Abstract
Cues associated with alcohol can stimulate subjective states that increase relapse. Alcohol-cue associations may be strengthened by enhancing adrenergic activity with yohimbine or weakened by blocking adrenergic activity with propranolol. Alcohol-cue associations may also be weakened by long cue exposure sessions or strengthened by short cue exposure sessions. A useful treatment approach for alcoholism may combine adrenergic manipulation with cue exposure sessions of a specific duration. The present study sought to determine if cue exposure during long- or short-duration extinction sessions with post-session yohimbine or propranolol would alter alcohol cue-induced responding and self-administration. Rats were trained to respond for alcohol during sessions that included an olfactory cue given at the beginning of the session and a visual/auditory cue complex delivered concurrently with alcohol. Cue-induced responding was assessed before and after the repeated extinction sessions. Repeated alcohol extinction sessions of long duration (45 min) or short duration (5 min) were followed immediately by injections of saline, yohimbine, or propranolol. After the second set of cue-induced responding tests, reacquisition of operant alcohol self-administration was examined. To determine if the experimental procedures were sensitive to memory manipulation through other pharmacological mechanisms, the NMDA receptor antagonist MK-801 was given 20 min prior to long-duration extinction sessions. Both the long- and short-duration extinction sessions decreased cue-induced responding. Neither yohimbine nor propranolol, given post-session, had subsequent effects on cue-induced responding or alcohol self-administration. MK-801 blocked the effect of extinction sessions on cue-induced responding but had no effect on self-administration. The present study shows that manipulation of the NMDA system in combination with alcohol cue exposure therapy during extinction-like sessions may be more effective than manipulation of the adrenergic system in reducing the strength of alcohol-cue associations in this specific model of alcohol relapse.
Keywords: ethanol, extinction, reinstatement, reconsolidation, propranolol, yohimbine, MK-801
1. Introduction
Drug and alcohol use as well as relapse are often considered context- and cue-specific. Alcohol-paired cues may cause subjective and/or physiological responses that are associated with increased alcohol consumption. For example, in human alcoholics, alcohol-paired cues can increase the urge to drink in addition to salivation, galvanic skin response, skin temperature, and heart rate (Monti et al., 1987; Pomerleau et al., 1983; Turkkan et al., 1989). This cue reactivity may have important therapeutic implications such as helping to identify individuals with more serious drinking problems and to predict drinking outcomes in treatment populations (Rohsenow et al., 1994). If alcohol cue reactivity predicts future drinking, craving and/or urges to drink, then reducing sensitivity to alcohol cues, via repeated cue exposure, may provide an alternative treatment for alcoholism.
Cue exposure therapy for addiction utilizes an approach that attempts to extinguish the learned relationship between drug-paired cues and their conditioned responses. This theoretical approach to treatment is based on multiple drug-cue learning models (for review see Heather and Greeley, 1990; Rohsenow et al., 1991). While cue exposure methods have been shown to be moderately effective in several alcohol studies (Blakey and Baker, 1980; Drummond and Glautier 1994; Rankin et al., 1983; Rohsenow et al., 2001; Sitharthan et al., 1997), one meta-analysis concluded that there was no consistent evidence for the efficacy of this approach for treating addiction (Conklin and Tiffany, 2002). Instead a combination of improved behavioral techniques and pharmacotherapy could be more useful in treating addiction disorders such as alcoholism. This combined approach has been validated in several anxiety and fear studies which provide support for altering adrenergic activity with drugs such as the alpha2-adrenergic antagonist yohimbine or the beta-adrenergic antagonist propranolol (Cain et al., 2004; Davis et al., 2006; Debiec and LeDoux, 2004; Powers et al., 2009; Soeter and Kindt, 2011). Altering adrenergic activity may affect the ability of fear-associated stimuli to produce a response and may do so by disrupting reconsolidation of the fear memory. Targeting the cue memory may be useful in decreasing the response to alcohol-paired cues and subsequent alcohol-seeking behavior.
