Abstract
Compared to socially housed (SH) rats, adult isolation-reared (IR) rats exhibit phenotypes relevant to schizophrenia (SZ), including reduced prepulse inhibition (PPI) of startle. PPI is normally regulated by the medial prefrontal cortex (mPFC) and nucleus accumbens (NAC). We assessed PPI, auditory-evoked local field potentials (LFPs) and expression of 7 PPI- and SZ-related genes in the mPFC and NAC, in IR and SH rats. Buffalo (BUF) rats were raised in same-sex groups of 2–3 (SH) or in isolation (IR). PPI was measured early (d53) and later in adulthood (d74); LFPs were measured approximately on d66. Brains were processed for RT-PCR measures of mPFC and NAC expression of Comt, Erbb4, Grid2, Ncam1, Slc1a2, Nrg1 and Reln. Male IR rats exhibited PPI deficits, most pronounced at d53; male and female IR rats had significantly elevated startle magnitude on both test days. Gene expression levels were not significantly altered by IR. PPI levels (d53) were positively correlated with mPFC expression of several genes, and negatively correlated with NAC expression of several genes, in male IR but not SH rats. Late (P90) LFP amplitudes correlated significantly with expression levels of 6/7 mPFC genes in male rats, independent of rearing. After IR that disrupts early adult PPI in male BUF rats, expression levels of PPI- and SZ-associated genes in the mPFC correlate positively with PPI, and levels in the NAC correlate negatively with PPI. These results support the model that specific gene-behavior relationships moderate the impact of early-life experience on SZ-linked behavioral and neurophysiological markers.
Keywords: catechol-O-methyltransferase, isolation rearing, medial prefrontal cortex, nucleus accumbens, prepulse inhibition, schizophrenia
Introduction
Animal models of the pathophysiology of schizophrenia have helped us understand the neurobiological and behavioral consequences of a number of neurodevelopmental insults, even if these insults cannot be definitively linked to schizophrenia per se. One such insult – isolation rearing (IR) in rats – is known to recreate a number of deficits associated with schizophrenia (Fone & Porkess, 2008; Powell, 2010) [1,2], including reductions in sensorimotor gating as measured by prepulse inhibition of startle (PPI) (Cilia et al. 2001; Geyer et al. 1993) [3,4] and sensory registration as measured by auditory evoked potentials (Stevens et al. 1997) [5].
The nature of forebrain disturbances that mediate the behavioral and neurophysiological impact of IR in rats has been a focus of several reports, with evidence implicating both the medial prefrontal cortex (mPFC) and nucleus accumbens (NAC) – two regions known to normally regulate PPI (Swerdlow et al. 2001) [6] - in PPI deficits detected after isolation rearing (Powell et al. 2003; Day-Wilson et al. 2006) [7,8]. IR-induced PPI deficits are associated with specific neurochemical and molecular changes in frontal cortex, including alterations in levels of dopamine (DA), immunologic and mitochondrial markers (Krolow et al. 2012) [9], synaptic proteins and receptor levels (Hermes et al. 2011) [10], expression of genes related to glutamate (Turnock-Jones et al. 2009; Murphy et al. 2010) [11,12] and GABA function (Murphy et al. 2010) [12], and structure, the latter attributed to a loss of mPFC neuropil (Day-Wilson et al. 2006) [8]. Such a long list of alterations in the mPFC suggests that the impact of IR on mPFC function is both pervasive and non-selective; it is not yet clear which of these changes – if any - are responsible for reduced PPI, a phenotype associated with neurodevelopmental brain disorders, including schizophrenia (Braff et al. 1978; cf. Swerdlow et al. 2008) [13,14].
We (Swerdlow et al. 2012) [15] and others (e.g. Flores et al. 2009) [16] have used gene expression patterns across brain regions as a means to understand the role of circuit dynamics in the regulation of behaviors – such as PPI – that are of relevance to schizophrenia. We reported significant differences in the cortical (mPFC), subcortical (nucleus accumbens; NAC) and ventral hippocampal (VH) expression of Comt, Grid2, Nrg1 and other genes associated with PPI deficits in schizophrenia patients (Stefansson et al. 2002; Sobin et al. 2005; Roussos et al. 2008; Kao et al. 2012; Quednow et al. 2010; Greenwood et al. 2011, 2012) [17–23] in outbred rat strains that also differed in PPI and PPI-sensitivity to dopamine agonists (Swerdlow et al. 2012; Shilling et al. 2008) [15,24]. More recently, we found that neonatal lesions of the ventral hippocampus (NVHLs) that disrupted PPI and auditory evoked local field potentials (LFPs) were also associated with an abnormal “coupling” of mPFC and NAC expression of several PPI-associated genes (Swerdlow et al. 2013) [25]. In the present study, we assessed the impact of IR on PPI, LFPs and the expression of PPI- and SZ-associated genes in the mPFC and NAC. Seven genes were selected for expression analyses, based on published reports of single nucleotide polymorphisms (SNPs) associated with PPI (COMT, GRID2, NRG1, NCAM, SLC1A2; Greenwood et al. 2011, 2012) [22,23] and/or schizophrenia (GRID2, NRG1, RELN, ERBB4; Greenwood et al. 2012) [23].
Methods
All procedures conformed to NIH guidelines and were approved by the UCSD Animal Subjects Committee. Female Buffalo (BUF: BUF/CrCrl) rats (Charles River; Portage, MI) were housed individually in a temperature-controlled room utilizing a reverse 12:12 light/dark cycle. Food and water were offered ad lib. BUF rats were selected for these gene expression studies because they are an inbred strain with a PPI phenotype very comparable to that of Sprague Dawley (SD) rats (Swerdlow et al. 2004) [26] that exhibit consistent PPI deficits after isolation rearing (Bakshi et al. 1998; Harte et al. 2007) [27,28]. Females were monitored daily until delivery (total # litters = 17; avg. size 3 – 4 pups/litter, typical of BUF rats; www.harlan.com). Pups were weaned on d24, and were then housed either in isolation (n=29; M:F = 13:16) or in same-sex SH groups of 2–3 (n=35; M:F=13:22). Weaning weights were comparable across rearing groups (Table 1). IR and SH rats were otherwise handled identically (primarily via tails) throughout the experiment.
Table 1.
Weight (g) (mean (SEM))
| Body: d24 | Body: d53 | Body: d74 | Brain | |
|---|---|---|---|---|
| Male SH | 59.46(3.56) | 208.62 (8.39) | 284.46 (10.20) | 1.73 (0.03) |
| Male IR | 58.77(3.42) | 210.77(8.31) | 287.23 (8.73) | 1.72(0.02) |
| Female SH | 58.96(2.17) | 155.91 (2.96) | 186.15(3.55) | 1.64(0.02) |
| Female IR | 53.19(2.47) | 158.38(4.30) | 193.33 (6.00) | 1.59(0.02) |
Startle chambers (SR-LAB; San Diego Instruments) consisted of Plexiglas cylinders (8.7 cm internal diameter) resting on Plexiglas stands in a sound-attenuated room (60 dB ambient noise). Stimuli were delivered by a mounted speaker located 24 cm above the cylinder. Startle magnitude was detected in 100 1-ms bins and recorded by a piezoelectric device located beneath the cylinder.
PPI testing first took place on PND 53, when deficits in IR rats have been reported previously (Bakshi & Geyer 1999) [29]; weights at this time were also comparable across groups (Table 1). Testing consisted of a 5 min acclimation period of 70 dB(A) background noise followed by 4 blocks: blocks 1 and 4 had 4 and 3 120 dB(A) 40 ms noise pulses (PULSE), respectively; block 2 and 3 had 5 trial types (1) PULSE; (2) 3 prepulse+PULSE trials (20 ms noise 3, 5, or 10 dB over background followed 100 ms by a PULSE); (3) behavioral measurement without stimulus delivery (NOSTIMs). Mean inter-trial interval was 15 s, (range 6–24 s), and total session length was 19 min; the session had a total of 106 trials, divided as follows: Blocks 1 & 4: 7 120 dB(A) pulses and 7 NOSTIM trials; Block 2–3: 16 PULSE trials, 30 PPI trials and 46 NOSTIMs.
