significant effects are highlighted. * P < 0.05, HASF Tg vs. WT; ** P <=0.05 HASF Tg: PKCε+/− vs. PKCε+/− control; #, P < 0.01 HASF Tg: PKCε−/− vs. HASF Tg: PKCε+/− or vs. HASF Tg: PKCε+/+. Data are presented as means ± SE of replicates, n= 4–5. Protein extracts (20μg) were immunoblotted for phospho-PKCε, phospho-Akt, cleaved caspase-3, phospho-ERK and actin.
To provide additional evidence for the role of PKCε as mediator of HASF actions, we tested whether selective inhibition of PKCε could block HASF function. For this, rat cardiomyocytes cells were pretreated with either 10 μmol/L PKCε inhibitor peptide (PKCε inh.), or its scramble control peptide, for 20–25 minutes followed by treatment with 100 nmol/L HASF for 5–10 minutes [17, 19]. The effects on ERK activation were then monitored. In an in vitro model of hypoxia/re-oxygenation adult rat cardiomyocytes subjected to H2O2 following pre-incubation with HASF and PKCε inh resulted in the abrogation of the positive effects of HASF upon mPTP closure and cell survival (Figure 4F). Untreated cells (Norm) and cells pre-treated with the control peptide and then challenged with H2O2 served as controls (Figure 4F) for the effects of H2O2 treatment in the absence of HASF. Protein extracts were immunoblotted for PKCe and Akt phosphorylation; the PKCe inhibitor completely inhibited PKCe phosphorylation, and hence activity (Figure 4F, right panel). However there was no effect on Akt, HASF activation of Akt phosphorylation was unaffected by the PKCe inhibitor (Figure 4F, right panel). Similar findings were observed when primary cortical neuronal cells isolated from E18 rat cortex were used instead of cardiomyocytes (Supplementary Figure 4A).
In-order to further clarify the importance of PKCε HASF transgenic animals were crossed with PKCε−/− knock-out mice. As expected, when HASF transgenic mice were subjected to cardiac I/R, their hearts showed a significant reduction in infarct size when compared to wild type littermate control mice (Figure 4G). These effects were lost in HASF transgenic mice crossed with PKCε knock-out animals confirming that the protective effects of HASF in vivo are modulated by PKCε (Figure 4G). These effects were not due to any differences in the initial injury (Supplementary Figure 4B). Protein extracts prepared from heart tissue were immunoblotted for various proteins (Figure 4G, right panel). HASF over-expression increased PKCε, Akt and ERK phosphorylation (Figure 4G, lane 4 versus lane 1) as expected. Similarly HASF over-expression decreased levels of cleaved caspase-3, a marker for apoptosis (Figure 4G, lane 4 versus lane 1). As expected no PKCe phosphorylation was observed in PKCε null mice (labeled +/+ in Figure 4G) irrespective of HASF over-expression (Figure 4G, lane 3 vs lane 1, lane 6 vs lane 3). Phospho-ERK levels were reduced PKCε null mice (Figure 4G, lane 3 vs lane 1, lane 6 vs lane 3). Cleaved caspase-3 levels were increased in PKCε null mice, indicating increased apoptosis (Figure 4G). HASF over-expression had no effect on cleaved caspase-3 levels in PKCε null mice (Figure 4G, lane 6 vs lane 3). Interestingly PKCε knockout inhibited HASF mediated activation of Akt in vivo (Figure 4G).
4. Discussion
Stem cells hold great promise for cell-based therapy of acute ischemic injury. When delivered into an ischemic environment stem cells have immediate paracrine effects through the release of proteins which promote cell survival, potentially enhancing tissue regeneration [10, 20, 21]. The identification of stem cell secreted proteins, as well as their downstream signaling pathways, is therefore of great biological and therapeutic importance. In recent years, our laboratory has developed the strategy of discovery therapeutic molecules by studying stem cell derived paracrine factors. We have recently shown that one such stem cell factor, HASF, promotes cardiomyocyte proliferation [8]. In this study, we were interested to determine whether HASF have direct cytoprotective effects on cardiomyocytes and if HASF could be used therapeutically. Our data showed that HASF protected the heart from the damage associated with myocardial infarction and that this required PKCε.
PKCε is known to play a key role in tissue protection from ischemic injury [22, 23]. Similarly, activation of PKCε has been shown to mediate the development of preconditioning, a powerful protective mechanism that can be induced by brief episodes of ischemia followed by reperfusion, or by the administration of pharmacological agents that mimic these effects [19, 24]. In this respect identifying agents, such as HASF, which stimulate the PKCε pathway are clearly of a therapeutic benefit. The identification of the HASF receptor and the mechanism by which HASF regulates PKCε are clearly required. Preliminary data in in our laboratory suggests that HASF does not activate traditional GPCR receptors however (data not shown). HASF has also been shown to endogenously expressed in the Golgi of some cell types and it is possible that Golgi-retained HASF may have important biological roles [25].
