Abstract
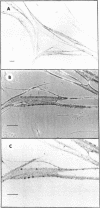
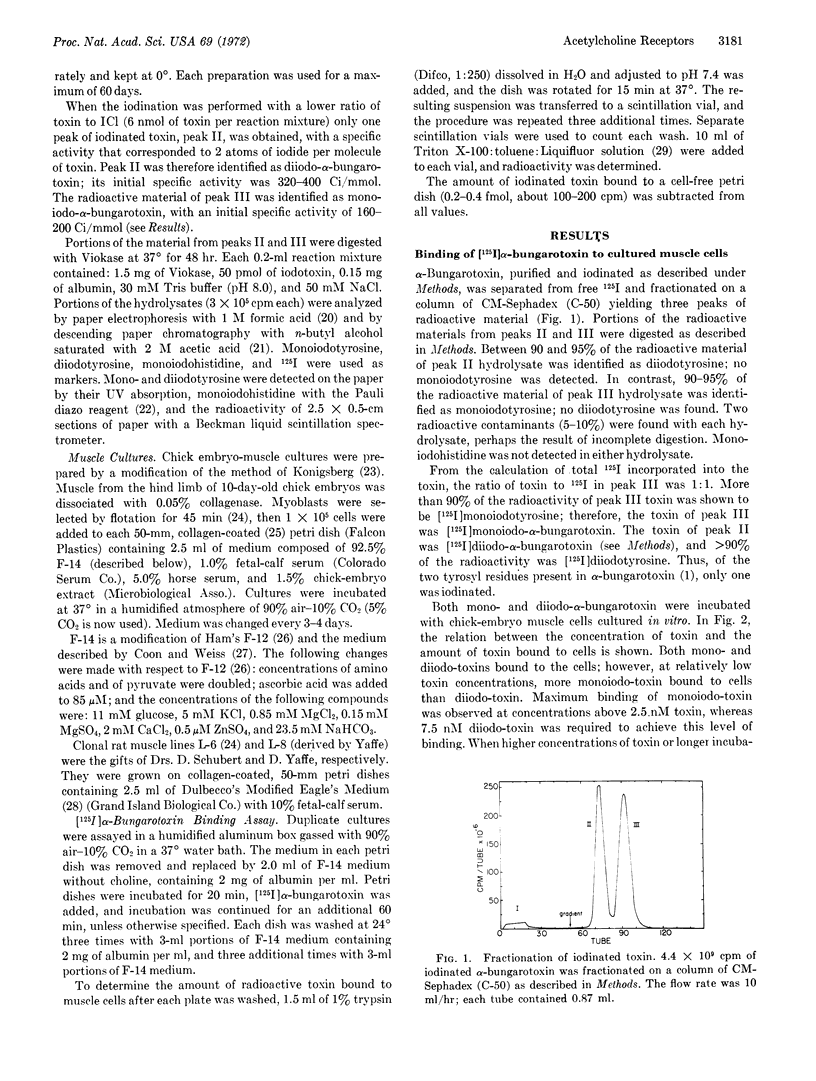
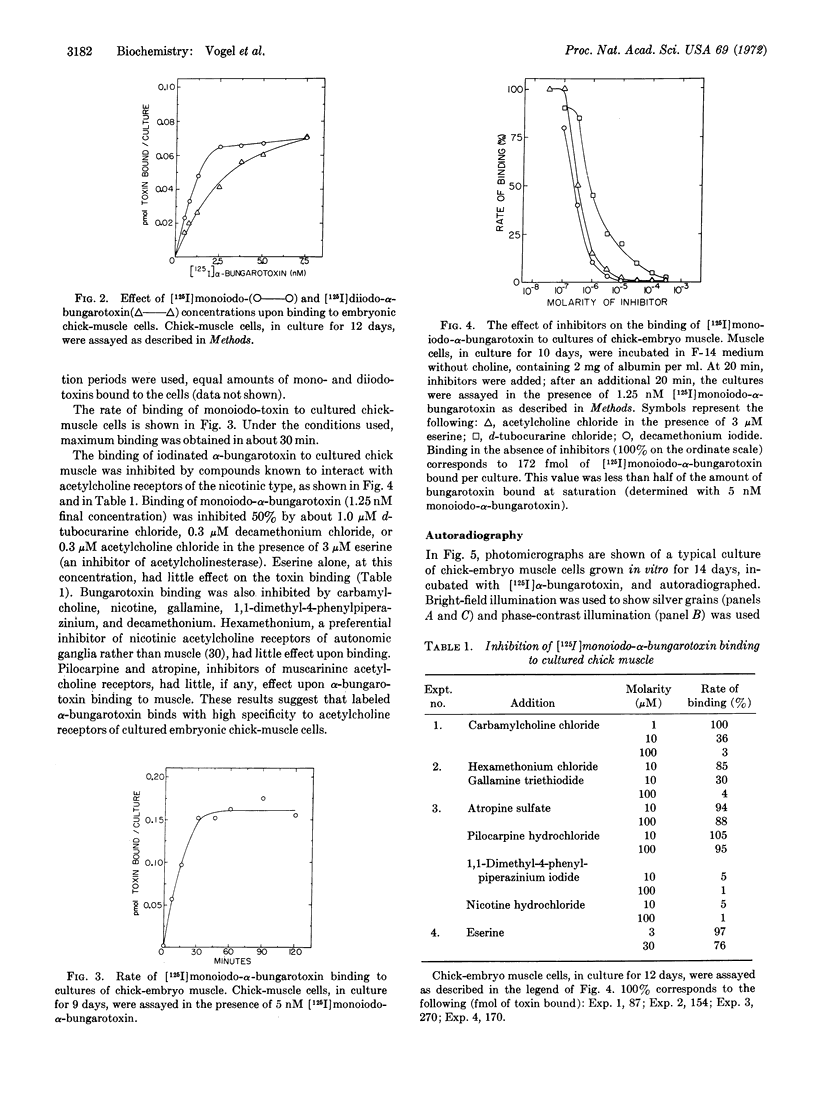
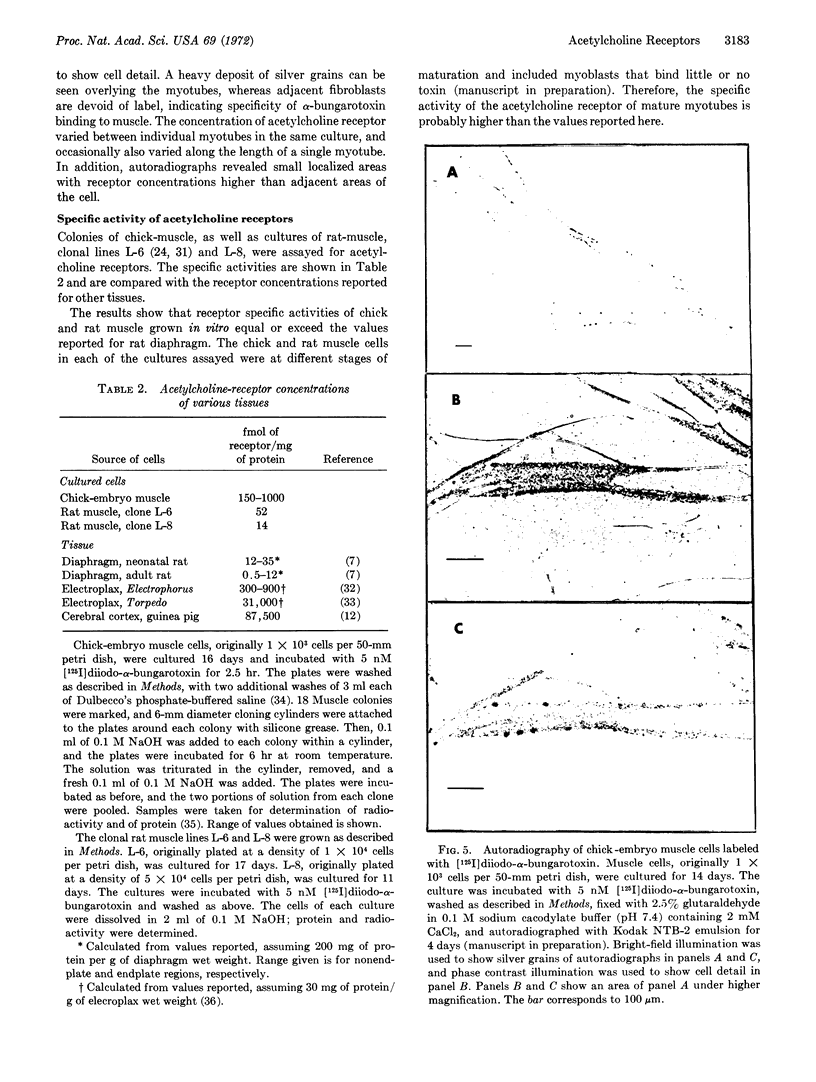
[125I]Monoiodo- and [125I]diiodo-α-bungarotoxin were synthesized and shown to bind specifically to the acetylcholine receptor of cultured embryonic chick- and rat-muscle cells. The pharmacologic properties of the receptor of cultured embryonic chick muscle resembled those of the nicotinic acetylcholine receptor of adult vertebrate muscle. Autoradiography of muscle cells labeled with toxin showed that acetylcholine receptors were distributed over the entire cell surface. In addition, discrete areas with a high receptor concentration were found.