The memory of drug-associated cues may undergo reconsolidation upon re-exposure to the cues. Reconsolidation is considered the process by which previously stored or consolidated memories are stabilized after retrieval (Tronson and Taylor, 2007). During this active retrieval process, these memories may become labile for a brief period allowing the molecular mechanism responsible for reconsolidation to become a pharmacological target. Drugs that decrease adrenergic activity may weaken the drug-cue association during reconsolidation. In rodent experiments with conditioned place preference (CPP), propranolol given immediately after cue re-exposure disrupted the later expression of a cocaine and morphine CPP (Bernardi et al., 2006; Fricks-Gleason and Marshall, 2008; Robinson and Franklin, 2007). Propranolol may disrupt reconsolidation when measured in a paradigm with an appetitive reinforcer as well. For example, the reinforcing properties of a conditioned stimulus (CS) associated with sucrose were attenuated when 10 mg/kg propranolol was given immediately after a 10 min extinction session (Milton et al. 2008). In that study, the authors suggested that the CS-sucrose association was “reactivated” during the short extinction session. This effect may transfer to alcohol self-administration. In rats trained to self-administer 12% alcohol, propranolol given immediately after each of three 20 min reactivation sessions decreased alcohol-seeking responses (Wouda et al., 2010). Together, these findings suggest that blocking adrenergic activity following CS reactivation sessions can weaken relapse-like behavior measured via cue-induced alcohol-seeking.
The duration of the cue re-exposure session may determine how adrenergic drugs affect memory and the subsequent behavioral response to cues. Short duration re-exposure to cues can be considered a reactivation session which triggers the process of reconsolidation further strengthening previously learned information. Long duration re-exposure to cues can be considered an extinction session which triggers the process of consolidation for the new extinction memory. The duration of cue re-exposure is a balancing act between reactivation (reconsolidation) and extinction (consolidation) which likely involve different neurochemical mechanisms (Quirk and Mueller, 2008; Tronson and Taylor, 2007). Although the precise duration at which the mechanisms shift from reactivation to extinction is not clear, the duration of the session has different behavioral effects. For example, in a cue-induced behavioral activation model, rats exposed to context cues associated with an alcohol-saccharin solution for 5 min increased overall responding during a subsequent operant session while slightly longer context cue exposure durations of 10 and 15 min had no effect (Pickering and Liljequist, 2003). Rats were differentially affected by session duration when exposed to a “memory retrieval session” that included olfactory cue presentation in operant chambers during sessions in which responses resulted in the delivery of an alcohol-paired CS (von der Goltz et al., 2009). A 5 min memory retrieval session enhanced cue-induced alcohol seeking 24 hr later whereas a 10 min session had no effect. Therefore, an extinction session as short as 5 min may serve as a memory reactivation session for operant self-administration in rats.
The purpose of the current experiments was to determine if cue exposure during extinction sessions of long or short duration in conjunction with post-session adrenergic manipulation would alter alcohol cue-induced responding and self-administration in rats. Rats were exposed to the alcohol-paired cues during long 45 min extinction sessions or short 5 min extinction sessions. Immediately after each extinction session, rats were injected with yohimbine (increasing adrenergic activation) or propranolol (decreasing adrenergic activation). Yohimbine was expected to decrease cue-induced alcohol (ethanol) responding and self-administration when repeatedly paired with the long extinction sessions, thus enhancing the consolidation of the newly learned extinction associations. In contrast, yohimbine should increase cue-induced ethanol responding and self-administration when given after short extinction sessions further enhancing reconsolidation of the drug-cue association. The exact opposite profile was expected with propranolol. To determine if the experimental procedures were sensitive to memory manipulation through other pharmacological mechanisms, the NMDA receptor antagonist MK-801 was given prior to long-duration extinction sessions. MK-801 was expected to decrease the consolidation of newly learned extinction associations when paired with long extinction sessions. MK-801 pretreatment has been shown to disrupt memory processes in several tasks in rats (Castellano et al., 2001; van der Staay et al., 2011) and an effect in our study would support the validity of the experimental procedures.