On PND 57–62, rats were surgically implanted with stainless steel EEG electrodes in the dentate gyrus. Rats received 0.1 ml atropine sulfate subcutaneously (Vedco, 0.054 mg/ml) 15–30 min before full anesthesia with sodium pentobarbital (Abbott, 60 mg/kg i.p.), and then were secured in a Kopf stereotaxic instrument in a flat skull position (tooth bar 3.3 mm below interaural line). An uncut tripole electrode (Plastics One, Roanoke, Va., USA, 0.010) was modified with Jam Nut fastener (Plastics One, 37761) placed on the head plug for precise placement of the connector cable (Plastics One, 100 cm TT2, 335-000). A 2-cm incision exposed the skull, and the skull surface was cleaned. Two holes were drilled for ground and reference wire placement on dura (AP: +2.0, L ± 1.0). Two holes were drilled for anchor screws (Plastics One, 0–80×3/32), and another for the recording electrode, positioned with its ventral tip in the dentate gyrus (DG: AP: −4.1, L: 1.0, DV −3.2). Acrylic dental cement (A-M Systems, Carlsborg, Wash., USA; Dental Cement Powder, 525000 and Solvent, 526000) firmly attached the electrode and anchor screws to the skull, and the incision was closed around the head plug. Rats recovered on a heating pad before being placed in their home cages; 3 female rats (1 IR, 2 SH) did not survive surgery.
EEG testing began 7d post-surgery. A single startle chamber (SR-LAB, San Diego Instruments, San Diego, Calif., USA) consisted of a Plexiglas cylinder 8.2 cm in diameter resting on a 12.5 × 25.5-cm Plexiglas frame within a ventilated enclosure (background noise: 65 dB(A)). The cylinder was modified with an elevated roof that allowed animals to move freely despite the presence of an EEG headpiece. The test chamber was also modified with electrical insulation, and an electrical interface cable that was fastened to the EEG headpiece. Otherwise, stimulus delivery methods were identical to those described above for startle testing. EEG signals were recorded via a preamplifier cable connection from the rat to an AM Systems 2 Channel Microelectrode AC Amplifier (Carlsborg, Wash., USA, Model 1800). The recording cable contained three male pins at the proximal end, which connected to the preamplifier, and three male amphenol pins at the distal end of the cable, to connect with the female pins in the head plug. The filter settings on the amplifier were 1.0 Hz low cutoff and 500 Hz high cutoff. The notch filter was in the ‘out’ position; the mode was set for record, and gain was set at 10 K. The amplified signals were recorded on the SR-LAB microcomputer. Auditory stimuli (53 ms duration) consisted of a frequent standard tone (8 KHz, 81.6 dB(A), 87.76% of trials), and two rare “oddball” tones (7.5 KHz or 8.5 KHz, 87.0 dB(A), 12.24% of trials) in an attempt to measure mismatch negativity (MMN), presented in pseudo-randomized sequences. After a 5 min acclimation period, each of 50 test blocks included 172 standard tones and 12 of oddball tone presentations, with a constant inter-stimulus interval of 300 ms; total session length was 59 minutes. After inspection of early data combined with separate audiometric evoked potential recordings revealed inadequate signal fidelity for the oddball stimuli, subsequent analyses focused on LFPs elicited in response to the standard tones.
EEG data files were imported into Brain Vision Analyzer (v2.0.2) and processed offline and blind to condition using an automated processing script. EEG responses to standard tones were centered across the 50 ms prestimulus baseline period and screened for artifacts (activity exceeding ±300 digital units). LFP Averaging for each rat was performed using remaining artifact-free segments (mean number of accepted sweeps = 6253, SD = 1309, with no significant SH vs. IR group differences). LFP peaks (screening window) were identified for P30 (20–40 ms) and N40 (30–60 msec). P90 was calculated as the mean voltage across the 65–105 ms range relative to the 50 ms prestimulus baseline.
Seven days after completion of EEG testing (d74), PPI testing was repeated; weights at this time remained comparable across groups (Table 1). Rats then remained housed for 7–14 d to minimize possible effects of startle and EEG testing on gene expression; they were then sacrificed, their brains removed, weighed and placed in ice-cold saline for 30 sec. Coronal tissue slabs were cut with a wire tissue slicer and the NAC and mPFC were removed bilaterally by free-hand razor dissection. Each bilateral tissue sample was placed onto dry ice and transferred to an RNase free tube, then stored at −80°C until analyzed for gene expression. Remaining tissue caudal to the thalamus was stored in a 10% formalin solution for histological assessment of EEG electrode placement. In this process, all utensils were cleaned in between each rat brain dissection with RNAlater (QIAGEN, Inc, Valencia, CA).
For Reverse Transcription Polymerase Chain Reaction (RT - PCR), total RNA was isolated from brain tissue using an RNeasy Mini kit (QIAGEN, Valencia, CA 91355) and protocols followed as per manufacturer (QIAGEN). Samples were spot checked for quality using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), which provides a RNA Integrity Number (RIN). An RIN > 7.0 indicates good quality RNA; the RIN for all samples analyzed was > 8.50. To measure RNA concentration, the optical density of 1.5 µL of total RNA at 260 nm was measured in a spectrophotometer (NanoDrop, ND-1000, NanoDrop Technologies, Wilmington, DE). Equal amounts of RNA /sample were used to make cDNA after DNase treatment. First strand cDNA was synthesized using qScript™ cDNA SuperMix as per manufacturer (Quanta Biosciences, Gaithersburg, MD). Real time RT-PCR was performed using Applied Biosystems’ TaqMan Gene Expression Assays in an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Each 20 µL RT-PCR reaction contained 10 µL of 2X Universal PCR Master Mix, 1 µL of primer/probe mix (900 nM/250 nM final concentration), 4 µL of nuclease-free water, and 5 µL of cDNA template (40 ng). Reactions were performed in MicroAmp Optical 384 well reaction plates (Applied Biosystems) as per manufacturer. Genes and assay ID numbers (Applied Biosystems) included: comt rn00561037_m1; nrg1 rn01482165_m1; grid2 rn00515053_m1; erbb4 rn00572447_m1; reln rn00589609_m1; slc1a2 rn00691548_m1; ncam1 rn00580526_m1 and glyceraldehyde 3-phosphate dehydrogenase (rgapdh) rn01775763_g1. Assays were performed in duplicate. Data were analyzed using SDS 2.3 software from Applied Biosystems. Amplification efficiencies were validated and relative expression values calculated after normalization to the rat GAPDH reference gene.
For histological assessment, 40 µm thick brain sections were mounted on microscope slides and Nissl stained. The ventral extend of all electrode tips were visualized within the dentate gyrus and plotted free-hand, blind to EEG results, onto a corresponding atlas page (Paxinos & Watson 1998) [30].
Statistical Analyses
Prepulse inhibition was defined as 100-[(startle amplitude on prepulse+pulse trials / startle amplitude on PULSE trials) × 100], and was analyzed by mixed-design analyses of variance (ANOVAs), with sex and rearing (SH vs. IR) as between factors and prepulse interval, trial block and age as within factors. Based on known significant sex differences in PPI and IR effects (Powell et al. 2002; Lehmann et al. 1999; Swerdlow et al. 2008) [31–33], separate analyses were then pursued in male and female rats. Similar analyses were used to assess IR effects on startle magnitude on PULSE trials, NOSTIM trials, and startle habituation (startle magnitude on PULSE trials in blocks 1 vs. 4). LFP amplitude for P30, N40 and P90 were treated as independent variables and subjected to one-way ANOVAs with housing and sex grouping factors. Litter effects were examined for measures exhibiting significant effects of housing or sex. For all comparisons, alpha was 0.05.
For gene expression data, fold change (FC) values for all genes were calculated relative to levels within a single SH group rat mPFC, to which FC values of 1.0 were assigned. Values were treated as continuous variables, and ANOVAs were conducted using rearing (SH vs. IR) as a between factor and region and gene as within factors. Post-hoc comparisons examined expression separately within each region. Correlations of gene expression with phenotypes (PPI, startle magnitude, LFPs), and both within and across regions was assessed using simple regression analyses and reported together with alpha values corrected for multiple comparisons; regression values across rearing groups were compared using the Fisher r-to-z transformation. For regression analyses, one rat was treated as an outlier for PPI (mean values for 3, 5 and 10 dB prepulses all > 90%; mean %PPI > 3 SD above group mean) and for P90 (magnitude > 5 SD below mean).