With respect to our previous study one question that could be asked is whether cardiomyocyte proliferation could explain the effect of HASF upon heart function and infarct size shown here. It is important to point out that the increased cardiomyocyte proliferation was observed under prolonged HASF treatment (>7 days) in vitro. It is unlikely that a single acute injection of HASF, which is probably cleared rapidly, is sufficient to drive sustained cardiomyocyte proliferation. We also reported that HASF does not affect cardiac progenitor cell (CPC) proliferation [8]. Furthermore, PKCε has been shown to negatively regulate CPC differentiation [26]. Consequently, we believe that the effects we show here are most likely due to HASF inhibition of apoptosis through PKCε. In order to conclusively settle the question of whether HASF protects the heart through the inhibition of apoptosis, cardiomyocyte proliferation, or a combination of both would require complex experiments beyond the scope of this study. Future experiments will assess whether HASF affects early events following myocardial infarction as well as the development of new cardiomyocytes following injury.
The protective effect of HASF was lost in PKCε null mice (Figure 4G). However, in PKCε null mice without HASF over-expression no effect on infarct size was observed. Compensatory endogenous mechanisms to protect the heart following injury are known to be very limited [5]. This relative inability to endogenously activate cardioprotective signaling may explain why in the absence of HASF the loss of PKCε expression had no effect on infarct size. One implication of our data is that exogenously delivered agents need to significantly activate PKCε in order to have a substantial cardioprotective effect.
In summary, our research has provided insights into the functional importance of HASF; a stem cell derived factor which confers paracrine cytoprotection in vivo through the activation of PKCε. These findings provide a better understanding of stem cell biology and actions. Further characterization of HASF may lead to the development of therapeutic agents for tissue protection in conditions of ischemic injury such as myocardial infarction.
Supplementary Material
Highlights.
Injected stem cells protect ischemic tissue through the release of paracrine factors.
HASF (Hypoxia and Akt induced Stem cell Factor) is secreted by MSCs
HASF prevents the damage associated with MI
HASF is cytoprotective through PKCε activation
Acknowledgments
Sincere thanks to Gina Briscoe, Brook Pyhtila and Prof. Harold P. Erickson and Christopher V. Nicchitta from the Department of Cell Biology of Duke University and Drs David Reczek, William Brondyk and Markryan Dwyer at Genzyme for their expert help. We also thank Dr. Molkentin and the Cincinnati core facility for generating the HASF transgenic mice and Dr. Lan Mao at Duke University for providing echocardiographic support in the study.
Sources of Funding
Research conducted in these studies was supported by NHLBI grants RO1 HL35610, HL81744, HL72010, and HL73219 (to V.J.D.); the Edna Mandel Foundation (to V.J.D. and M.M.); the Foundation Leducq (to V.J.D.). and the American Heart Association (M.M.; 10SDG4280011).
Footnotes
Disclosures
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–43. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. Journal of Neuroscience Research. 2009;87:2183–200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 5.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–9. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–9. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 7.Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010;21:1513–26. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigi F, Schmeckpeper J, Pow-Anpongkul P, Payne JA, Zhang L, Zhang Z, et al. C3orf58, a Novel Paracrine Protein, Stimulates Cardiomyocyte Cell Cycle Progression Through the PI3K-AKT-CDK7 Pathway. Circ Res. 2013;113:372–80. doi: 10.1161/CIRCRESAHA.113.301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. The Journal of Biological Chemistry. 1991;266:24613–20. [PubMed] [Google Scholar]
- 10.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 12.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, et al. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27:971–9. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14:536–48. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 15.Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–68. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 16.Duquesnes N, Lezoualc’h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers? Journal of Molecular and Cellular Cardiology. 2011;51:665–73. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Sivaraman V, Hausenloy DJ, Kolvekar S, Hayward M, Yap J, Lawrence D, et al. The divergent roles of protein kinase C epsilon and delta in simulated ischaemia-reperfusion injury in human myocardium. Journal of Molecular and Cellular Cardiology. 2009;46:758–64. doi: 10.1016/j.yjmcc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Palaniyandi SS, Sun L, Ferreira JC, Mochly-Rosen D. Protein kinase C in heart failure: a therapeutic target? Cardiovasc Res. 2009;82:229–39. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70:222–30. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–93. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 21.Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Muller S, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–40. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budas GR, Mochly-Rosen D. Mitochondrial protein kinase Cepsilon (PKCepsilon): emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans. 2007;35:1052–4. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- 23.Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, et al. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28:4172–82. doi: 10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–99. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 25.Takatalo M, Jarvinen E, Laitinen S, Thesleff I, Ronnholm R. Expression of the novel Golgi protein GoPro49 is developmentally regulated during mesenchymal differentiation. Dev Dyn. 2008;237:2243–55. doi: 10.1002/dvdy.21646. [DOI] [PubMed] [Google Scholar]
- 26.Galli D, Gobbi G, Carrubbi C, Di Marcantonio D, Benedetti L, De Angelis MG, et al. The role of PKCepsilon-dependent signaling for cardiac differentiation. Histochem Cell Biol. 2013;139:35–46. doi: 10.1007/s00418-012-1022-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.