2. Materials and Methods
2.1 Animals and Housing
Male Long-Evans rats weighing 101 – 125 g at the beginning of the studies (Charles River, Wilmington, MA) were individually housed and maintained in a temperature and humidity controlled room with a 12-h reversed light/dark cycle. Operant experiments were conducted during the dark cycle. The rats had free access to food and water in their home cages except during initial training. The protocol was approved by Institutional Animal Care and Use Committee and the rats were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2 Apparatus
Experiments were conducted in standard operant chambers (Med Associates, St. Albans, VT) housed in melamine sound-attenuated cubicles. Each chamber (30.5 × 24.2 × 29.2 cm) contained 2 rolled-edge standard levers approximately 7 cm from the grid floor on the right wall. One lever was located near the back of the chamber while the other was located near the front of the chamber. A receptacle cup was located on the center of the right wall between the levers approximately 3 cm from the grid floor. The cup was fitted to receive food pellets or fluid deliveries from a syringe pump via 18 gauge stainless steel tubing connected to the cup. A white stimulus light was located above the cup. The house light was on the center of the left wall near the top of the chamber. Operant chambers were controlled with programs written in Med-PC Medstate Notation version IV (Med Associates).
2.3 Training
Lever-press response training started after food depriving the rats for 24 h. Rats began lever-pressing for food pellets during shaping sessions that lasted 10 – 20 min and were conducted twice per day for up to four days. When the rats acquired the lever-press response, 20% sucrose was substituted for the food pellets for a single 20-min session to allow the rats to transition from solid food reinforcers to fluid reinforcers. On the following day, the rats responded for 5% ethanol (weight/volume) mixed in 0.1% saccharin (w/v). The operant sessions began with a time out period (1 min) during which the chamber was dark and responses on the lever had no consequence. A 20-min response period followed the time out period. During the response period, the rats could earn fluid reinforcers by lever-pressing on a continuous reinforcement schedule. Fluid reinforcers of 0.1 ml were delivered by activating the syringe pump. Accuracy of volume delivery was confirmed during all sessions by measuring the fluid remaining in the syringe at the end of the session. A 3 ml syringe fitted with a 16-gauge needle was used to extract the excess fluid from the cup. If any fluid was found, then that amount was subtracted from the amount measured from the syringe pump. This final number was used to calculate the intakes (g/kg). After the first week of training, the rats rarely left fluid in the cup. During the fluid delivery, the house light was extinguished for 4 sec and responding had no consequence. Additionally, a clicking sound (auditory cue) was presented and the stimulus light over the receptacle cup flashed (visual cue). At the beginning of a session, six drops of peppermint extract (McCormick, Sparks, MD) were put into the waste pan inside the chamber to serve as an olfactory discriminative stimulus signaling ethanol availability for the session. Changes were made over the next 2 weeks that included a gradual increase in the ethanol concentration and the response requirement such that rats were responding for a solution of 10% ethanol mixed in 0.1% saccharin on a fixed ratio schedule of reinforcement (FR2). Self-administration sessions were conducted on the above schedule for approximately 12 days to allow responding to stabilize. A minimum average of 0.4 g/kg ethanol consumption was set as the requirement for the rats to be included in the manipulations described below.