The average litter size for BUF rats did not yield full representation of the 4 possible sex × housing condition groups in each litter. Nonetheless, subsets of groups did have adequate representation to demonstrate that litter (as a grouping factor) did not interact significantly with either sex or housing factors in analyses of PPI (F<1 for sex and housing), startle magnitude (F<1 for sex; F = 1.03 for housing) or gene expression levels (F<1 and F<1.07 for NAC; F<1.29 and F<1.19 for mPFC). Thus, litter was not used as a between-subject factor in most subsequent analyses.
Lastly, structural equation modeling (SEM; Bollen 1989) [34] was used in exploratory analyses via MPlus software, version 7 (Muthen & Muthen, 2012) [35] to understand any potential relationships among regional patterns of gene expression, and PPI and startle abnormalities in IR rats. SEM can simultaneously estimate both a measurement model (confirmatory factor analysis) and a structural model (path/regression analyses), and can specify latent variables that reflect unmeasured constructs estimated by measured variables.
Results
Housing effects on growth and brain weight
No significant effects of housing condition were detected on body weight at days 53 or 74, or on brain weight at time of sacrifice (Table 1); expected M > F differences were detected in both measures, irrespective of housing status.
Behavior
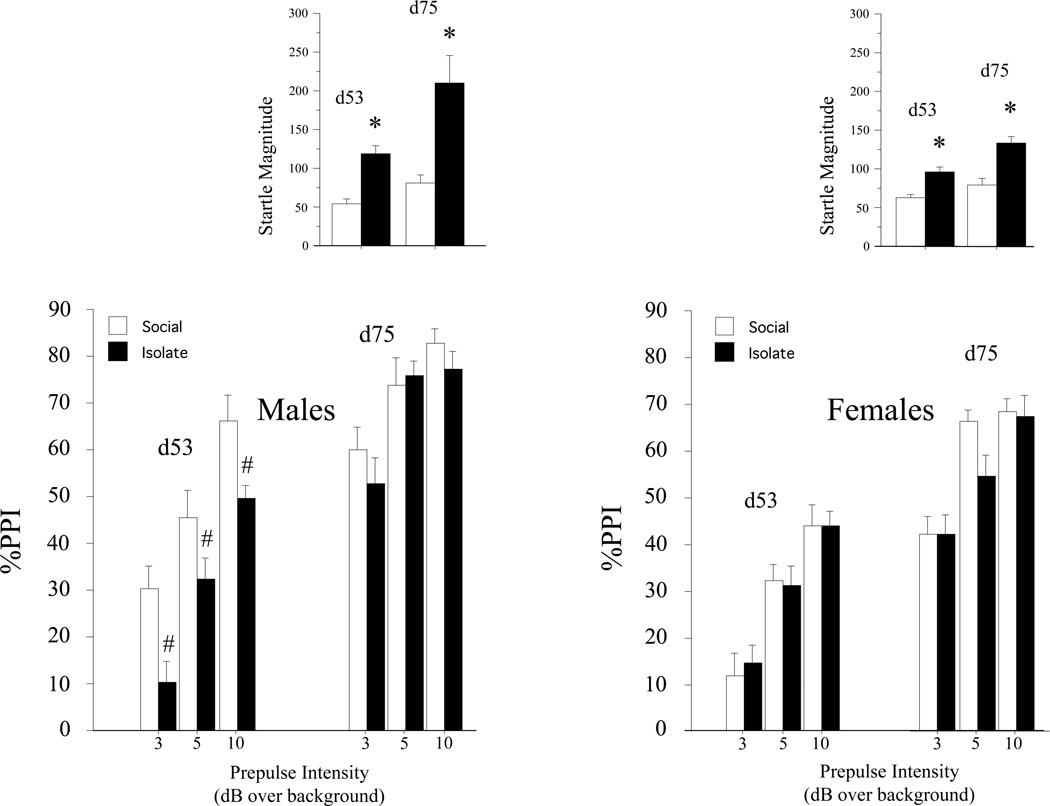
No gross behavioral disturbances were noted in IR vs. SH rats. Startle and PPI data are displayed in Figure 1; statistical terms are seen in Table 2. Repeated measures ANOVA of startle magnitude, with sex and housing condition as between-factors, and age and trial block as within-factors, revealed that startle magnitude was significantly potentiated among male and female IR rats, that this effect of housing was somewhat more robust in males, and actually increased with age. ANOVA restricted to litters in which both housing conditions were represented detected a significant effect of housing (F=7.07, df 1,10, p<0.02) but not litter (F<1), and no significant interaction of litter × housing (F<1).
Figure 1.
Effects of IR vs. SH on PPI in male (A) and Female (B) BUF rats. Startle magnitude (mean (SEM)) on pulse alone trials is shown in insets. Statistical terms are shown in Table 2.
Table 2.
Startle Statistical Terms - Significant Findings
| Measure | Factor (s) | F | P |
|---|---|---|---|
| Startle magnitude - PA Trials | Housing (H) | 28.43 | <0.0001 |
| H × Sex | 4.15 | <0.05 | |
| Age | 16.21 | <0.0003 | |
|
Startle magnitude - PA and Prepulse Trials |
H | 22.90 | <0.0001 |
| Trail Type (T) | 35.02 | <0.0001 | |
| T × Sex | 6.89 | <0.0003 | |
| T×H | 16.29 | <0.0001 | |
| T × H × Sex | 3.57 | <0.02 | |
| T × H × Sex × Age | 2.75 | <0.05 | |
| %PPI All Rats | Sex | 12.73 | <0.001 |
| H | 3.04 | <0.09 | |
| H × Sex × Age | 3.06 | <0.09 | |
| % PPI Male Rats | H | 4.65 | <0.05 |
| H × Age | 3.26 | <0.09 | |
| % PPI Males Rats d53 | H | 8.40 | <0.009 |
Unlike startle magnitude, large sex differences (M > F) were detected in measures of %PPI of SH rats at both ages. Significant PPI-reducing effects of IR were detected in male, but not female rats; this effect in males was most robust at day 53, and largely faded by day 74. Significant IR-associated PPI deficits in males at day 53 were evident at all prepulse intensities, and remained robust among subgroups matched for startle magnitude, confirming the statistical independence of PPI-reducing and startle-increasing effects of IR. Furthermore, analysis of startle magnitude on PULSE and prepulse+PULSE trials among PULSE startle-matched males confirmed that the loss of %PPI in male IR rats reflected a true loss of sensorimotor gating, i.e. a reduction in the ability of the prepulse to inhibit the magnitude of the response to the PULSE (Table 2). As with startle magnitude, housing effects on PPI were unrelated to litter identity: among litters with males in both housing conditions, ANOVA detected a significant effect of housing (F=6.14, d 1,11, p<0.02) but not litter (F<1), with no interaction of housing × litter (F=1.40, df 11,27, ns).