Keywords: α-bungarotoxin, iodination, cholinergic drugs, autoradiography
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Wieckowski J., Chiu T. H. Cholinergic receptor molecules and cholinesterase molecules at mouse skeletal muscle junctions. Nature. 1971 Nov 26;234(5326):207–209. doi: 10.1038/234207a0. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Kelly R. B., Sargent P. B., Williamson P., Hall Z. W. Binding of -bungarotoxin to acetylcholine receptors in mammalian muscle (snake venom-denervated muscle-neonatal muscle-rat diaphragm-SDS-polyacrylamide gel electrophoresis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):147–151. doi: 10.1073/pnas.69.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B. Acetylcholine receptor. I. Identification and biochemical characteristics of a cholinergic receptor of guinea pig cerebral cortex. J Biol Chem. 1972 Jan 10;247(1):130–145. [PubMed] [Google Scholar]
- CHANG C. C., LEE C. Y. ISOLATION OF NEUROTOXINS FROM THE VENOM OF BUNGARUS MULTICINCTUS AND THEIR MODES OF NEUROMUSCULAR BLOCKING ACTION. Arch Int Pharmacodyn Ther. 1963 Jul 1;144:241–257. [PubMed] [Google Scholar]
- Chang C. C., Yang C. C., Hamaguchi K., Nakai K., Hayashi K. Studies on the status of tyrosyl residues in cobrotoxin. Biochim Biophys Acta. 1971 Apr 27;236(1):164–173. doi: 10.1016/0005-2795(71)90161-9. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Meunier J. C., Huchet M. Studies on the cholinergic receptor protein of Electrophorus electricus. I. An assay in vitro for the cholinergic receptor site and solubilization of the receptor protein from electric tissue. Mol Pharmacol. 1971 Sep;7(5):538–553. [PubMed] [Google Scholar]
- Coon H. G., Weiss M. C. A quantitative comparison of formation of spontaneous and virus-produced viable hybrids. Proc Natl Acad Sci U S A. 1969 Mar;62(3):852–859. doi: 10.1073/pnas.62.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covelli I., Wolff J. Iodination of the normal and buried tyrosyl residues of lysozyme. I. Chromatographic analysis. Biochemistry. 1966 Mar;5(3):860–866. doi: 10.1021/bi00867a008. [DOI] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden W. F. Development of acetylcholine sensitivity in cultured skeletal muscle. Experientia. 1970 Sep 26;26(9):984–986. doi: 10.1007/BF02114146. [DOI] [PubMed] [Google Scholar]
- EHRMANN R. L., GEY G. O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956 Jun;16(6):1375–1403. [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T. Characterization and partial purification of the acetylcholine receptor from Torpedo electroplax. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1776–1780. doi: 10.1073/pnas.69.7.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Fambrough D., Rash J. E. Development of acetylcholine sensitivity during myogenesis. Dev Biol. 1971 Sep;26(1):55–68. doi: 10.1016/0012-1606(71)90107-2. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D. Synaptic potentials recorded in cell cultures of nerve and muscle. Science. 1970 Sep 25;169(3952):1331–1333. doi: 10.1126/science.169.3952.1331. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp R. W., Contreras M. A., Bale W. F. I-131-labeling of proteins by the iodine monochloride method. Int J Appl Radiat Isot. 1967 Nov;18(11):737–746. doi: 10.1016/0020-708x(67)90011-7. [DOI] [PubMed] [Google Scholar]
- Kano M., Shimada Y. Innervation and acetylcholine sensitivity of skeletal muscle cells differentiated in vitro from chick embryo. J Cell Physiol. 1971 Oct;78(2):233–242. doi: 10.1002/jcp.1040780210. [DOI] [PubMed] [Google Scholar]
- Karlin A., Prives J., Deal W., Winnik M. Affinity labeling of the acetylcholine receptor in the electroplax. J Mol Biol. 1971 Oct 14;61(1):175–188. doi: 10.1016/0022-2836(71)90214-2. [DOI] [PubMed] [Google Scholar]
- Konigsberg I. R. Diffusion-mediated control of myoblast fusion. Dev Biol. 1971 Sep;26(1):133–152. doi: 10.1016/0012-1606(71)90113-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y., Chang C. C. Modes of actions of purified toxins from elapid venoms on neuromuscular transmission. Mem Inst Butantan. 1966;33(2):555–572. [PubMed] [Google Scholar]
- Lee C. Y., Tseng L. F., Chiu T. H. Influence of denervation on localization of neurotoxins from clapid venoms in rat diaphragm. Nature. 1967 Sep 9;215(5106):1177–1178. doi: 10.1038/2151177a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Mebs D., Narita K., Iwanaga S., Samejima Y., Lee C. Y. Amino acid sequence of -bungarotoxin from the venom of Bungarus multicinctus. Biochem Biophys Res Commun. 1971 Aug 6;44(3):711–716. doi: 10.1016/s0006-291x(71)80141-9. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- Morton H. J. A survey of commercially available tissue culture media. In Vitro. 1970 Sep-Oct;6(2):89–108. doi: 10.1007/BF02616112. [DOI] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. J. The pharmacological actions of polymethylene bistrimethyl-ammonium salts. Br J Pharmacol Chemother. 1949 Dec;4(4):381–400. doi: 10.1111/j.1476-5381.1949.tb00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- WASER P. G., LUTHI U. Autoradiographische Lokalisation von 14C-Calebassen-Curarin I und 14C-Decamethonium in der motorischen Endplatte. Arch Int Pharmacodyn Ther. 1957 Nov 1;112(3-4):272–296. [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]