Abstract
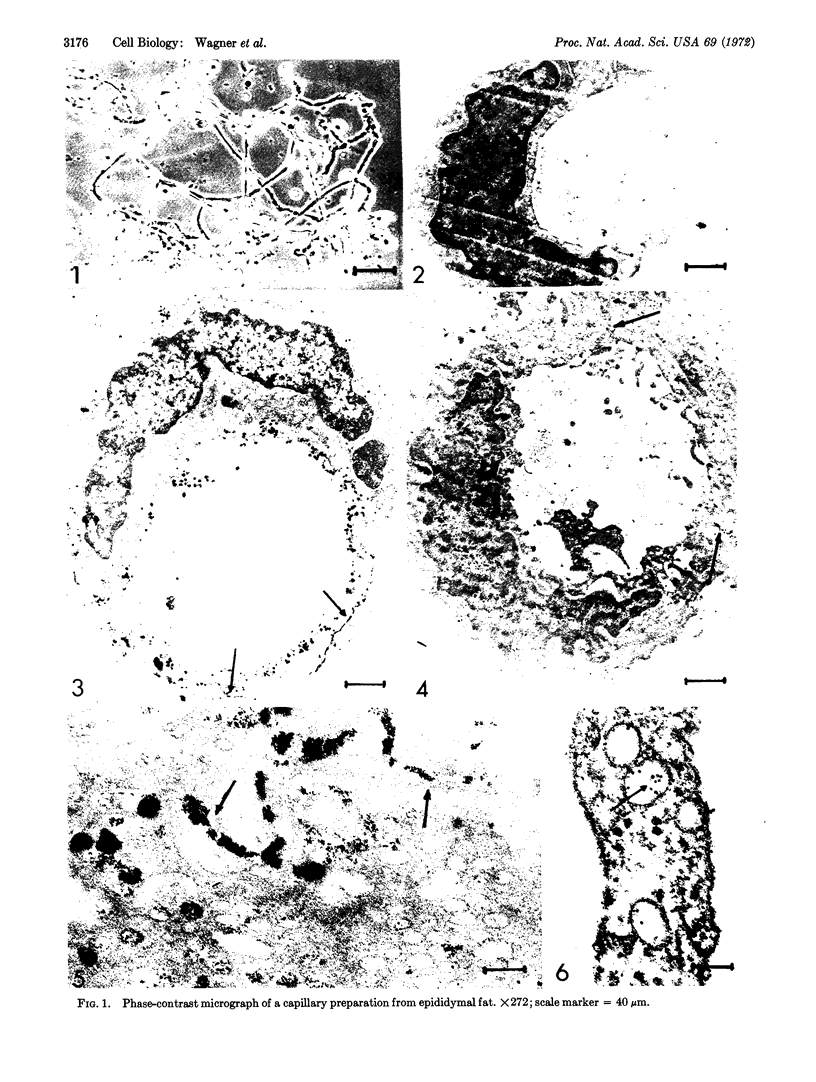
Capillaries were isolated from epididymal fat, and a catecholamine-sensitive adenylate cyclase found in these capillaries was characterized. The effect of various hormones on the accumulation of adenosine 3′:5′-cyclic monophosphate in capillary endothelial cells was determined and the cyclase was found to exhibit mixed alpha and beta characteristics. Cyclase was cytochemically localized in these endothelial cells with 5′-adenylyl-imidodiphosphate as a specific cyclase substrate and alloxan as a specific cyclase inhibitor. Lead imidodiphosphate was precipitated at or near the site of cyclase activity upon hydrolysis of 5′-adenylyl-imidodiphosphate by cyclase. This reaction product was observed primarily on the luminal surface of intact capillaries, in micropinocytic invaginations, in free vesicles within the cytoplasm, and in the intracellular junctions.
Keywords: micropinocytosis, AMP-PNP, alloxan, intercellular junctions
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitensky M. W., Gorman R. E., Miller W. H. Digitonin effects on photoreceptor adenylate cyclase. Science. 1972 Mar 24;175(4028):1363–1364. doi: 10.1126/science.175.4028.1363. [DOI] [PubMed] [Google Scholar]
- Bruns R. R., Palade G. E. Studies on blood capillaries. II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol. 1968 May;37(2):277–299. doi: 10.1083/jcb.37.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casley-Smith J. R., Chin J. C. The passage of cytoplasmic vesicles across endothelial and mesothelial cells. J Microsc. 1971 Jun;93(3):167–189. doi: 10.1111/j.1365-2818.1971.tb02280.x. [DOI] [PubMed] [Google Scholar]
- Cohen K. L., Bitensky M. W. Inhibitory effects of alloxan on mammalian adenyl cyclase. J Pharmacol Exp Ther. 1969 Sep;169(1):80–86. [PubMed] [Google Scholar]
- Gorman R. E., Bitensky M. W. Selective activation by short chain alcohols of glucagon responsive adenyl cyclase in liver. Endocrinology. 1970 Nov;87(5):1075–1081. doi: 10.1210/endo-87-5-1075. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARCHESI V. T., BARRNETT R. J. The demonstration of enzymatic activity in pinocytic vesicles of blood capillaries with the electron microscope. J Cell Biol. 1963 Jun;17:547–556. doi: 10.1083/jcb.17.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reik L., Petzold G. L., Higgins J. A., Greengard P., Barrnett R. J. Hormone-sensitive adenyl cyclase: cytochemical localization in rat liver. Science. 1970 Apr 17;168(3929):382–384. doi: 10.1126/science.168.3929.382. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. The role of mucopolysaccharides in vesicle architecture and endothelial transport. An electron microscope study of myocardial blood vessels. J Cell Biol. 1972 Jan;52(1):198–206. doi: 10.1083/jcb.52.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]