Electrophysiology
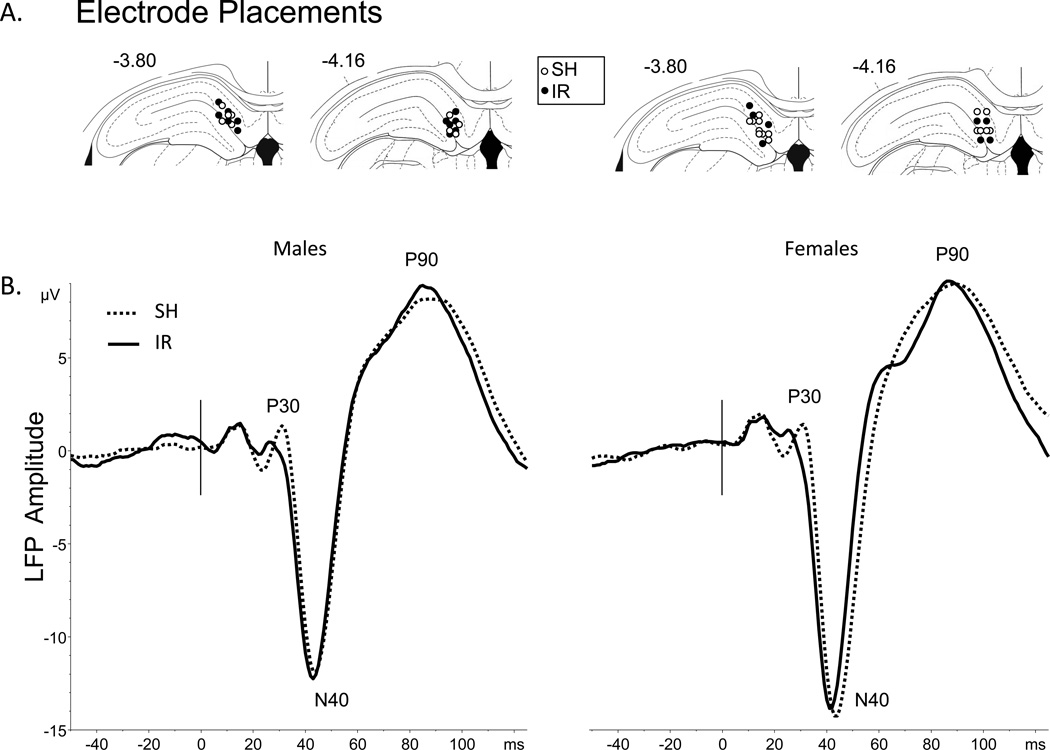
Electrode placements could be localized to the DG region (Figure 2A). Grand average LFPs are seen in Figure 2B. Separate ANOVAs were conducted on the amplitudes of three evoked field potential components: P30, N40 and P90. None of these components demonstrated significant effects of either sex or housing, or sex × housing interactions.
Figure 2.
Local field potentials. A. Areas in which ventral tips of recording electrodes were located blind to LFP results. Male placements are shown on the left and female placements are shown on the right; many placements are overlapping. B. Grand average LFPs across 4 groups, labeled for specific temporal components.
We examined the relationships between LFP amplitude and startle magnitude, focusing on P90 amplitude based on its known sensitivity to early developmental manipulations [25]. Among SH rats, significant correlations were detected between P90 and startle magnitude at d53 (r = 0.53, p<0.002) and at d74 (r = 0.40, p<0.025). In contrast, no such relationships were detected in IR rats (r’s = 0.15 at d53 and −0.18 at d74). We then examined the relationships between LFP amplitude and PPI. P90 magnitude was strongly correlated with d53 PPI among all IR rats (r = 0.43, p<0.025), and particularly among male IR rats (r = 0.71, p<0.01), but not among all SH rats (r = −0.35, ns) or selectively male SH rats (r =−0.51, ns). These relationships of P90 magnitude to d53 PPI were significantly different in SH vs. IR rats when all rats, or only male rats, were compared (p’s < 0.005 or 0.002, respectively).”
Gene Expression
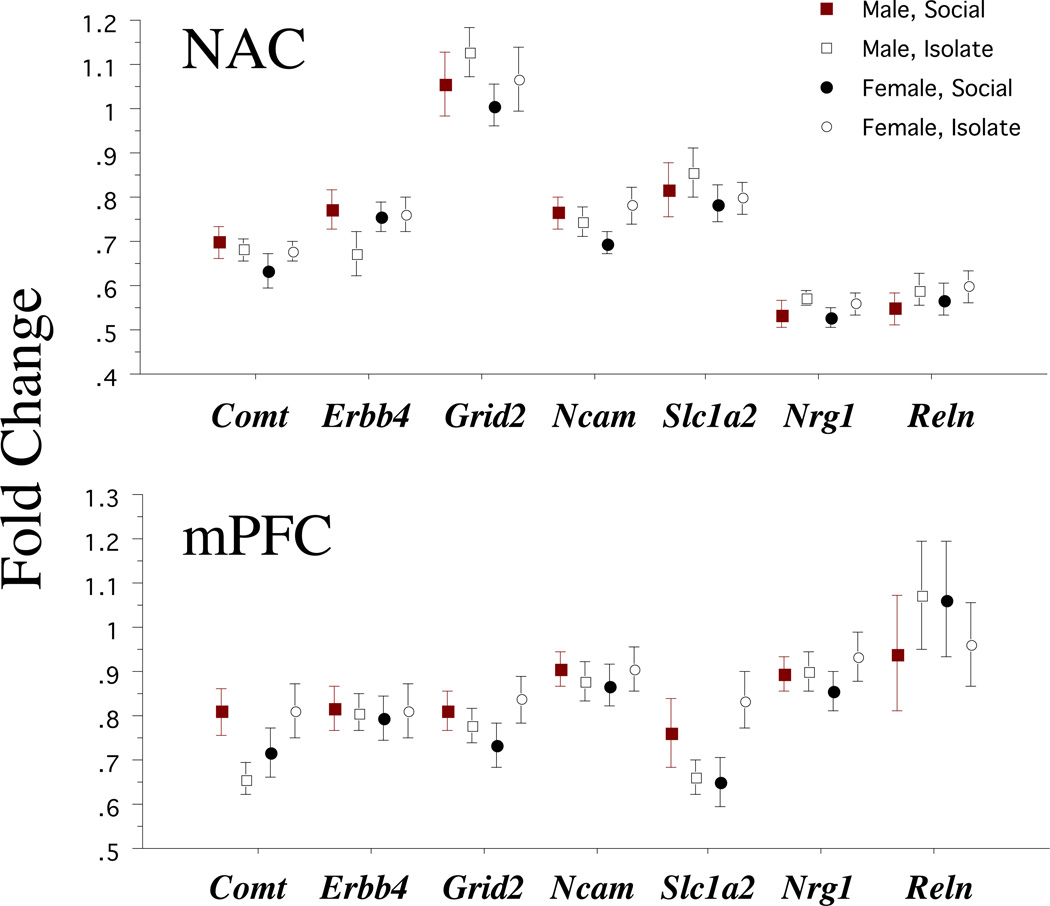
Rearing condition had no significant effects on the level of expression of the 7 genes assessed in either the NAC or mPFC (Figure 3). ANOVAs of fold-change values in the NAC and mPFC detected no main effect of rearing or sex, and significant effects of gene (i.e. different expression levels were detected among the 7 genes). The only significant interaction term was a 3-way interaction for mPFC expression levels of rearing × gene × sex (F=2.79, df 6,342, p<0.015); this interaction reflected significant increases in expression of mPFC Comt (p<0.025) and Slc1a2 (p<0.02) in IR females, and non-significant reductions in these expression levels in IR males. Neither of these increases remained significant when alpha was corrected for 14 (2 sexes × 7 genes) comparisons.
Figure 3.
Levels of gene expression (Fold Change, mean (SEM)) in the NAC and mPFC in SH and IR male and female BUF rats.
While rat litter did not interact significantly with either sex or housing conditions in analyses of the major behavioral variables in this study, ANOVAs restricted to litters with more than one pup did identify a significant main effect of litter on mPFC gene expression levels (F=4.44, df 13,44, p<0.0001), and a significant interaction of gene × litter (F=1.85, df 78, 264, p<0.0003). For the NAC, the main effect of litter did not reach significance (F<1), though the interaction of litter × gene did (F=1.69, df 78, 264, p<0.002). Correcting for multiple (14) comparisons, within the NAC, no individual gene exhibited significant litter effects, but within the mPFC, litter effects reached corrected levels of significance for Comt, Grid2 and Slc1a2 (all p’s<0.0001), Nrg1 (p<0.0005) and Erbb4 (p<0.002), with uncorrected significance levels achieved by Ncam1 (p<0.015). Thus, within this inbred strain, maternal factors may play a role in the levels of brain regional gene expression, particularly within the mPFC, but in this process, these maternal factors do not appear to interact with either sex or housing condition.