2.4 Ethanol Self-Administration
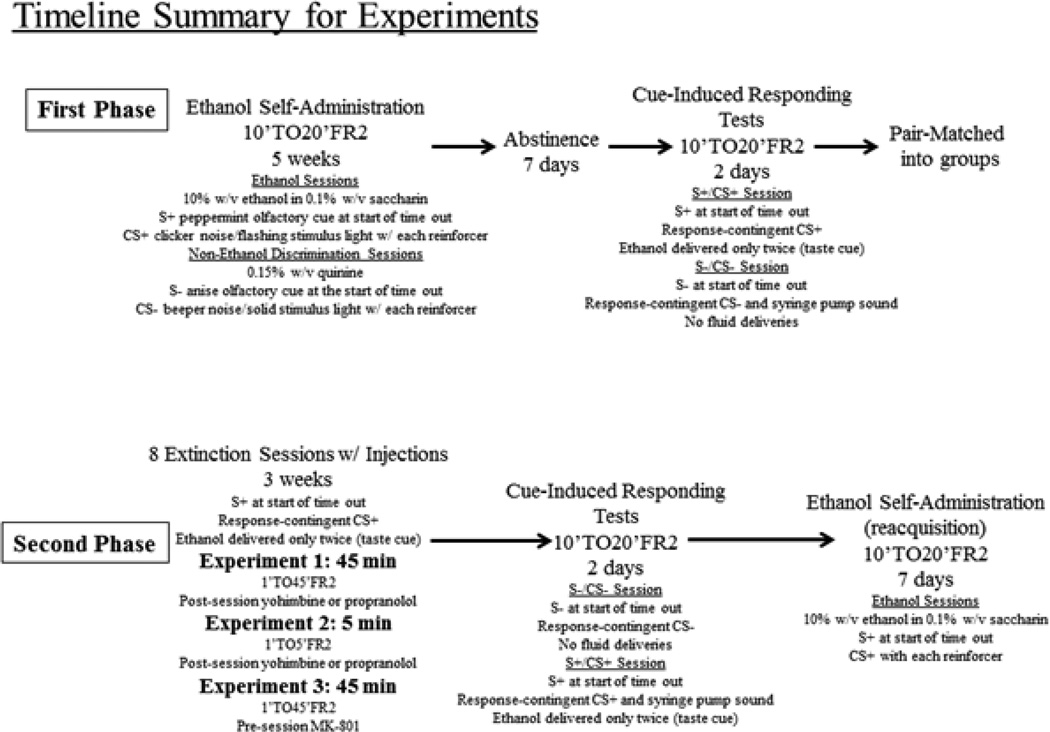
Ethanol self-administration continued for approximately 5 weeks on an FR2 schedule with a 10-min time out followed by a 20-min response period (10’TO20’FR2). The increased time out duration allowed exposure to a distinct olfactory stimulus (S+) that predicted an operant session with ethanol available. During the 20-min response period, the conditioned auditory and visual stimuli (CS+) were exclusively paired with the ethanol delivery. Ten non-ethanol sessions were conducted randomly wherein the ethanol solution was replaced with a bitter-tasting quinine solution (0.15% w/v). The non-ethanol session discriminative stimulus (S−) was 6 drops of anise extract (McCormick, Sparks, MD) delivered into the waste pan at the beginning of the time out. When quinine was delivered during the response period, an intermittent beeper was activated and the stimulus light was constantly illuminated (CS−). The stimuli used in the current experiments during the conditioning phase are similar to the predictive olfactory cues (used as either the S+ or the S−) and response-contingent visual and auditory cues used in other studies (e.g., Backstrom and Hyytia, 2005; Ciccocioppo et al., 2002; 2003; Economidou et al., 2007, Liu and Weiss, 2002). Following these last 5 weeks of ethanol self-administration and discrimination training, the rats remained in their home cages for 7 days with free access to food and water only. This forced abstinence model may better reproduce features in human addiction treatment compared to the more traditional reinstatement model of relapse after extinction (Reichel and Bevins, 2009). Following the 7-day abstinence period, cue-induced ethanol responding was tested by exposing the rats to S+/CS+ cues on the first day and S−/CS− cues on the second day. The cue presentation was not counter-balanced within rat groups because each group of rats was run at the same time each day in the same operant room. Under these circumstances, counter-balancing the olfactory context cues using both extracts in the same room on the same day might allow the rats to detect both olfactory cues at the same time which could affect subsequent responding. All cue-induced responding sessions began at the same time of day as the regular operant self-administration sessions in order to maintain stable operant responding across days. The cue-induced ethanol responding sessions began with the appropriate olfactory cue placed in the pan at the beginning of the time-out period. During the response period, the house light was illuminated and a response on the lever resulted in the presentation of the appropriate auditory stimulus and the visual stimulus. During the S+/CS+ sessions, the first two lever presses resulted in a clicking sound, flashing stimulus light, and an ethanol fluid delivery. The ethanol delivery acted as an additional taste cue, which has been shown to further enhance reinstatement responding (Backstrom and Hyytia, 2005, von der Goltz et al., 2009). The ethanol was not likely to act as a drug primer because the average intake after 2 ethanol deliveries was extremely small (about 0.04 g/kg) and unlikely to produce pharmacological effects. Oral ethanol doses act as a drug primer at doses closer to 0.4 – 0.5 g/kg (Le et al., 1998). After the first two lever presses, only the ethanol-paired auditory and visual stimuli were presented following an FR completion (no ethanol was delivered). During the S−/CS− sessions, a