Correlations among genes
Consistent with our previous reports (Swerdlow et al. 2012; 2013) [15,25], gene expression levels were generally correlated within brain regions, but not across brain regions (Table 3). Within both the NAC and mPFC, of the 42 possible pair-wise relationships among the 7 genes in each region (e.g. NAC Comt vs. NAC Erbb4), 40 regression terms were positive, 35 reached corrected significance levels (0.05/21=0.0024), and 4 reached uncorrected significance levels (p<0.05); similar patterns were detected among each of the 4 subgroups based on sex and rearing (Supplemental Table 1). In contrast, across regions (e.g. NAC Comt vs. mPFC Comt), among the inclusive sample of rats, 7 out of 7 regression terms were negative (R’s = −0.01 – (−0.24)), none reaching statistical significance.
Table 3.
Correlated gene expression (FC) within brain regions (n=61)
| Comt | Erbb4 | Grid2 | Ncaml | Slcla2 | Nrgl | Reln | ||
|---|---|---|---|---|---|---|---|---|
| Comt | .493** | .664** | .447** | .826** | .591** | −.004 | mPFC | |
| Erbb4 | .337* | .749** | .794** | .311* | .805** | .763** | ||
| Grid2 | .490** | .538** | .757** | .490** | .882** | .367* | ||
| Ncam1 | .514** | .539** | .823** | .173 | .886** | .746** | ||
| Slcla2 | .567** | .474** | .760** | .641** | .385** | −.160 | ||
| Nrgl | .644** | .497** | .750** | .746** | .668** | .484* | ||
| Reln | 797** | .370* | .511** | .546** | .602** | .620** | ||
| NAC | ||||||||
p < 0.05
p < 0.0024
Gene – behavior correlations
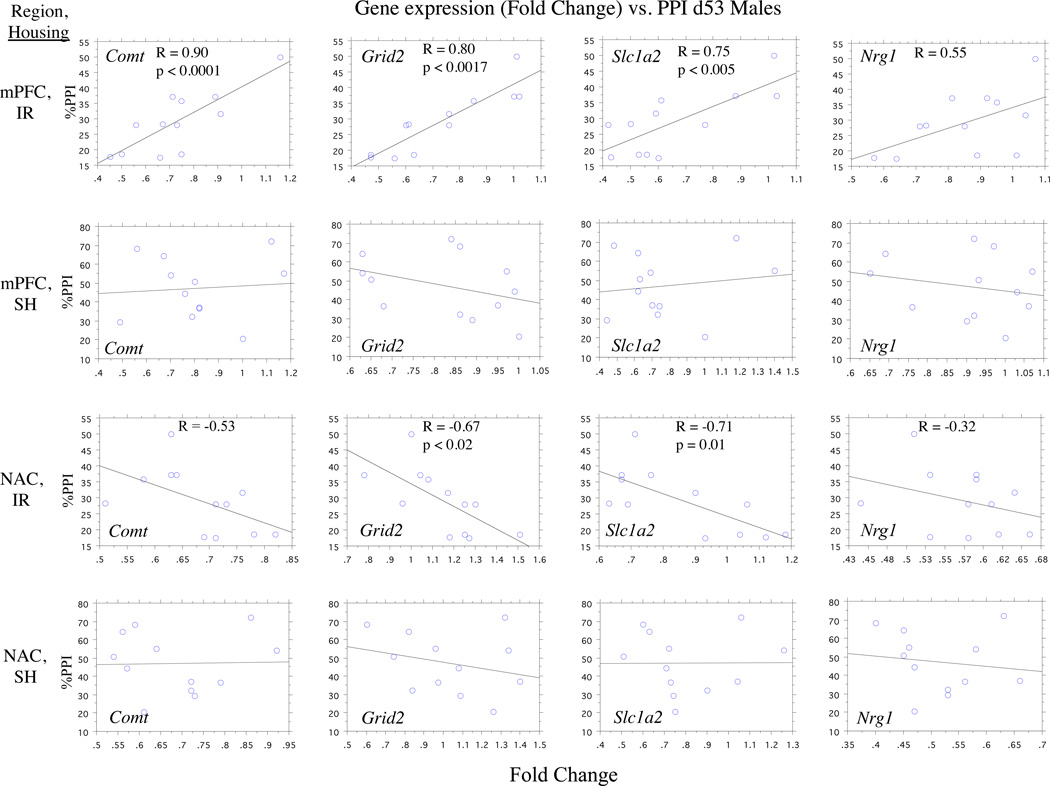
In general, levels of gene expression in the NAC and mPFC did not correlate significantly with mean PPI levels; this was true in analyses that included all data from tests in which rats exhibited “normal” PPI levels, including females (d53, d74, SH, IR) and males (d53 SH, d74 SH and IR). Of the 98 possible correlations (7 genes × 2 regions (NAC, mPFC) × 7 groups), only 3 correlations achieved uncorrected significance (p<0.02–0.045). However, in analyses limited to rats with IR-induced PPI deficits (d53 IR male rats), mean PPI levels appeared to be associated with several gene expression levels in both the mPFC (positively) and NAC (negatively) (5 values reaching p’s < 0.02-0.0001; Table 4; Figure 4).
Table 4.
Correlations of Mean %PPI and Gene Expression (Fold Change)
| NAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| d53 | d74 | ||||||||
| Male | Female | Male | Female | ||||||
| IR | SH | IR | SH | IR | SH | IR | SH | ||
| Comt | −0.53 | 0.03 | 0.41 | −0.07 | 0.49 | −0.42 | −0.03 | −0.30 | |
| Erbb4 | −0.33 | −0.67* | 0.53 | −0.26 | 0.08 | −0.54 | 0.12 | 0.05 | |
| Grid2 | −0.67* | −0.27 | −0.06 | −0.07 | 0.30 | −0.07 | 0.04 | −0.01 | |
| Ncaml | −0.02 | −0.24 | 0.21 | −0.13 | −0.04 | −0.25 | 0.08 | 0.08 | |
| Slcla2 | −0.71* | 0.11 | −0.18 | −0.17 | 0.35 | −0.30 | −0.38 | −0.06 | |
| Nrgl | −0.32 | −0.14 | 0.25 | −0.20 | 0.19 | −0.08 | 0.13 | −0.11 | |
| Reln | −0.09 | −0.05 | 0.22 | −0.16 | 0.37 | −0.26 | −0.24 | −0.23 | |
| mPFC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| d53 | d74 | ||||||||
| Male | Female | Male | Female | ||||||
| IR | SH | IR | SH | IR | SH | IR | SH | ||
| Comt | 0.90*** | 0.08 | 0.17 | 0.03 | −0.34 | 0.04 | 0.54 | 0.41 | |
| Erbb4 | 0.40 | −0.09 | −0.11 | 0.24 | 0.23 | 0.16 | 0.12 | −0.13 | |
| Grid2 | 0.80** | −0.37 | 0.00 | 0.02 | −0.13 | 0.30 | 0.18 | 0.39 | |
| Ncaml | 0.47 | −0.27 | 0.08 | 0.28 | −0.19 | 0.41 | 0.24 | 0.12 | |
| Slcla2 | 0.75** | 0.14 | −0.01 | 0.08 | −0.18 | 0.05 | 0.38 | 0.29 | |
| Nrgl | 0.55 | −0.21 | 0.07 | 0.18 | −0.17 | 0.34 | 0.11 | 0.25 | |
| Reln | −0.06 | 0.10 | −0.04 | 0.19 | 0.39 | 0.45 | −0.18 | −0.30 | |
p <0.02
p < 0.005
p < 0.0001
Figure 4.
Correlations of NAC and mPFC gene expression with PPI in SH vs. IR rats.
Fisher’s r-to-Z transformation confirmed significant differences between gene-PPI correlations among d53 IR vs. SH males for mPFC genes (Comt and Grid2 achieving significance with corrected alpha (p’s < 0.0035-0.0015); Ncam1, Slc1a2 and Nrg1 achieving trend levels); among IR rats, correlations with PPI were significantly greater for genes in the mPFC than for those in the NAC (Comt, Grid2 and Slc1a2 achieving significance with corrected alpha (p’s<0.0001) and Nrg1 achieving significance with uncorrected alpha (p<0.045)).
Startle magnitude on PULSE trials was elevated among both male and female IR rats, and NAC gene expression levels were generally positively correlated with d53 PULSE amplitude in IR males and females. Of the 14 possible d53 correlations in these groups (2 sexes × 7 genes), all 14 values were positive, one achieved corrected significance (NAC Comt levels in males, p<0.0025), and 6 achieved uncorrected significance (p’s < 0.046-0.011) (Table 5). In contrast, among SH males and females, these correlations were evenly split between negative and positive values, none of which achieved even uncorrected alpha levels (Supplemental Table 1).
Table 5.
Correlations of Mean Startle Magnitude and Gene Expression (Fold Change) NAC
| NAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| d53 | d74 | ||||||||
| Male | Female | Male | Female | ||||||
| IR | SH | IR | SH | IR | SH | IR | SH | ||
| Comt | −0.65* | −0.47 | 0.03 | 0.21 | −0.48 | −0.35 | −0.02 | 0.66** | |
| Erbb4 | −0.31 | −0.04 | 0.24 | −0.41 | −0.04 | 0.07 | 0.10 | 0.10 | |
| Grid2 | −0.15 | 0.09 | 0.60* | 0.05 | −0.12 | −0.08 | 0.25 | 0.16 | |
| Ncaml | −0.49 | −0.26 | 0.48 | −0.01 | 0.09 | −0.30 | 0.22 | 0.10 | |
| Slcla2 | −0.41 | −0.24 | 0.37 | −0.06 | −0.42 | −0.22 | 0.37 | 0.21 | |
| Nrgl | −0.54 | −0.39 | 0.27 | 0.08 | −0.05 | −0.44 | −0.10 | 0.38 | |
| Reln | −0.31 | −0.46 | 0.30 | 0.01 | −0.31 | −0.48 | 0.42 | 0.47 | |
| mPFC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| d53 | d74 | ||||||||
| Male | Female | Male | Female | ||||||
| IR | SH | IR | SH | IR | SH | IR | SH | ||
| Comt | −0.07 | 0.09 | −0.24 | −0.17 | 0.22 | −0.03 | −0.45 | −0.52* | |
| Erbb4 | −0.21 | 0.49 | −0.38 | −0.15 | −0.26 | 0.24 | −0.49 | −0.13 | |
| Grid2 | −0.07 | 0.77** | −0.28 | −0.11 | 0.03 | 0.39 | −0.39 | −0.29 | |
| Ncaml | −0.05 | 0.61 | −0.21 | −0.05 | 0.08 | 0.27 | −0.42 | −0.22 | |
| Slcla2 | −0.10 | 0.10 | −0.03 | −0.08 | −0.07 | −0.07 | −0.04 | −0.09 | |
| Nrgl | −0.09 | 0.68* | −0.20 | −0.18 | 0.12 | 0.39 | −0.32 | −0.35 | |
| Reln | −0.28 | 0.32 | −0.52 | 0.00 | −0.35 | −0.14 | −0.41 | 0.08 | |
p <0.02
p < 0.005
p < 0.0001
Correlations of gene expression levels with P90 LFP amplitudes exhibited patterns moderated by sex but not housing condition (Supplemental Table 2).
Structural equation modeling (SEM)
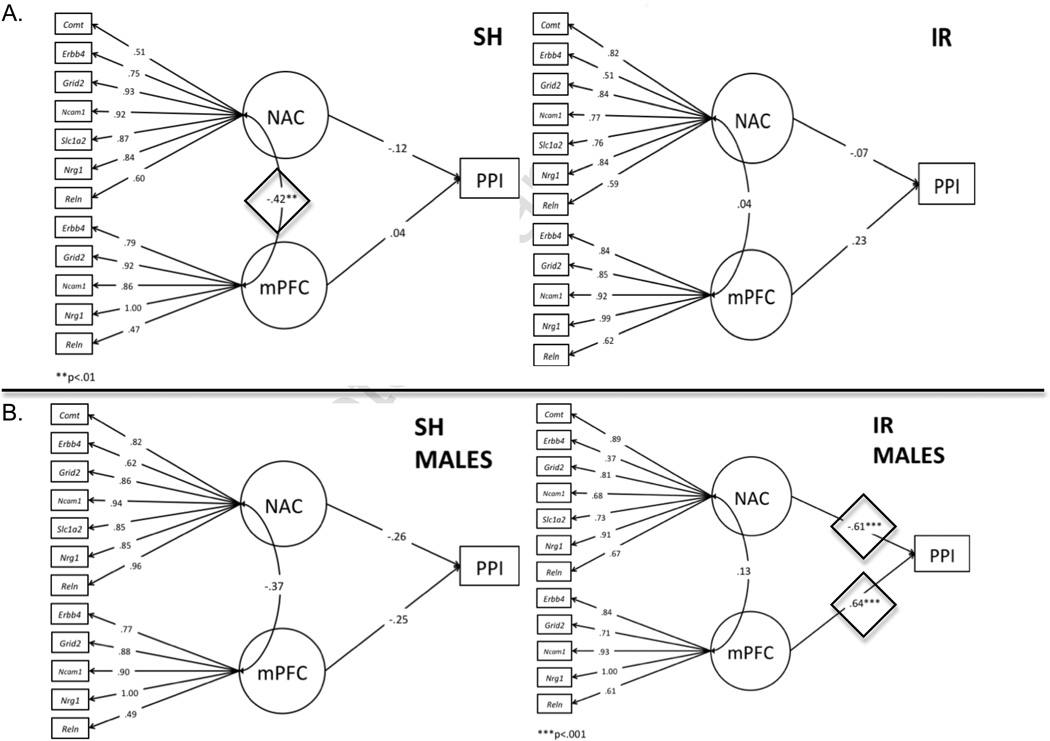
Lastly, we estimated a series of models to establish the relations between the measured levels of gene expression and the expression patterns of interest. Preliminary analyses first focused on the expression values of the 7 genes in the two brain regions. Our a priori hypotheses (which served as the basis for selecting these two structures for analysis) and preliminary bivariate correlations led to the prediction that the measured levels of gene expression would load on separate latent factors specific to each brain region examined (NAC, mPFC). Exploratory factor analysis showed that the observed gene expression variables for the NAC appeared to load on a single latent factor; for the mPFC, however, expression levels loaded on two latent factors, with some genes (Grid2, Nrg1) cross-loading on both factors. These results were used to construct latent variables within an SEM framework in MPlus. All 7 gene expression variables were used as indicators for NAC; the latent factor for the mPFC that accounted for the most variance (indicated by 5 gene expression variables) was retained for these analyses in order to maximize the model fit to the data. The outcome variables were regressed on both latent variables for the whole sample; however there was no significant effect between NAC and mPFC expression patterns (Figure 5A). When these paths were examined separately for the housing subgroups, there was a significant negative correlation between the NAC and mPFC latent factors in SH rats (b = −0.42, p=0.005) but not IR rats (b=0.04, ns). In other words, the NAC and mPFC regional “gene expression” factors were inversely related among SH rats, but this normal inverse relationship was eliminated in IR rats.
Figure 5.
Measurement model from confirmatory factor analysis shows pathways inter-relating gene expression, brain regions and behavioral phenotypes in all SH and IR rats (A) and in males only (B). Statistically significant pathways between NAC and mPFC (Figure 5A, SH) and between brain regions and PPI (Figure 5B, IR) are shown within diamonds.
Because phenotypic deficits in IR rats were sex- and time-dependent, we examined these “phene-gene” paths in the relevant subgroups of rats. Among IR males, the NAC latent factor was negatively related to d53 PPI (b=−0.61, p=0.001) and the mPFC latent factor was positively related to d53 PPI (b=0.64, p<0.001); the latent factors did not predict d53 PPI among SH males (Figure 5B). Gene expression also predicted PULSE magnitude elevations in male and female IR rats. For IR males, the NAC latent factor was negatively related to d53 PULSE magnitude (b=−0.75, p<0.001), but the effect of the mPFC latent factor was not significant. For SH males, the opposite was true: the mPFC latent factor was positively related to d53 PULSE magnitude (b=−0.62, p=0.001), but the effect of the NAC latent factor was not significant. In female IR rats, the NAC latent factor was positively related to d53 PULSE magnitude (b=0.61, p<0.001), but the effect of the mPFC latent factor was not significant. In SH females, neither factor was related to d53 PULSE magnitude.