response resulted in the presentation of the quinine-paired stimuli (intermittent beeper and solid stimulus light). To provide an additional S−/CS− auditory stimulus, the syringe pump was also activated following each completed FR (Backstrom and Hyytia, 2005, Williams and Schimmel, 2008). No fluid was delivered during the S−/CS− sessions; the syringe pump was empty. Rats were assigned to groups such that responding was equivalent across groups for the S+/CS+ session. All rats were treated similarly during the above conditions. The timeline for the first phase of the experiments is depicted in the upper panel of Fig 1 and second phase described below is depicted in the lower panel of Fig 1.
Fig. 1.
Diagrams illustrating the timeline of experimental manipulations during Experiment 1 and Experiment 2. The timeline for Experiment 3 was similar to experiment 1 with the exception of the order of cue-induced responding presentation and the drug treatment during the 8-session extinction period and an additional 2 days during the reacquisition period.
2.5 Experiment 1: Repeated, long-duration extinction sessions with adrenergic manipulation
This experiment was designed to expose rats to the ethanol-paired cues during long-duration sessions to stimulate extinction-like processes. Three of the four rat groups (n=7 rats per group) were exposed to S+/CS+ sessions similar to those described above. Time parameters were altered such that each session began with a 1-min time out followed by a longer 45-min response period. Immediately after each session, the rats received intraperitoneal injections of saline, 1.25 mg/kg yohimbine, or 10 mg/kg propranolol. The fourth group of rats (Home-Cage Control) remained in their home cages but were handled and weighed on the same day that the other groups received a S+/CS+ session. These long-duration extinction sessions were conducted 8 times during the next 3 weeks and each session was separated by at least 48 – 72 hours to allow elimination of the drugs between sessions. Twenty-four hours after the final session, all rats (including the Home-Cage Control group) were given another set of cue-induced responding tests during 2 consecutive daily sessions (10’TO20’FR2) that were in place prior to the 8 extinction sessions. For all rats, the cue conditions were presented in the opposite order relative to the tests just after the abstinence period; S−/CS− conditions were presented on the first day and the S+/CS+ conditions were presented on the second day. Rats were allowed to self-administer ethanol for the next 7 days (reacquisition of ethanol self-administration).
2.6 Experiment 2: Repeated, short-duration extinction sessions with adrenergic manipulation
This experiment was designed to expose rats to the ethanol-paired cues during short-duration sessions to stimulate excitatory processes and reconsolidation of the drug-paired cues. Three groups of rats (n=8 rats per group) were exposed to S+/CS+ extinction sessions similar to those described above. The session duration was altered such that each session contained a 5-min response period instead of a 45-min response period. Immediately after each session, the rats received injections of saline, 1.25 mg/kg yohimbine, or 10 mg/kg propranolol. For Experiment 2, the Home-Cage Control group was eliminated to avoid unnecessary replication of the same data received from Experiment 1. The same general procedures described above were used to test cue-induced responding and reacquisition of ethanol self-administration.