Discussion
Consistent with many previous reports, isolation rearing was associated with two behavioral phenotypes in this study: elevated startle magnitude, and reduced PPI. Startle potentiation was evident in both males and females, while reduced PPI was evident only in males. Studies independently report IR-induced PPI deficits in females (e.g. Powell et al. 2003) [7] and in males (e.g. Cilia et al. 2005) [36], though few studies assess both sexes. We have observed strain differences in sex-specific IR effects in rats (Powell et al. 2002) [31], and others have reported male > female IR effects on various behaviors in mice (Pietropaolo et al. 2008) [37]; the present study is the first involving IR that we could identify in inbred BUF rats.
It is conceivable that substantial male > female PPI differences in SH rats in the present study created a “floor effect” that obviated IR-induced deficits in females: at d53, PPI levels in SH females were actually lower than those in IR males. While PPI and startle magnitude are both startle phenotypes, the relative independence of IR-induced PPI deficits and startle potentiation is suggested by four findings: 1) sex differences were detected in the former but not the latter; 2) startle-potentiating effects of IR persisted through the final test, while IR effects on PPI did not; 3) IR-induced PPI deficits were evident among subgroups of SH and IR rats matched for comparable levels of startle magnitude.
Robust IR-induced PPI deficits in male BUF rats were evident only during the first test session. In previous reports, IR-induced PPI deficits have been reported to diminish with repeated testing (Geyer et al., 1993; Lim et al. 2011; Weiss & Feldon, 2001) [4,38,39] and with handling immediately post-weaning (Krebs-Thomson et al. 2001) [40], though the biological basis of this phenomenon is not clear. Other studies have shown the enduring effects of isolation with repeated testing (Varty & Higgins 1995; Cilia et al. 2001) [36,41]. In the present study, it is impossible to determine whether the recovery of PPI among IR rats by day 74 reflects the impact of repeated testing, aging, surgery for electrode implantation, handling, or some combination of these processes.
Despite its potent effects on startle behavior, IR did not significantly alter expression levels of 7 PPI- and/or SZ-associated genes, within two brain regions known to regulate PPI. This observation is consistent with the lack of impact on expression levels of these genes by another potent early developmental manipulation – NVHLs – that also disrupts PPI (Swerdlow et al. 2013) [25]. However, the fact that PPI levels had largely recovered to SH levels by the time of tissue collection makes the present “negative” finding (i.e. no difference in gene expression levels between SH and IR rats) difficult to interpret. Nonetheless, evidence that expression levels were not altered among male IR rats whose PPI levels were lowest at either day 53 or 74, or among those whose startle magnitude was elevated at both test dates, suggests that processes that generated IR-induced behavioral changes in this study did not generate changes in the expression levels of these 7 genes in the NAC or mPFC.
The present results confirm that the expression of these 7 genes is strongly correlated within brain regions, but not across two highly interconnected brain regions (Swerdlow et al. 2012, 2013) [15,25]. We previously reported that mPFC and NAC expression levels of Comt, Slc1a2 and Ncam1 became “coupled,” or significantly positively correlated, in adult NVHL rats, and we hypothesized that, developing in the absence of ventral hippocampal inputs, these two brain regions established stronger functional interconnections (Swerdlow et al. 2013) [25]. A similar “coupling” of these mPFC and NAC genes was not detected in IR rats in the present study. However, in the present study, we did observe that PPI deficits among male IR rats correlated significantly with expression levels of several genes – particularly Comt and Grid2 in both the mPFC and NAC, with positive relationships exclusively detected in the mPFC, and negative relationships exclusively detected in the NAC. Thus, as with NVHLs, a developmental manipulation that reduced PPI also altered the forebrain expression of several PPI- and/or SZ-associated genes; in the case of IR, this alteration resulted in an aberrant and more pronounced relationship between expression levels and PPI levels.
Consistent with our past report (Swerdlow et al. 2012) [15], observed gene expression variables loaded on a single latent factor for the NAC, and to a lesser degree for the mPFC. Regressing these latent factors with the IR behavioral phenotypes identified significant relations for the NAC (negative for PPI, positive for startle magnitude) and mPFC (positive for PPI). This observation does not preclude the possibility that these phenotypes are biologically linked to one or more of these individual genes, but suggests an alternative explanation: that IR-induced changes in these phenotypes reflects a more generalized impact on NAC and mPFC function, e.g. changes in the levels of metabolic or cellular activity within these regions, or alterations in one or more signaling pathways that engage a number of these different genes.
PPI is a complex phenotype, regulated by numerous brain regions and neurotransmitters (cf. Swerdlow et al. 2008) [14]. It is thus not surprising that, in its “baseline” state, PPI levels do not correlate significantly with the expression levels of a handful of forebrain genes, even ones selected specifically based on associations between PPI and specific SNPs in humans, and which in many cases influence dopaminergic and glutamatergic activity in brain regions known to regulate PPI (Greenwood et al. 2011, 2012) [22,23]. However, the fact that these expression levels do become correlated with IR-impaired PPI suggests that the developmental intervention of isolation rearing may: 1) independently impact both the phenotype and the gene expression levels, such that the stronger correlations reflect a shared influence of IR; or 2) alter forebrain organization in a manner that gives activity levels in the mPFC and NAC more potent control over this phenotype. The present findings cannot distinguish between these possible explanations.
IR did not significantly alter the amplitude of either short or longer latency local field potentials in this study. Interesting and systematic relationships were detected between LFP magnitudes and levels of startle phenotypes, and some of these relationships appeared to be moderated by rearing conditions. Superficially, these observations are not surprising, as both LFPs and startle phenotypes reflect neural mechanisms relevant to sensory processing, hippocampal function and interconnected hippocampal-frontal circuitries. However, beyond such a pseudo-mechanistic explanation, the lack of robust a priori hypotheses related to the relationships of these complex phenotypes makes it prudent to treat the observed LFP-startle and LFP-PPI correlations, and their modification by IR, as preliminary and in need of replication and extension.
A strength of present findings is that clear effects of IR were detected on PPI and startle magnitude, in a heretofore unstudied inbred rat strain, that are generally consistent with a literature rife with repeatable and often enduring (e.g. Cilia et al. 2005) [36], but sometimes “fragile” effects that exhibit sex and strain differences, and sometimes fade with repeated testing (Lim et al. 2011) [38]. A major weakness of the present findings is the fact – beyond the largely “expected’ patterns of IR effects - the novel observations in this study related to the effects of IR were detected via a plethora of simple regression analyses, albeit with corrections for multiple comparisons and potential family-wise error rates. Some coherence to these multiple comparisons emerged from the use of Structural Equation Modeling and Confirmatory Factor Analyses. Making these novel findings even more surprising is that many were detected only among groups with a compressed range of PPI values, based on PPI deficits. The main 2 × 2 (sex, housing) experimental design was selected to test simple effects of IR on the mean expression levels of specific PPI- and/or schizophrenia-associated genes, and the most parsimonious conclusion based on our failure to detect such effects might be that IR does not produce behavioral changes by altering levels of these genes.
However, another reasonable conclusion from the present data is that modification of the postweaning environment by IR impacted mPFC and NAC circuitry in a manner that strengthened the relationships between activity in these regions (and perhaps secondarily, levels of gene expression) and PPI, startle magnitude and P90 LFPs. We can only speculate that similar (or more robust) relationships would have been detected had gene expression been assessed at d53, and the fact that these relationships persisted after normalization of PPI levels suggests that these patterns of gene expression were not sufficient to produce IR-induced PPI deficits. Nonetheless, it would not be surprising if post-weaning social isolation alters brain-behavior relationships by reorganizing existing and developing neural elements, in a manner that is more subtle and complex than we might expect from a lesion, or even from an in-utero intervention. To the extent that they occurred here, these more complex processes escaped detection by simple means comparisons and ANOVAs. That such reorganization would result not in an absolute change in gene expression levels, but rather in a significantly stronger connection of those levels to an aberrant phenotype, might have implications for the biological mechanisms of IR-induced syndromes, and for the strategies to correct the clinical conditions that they model.
Supplementary Material
Highlights.
PPI was reduced in isolation reared (IR) vs. social housed (SH) adult male BUF rats
IR did not alter mPFC and NAC expression of 7 PPI- and schizophrenia-linked genes
In IR but not SH males, PPI correlated positively with mPFC gene expression
In IR but not SH males, PPI correlated negatively with NAC gene expression
IR effects on brain function are expressed via altered gene-behavior relationships
Acknowledgements
Supported by MH042228 and MH059803. The authors are grateful for assistance in manuscript preparation provided by Ms. Maria Bongiovanni. NRS has received Consulting fees from Neurocrine, Inc. GAL has received Consulting fees from Astellas Pharma, Inc. This work was performed with the support of the Genomics and Sequencing Core at the UC San Diego Center for AIDS Research (P30 AI036214), the VA San Diego Healthcare System, and the Veterans Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Powell SB. Models of neurodevelopmental abnormalities in schizophrenia. Curr Top Behav Neurosci. 2010;4:435–481. doi: 10.1007/7854_2010_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cilia J, Reavill C, Hagan JJ, Jones DN. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats. Psychopharmacology (Berl) 2001;156:327–337. doi: 10.1007/s002130100786. [DOI] [PubMed] [Google Scholar]
- 4.Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- 5.Stevens KE, Johnson RG, Rose GM. Rats reared in social isolation show schizophrenia-like changes in auditory gating. Pharmacol Biochem Behav. 1997;58:1031–1036. doi: 10.1016/s0091-3057(97)00306-7. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: Current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 7.Powell SB, Geyer MA, Preece MA, Pitcher LK, Reynolds GP, Swerdlow NR. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience. 2003;119:233–240. doi: 10.1016/s0306-4522(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 8.Day-Wilson KM, Jones DN, Southam E, Cilia J, Totterdell S. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 141:1113–1121. doi: 10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 9.Krolow R, Noschang C, Weis SN, Pettenuzzo LF, Huffell AP, Arcego DM, Marcolin M, Mota CS, Kolling J, Scherer EB, Wyse AT, Dalmaz C. Isolation stress during the prepubertal period in rats induces long-lasting neurochemical changes in the prefrontal cortex. Neurochem Res. 2012;37:1063–1073. doi: 10.1007/s11064-012-0709-1. [DOI] [PubMed] [Google Scholar]
- 10.Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnock-Jones JJ, Jennings CA, Robbins MJ, Cluderay JE, Cilia J, Reid JL, Taylor A, Jones DN, Emson PC, Southam E. Increased expression of the NR2A NMDA receptor subunit in the prefrontal cortex of rats reared in isolation. Synapse. 2009;63:836–846. doi: 10.1002/syn.20665. doi: 10.1002/syn.20665. [DOI] [PubMed] [Google Scholar]
- 12.Murphy KJ, Ter Horst JP, Cassidy AW, DeSouza IE, Morgunova M, Li C, Connole LM, O'Sullivan NC, Loscher JS, Brady AT, Rombach N, Connellan J, McGettigan PA, Scully D, Fedriani R, Lukasz B, Moran MP, McCabe OM, Wantuch CM, Hughes ZA, Mulvany SK, Higgins DG, Pangalos MN, Marquis KL, O'Connor WT, Ring RH, von Schack D, Regan CM. Temporal dysregulation of cortical gene expression in the isolation reared Wistar rat. J Neurochem. 2010;113:601–614. doi: 10.1111/j.1471-4159.2010.06617.x. [DOI] [PubMed] [Google Scholar]
- 13.Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swerdlow NR, Shilling PD, Breier M, Trim RS, Light GA, Saint Marie R. Fronto-temporal-mesolimbic gene expression and heritable differences in amphetamine-disrupted sensorimotor gating in rats. Psychopharmacology. 2012;224:349–362. doi: 10.1007/s00213-012-2758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores G, Alquicer G, Silva-Gómez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am J Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roussos P, Giakoumaki SG, Rogdaki M, Pavlakis S, Frangou S, Bitsios P. Prepulse inhibition of the startle reflex depends on the catechol O-methyltransferase Val158Met gene polymorphism. Psychol Med. 2008;38:1651–1658. doi: 10.1017/S0033291708002912. [DOI] [PubMed] [Google Scholar]
- 20.Kao WT, Wang Y, Kleinman JE, Lipska BK, Hyde TM, Weinberger DR, Law AJ. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proc Natl Acad Sci USA. 2010;107:15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quednow BB, Wagner M, Mössner R, Maier W, Kuhn KU. Sensorimotor gating of schizophrenia patients depends on catechol O-methyltransferase Val158Met polymorphism. Schizophr Bull. 2010;36:341–346. doi: 10.1093/schbul/sbn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, et al. Analysis of 94 candidate genes and twelve endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS Genet. 2012;7:e1002134. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shilling PD, Saint Marie RL, Shoemaker JM, Swerdlow NR. Strain differences in the gating-disruptive effects of apomorphine: relationship to gene expression in nucleus accumbens signaling pathways. Biol Psychiatry. 2008;63:748–758. doi: 10.1016/j.biopsych.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swerdlow NR, Powell SB, Breier MR, Hines SR, Light GA. Coupling of gene expression in medial prefrontal cortex and nucleus accumbens after neonatal ventral hippocampal lesions accompanies deficits in sensorimotor gating and auditory processing in rats. Neuropharmacology. 2013;75C:38–46. doi: 10.1016/j.neuropharm.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacol Biochem Behav. 2004;77:291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Bakshi VP, Swerdlow NR, Braff DL, Geyer MA. Reversal of isolation rearing-induced deficits in prepulse inhibition by Seroquel and olanzapine. Biol Psychiatry. 1998;43:436–445. doi: 10.1016/s0006-3223(97)00246-1. [DOI] [PubMed] [Google Scholar]
- 28.Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- 29.Bakshi VP, Geyer MA. Ontogeny of isolation rearing-induced deficits in sensorimotor gating in rats. Physiol Behav. 1999;67:385–392. doi: 10.1016/s0031-9384(99)00082-7. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. fourth ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 31.Powell SB, Swerdlow NR, Pitcher LK, Geyer MA. Isolation rearing-induced deficits in prepulse inhibition and locomotor habituation are not potentiated by water deprivation. Physiol Behav. 2002;77:55–64. doi: 10.1016/s0031-9384(02)00817-x. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann J, Pryce CR, Feldon J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav Brain Res. 1999;104:113–117. doi: 10.1016/s0166-4328(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 33.Swerdlow NR, Breier M, Mora AB, Ko D, Shoemaker JM. A novel rat strain with enhanced sensitivity to the effects of dopamine agonists on startle gating. Pharmacol Biochem Behav. 2008;88:280–290. doi: 10.1016/j.pbb.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bollen KA. Structural Equations with Latent Variables. Wiley: New Jersey; 1989. [Google Scholar]
- 35.Muthén LK, Muthén BO. Mplus User’s Guide. Sixth Edition. Los Angeles: Author; 1998–2010. [Google Scholar]
- 36.Cilia J, Hatcher PD, Reavill C, Jones DN. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats: an update. Psychopharmacology. 2005;180:57–62. doi: 10.1007/s00213-004-2139-5. [DOI] [PubMed] [Google Scholar]
- 37.Pietropaolo S, Singer P, Feldon J, Yee BK. The postweaning social isolation in C57BL/6 mice: preferential vulnerability in the male sex. Psychopharmacology. 2008;197:613–628. doi: 10.1007/s00213-008-1081-3. doi: 10.1007/s00213-008-1081-3. [DOI] [PubMed] [Google Scholar]
- 38.Lim AL, Taylor DA, Malone DT. Isolation rearing in rats: effect on expression of synaptic, myelin and GABA-related immunoreactivity and its utility for drug screening via the subchronic parenteral route. Brain Res. 2011;1381:52–65. doi: 10.1016/j.brainres.2011.01.017. doi: 10.1016/j.brainres.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Weiss IC, Feldon J. Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology. 2001;156:305–326. doi: 10.1007/s002130100800. [DOI] [PubMed] [Google Scholar]
- 40.Krebs-Thomson K, Giracello D, Solis A, Geyer MA. Post-weaning handling attenuates isolation-rearing induced disruptions of prepulse inhibition in rats. Behav Brain Res. 2001;120:221–224. doi: 10.1016/s0166-4328(00)00374-0. [DOI] [PubMed] [Google Scholar]
- 41.Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology. 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.