2.7 Experiment 3: Repeated, long-duration extinction sessions with MK-801 pretreatment
This experiment was designed to demonstrate that the procedures used in our study were sensitive to manipulation of memory through other pharmacological mechanisms such as blockade of NMDA receptors. Two groups of rats (n=7 rats per group) were exposed to 8 long-duration S+/CS+ extinction sessions similar to those described for the first experiment with injections of saline or 0.1 mg/kg MK-801 given 20 min prior to each session. A third group of rats (n=7) received injections of 0.1 mg/kg MK-801 in the home-cage room in the absence of extinction sessions. With this third experiment, we attempted to address the issue of counterbalancing cue presentation conditions by reversing the order of the cue-induced responding sessions. For example, following the 7-day abstinence period, the S−/CS− cues were tested on the first day and the S+/CS+ cues were tested on the second day. After the 8 extinction sessions, the S+/CS+ cues were tested on the first day and the S−/CS− cues were tested on the second day.
2.8 Drugs
Ethanol solutions were prepared by mixing appropriate volumes of 95% w/v ethanol (Pharmco Products Inc., Brookfield, CT) and deionized water. Ethanol solutions were sweetened by adding 0.1% w/v sodium saccharin (Sigma Aldrich, St. Louis, MO). Sucrose solution (20% w/v) was made by dissolving granulated cane sugar in deionized water. Quinine solution (0.15% w/v) was made by dissolving quinine hydrochloride (Sigma Aldrich, St. Louis, MO) in deionized water. Yohimbine HCl, Propranolol HCl, and (+)-MK-801 hydrogen maleate (Sigma Aldrich, St. Louis, MO) were dissolved in 0.9% saline. The injections were given as intraperitoneal injections each in a fluid volume of 1 ml/kg.
2.9 Data Analysis
For Experiment 1, an initial pair-wise sort was conducted using the average number of responses during the S+/CS+ session on the day after the 7-day abstinence period. A one-way ANOVA was applied to confirm that the average responding across groups was not significantly different. The same analysis was applied to the average number of responses for ethanol for the 3 days just prior to the 7-day abstinence period to ensure that groups were similar across multiple measures prior to experimental manipulation. The average of the last 3 days was used to represent the most accurate estimate of current responding just prior to the 7-day abstinence. The significance level for all tests was set at p < 0.05 and all data are displayed as the mean ± standard error (SEM). To show that the rats could discriminate between the ethanol cues and the non-ethanol cues, a two-way mixed model ANOVA was applied to the data analyzing “treatment” (saline, yohimbine, propranolol, home-cage) and “cue type” (S+/CS+ vs. S−/CS−). If the F values were statistically significant, a Tukey’s HSD test was applied as a post-hoc test for this and all other ANOVAs. To determine if the post-session injections had an effect on responding during the 8 extinction sessions, a two-way mixed model ANOVA was used analyzing “treatment” (saline, yohimbine, propranolol) and “days” (8 extinction sessions). In addition, a one-way ANOVA was used to analyze the average number of responses during the last 3 sessions of the 8-session treatment period. The average of the last 3 sessions was used because responding seemed to stabilize after approximately 5 sessions.
To test the effect of the 8-session extinction treatment on subsequent ethanol cue-induced responding a two-way repeated measures mixed model ANOVA was applied to the data analyzing “treatment” and “time” (S+/CS+ prior to extinction session and S+/CS+ after extinction sessions). Because the interaction effect was significant, we conducted repeated two-tailed t-tests with Bonferroni Correction for planned between-group comparisons of responding during the S+/CS+ session after the 8-session treatment period in addition to planned within-group comparisons of responding before vs. after the 8-session treatment. A similar analysis was used to determine if the rats continued to discriminate between the S+/CS+ and the S−/CS− (“cue type”).
To test the effect of 8-session extinction treatment on subsequent responding during reacquisition of ethanol self-administration, a two-way mixed model ANOVA was applied analyzing “treatment” and “time” (7 days of reacquisition). If the interaction effect was significant, then we conducted repeated two-tailed t-tests with Bonferroni Correction for planned comparisons. The 7-day average responding during reacquisition was analyzed across groups using a one-way ANOVA.
For Experiment 2 and 3, the data were handled in the same manner described above and the same statistical tests were applied to the corresponding data.
3. Results
3.1 Experiment 1: Repeated, long-duration extinction sessions with adrenergic manipulation
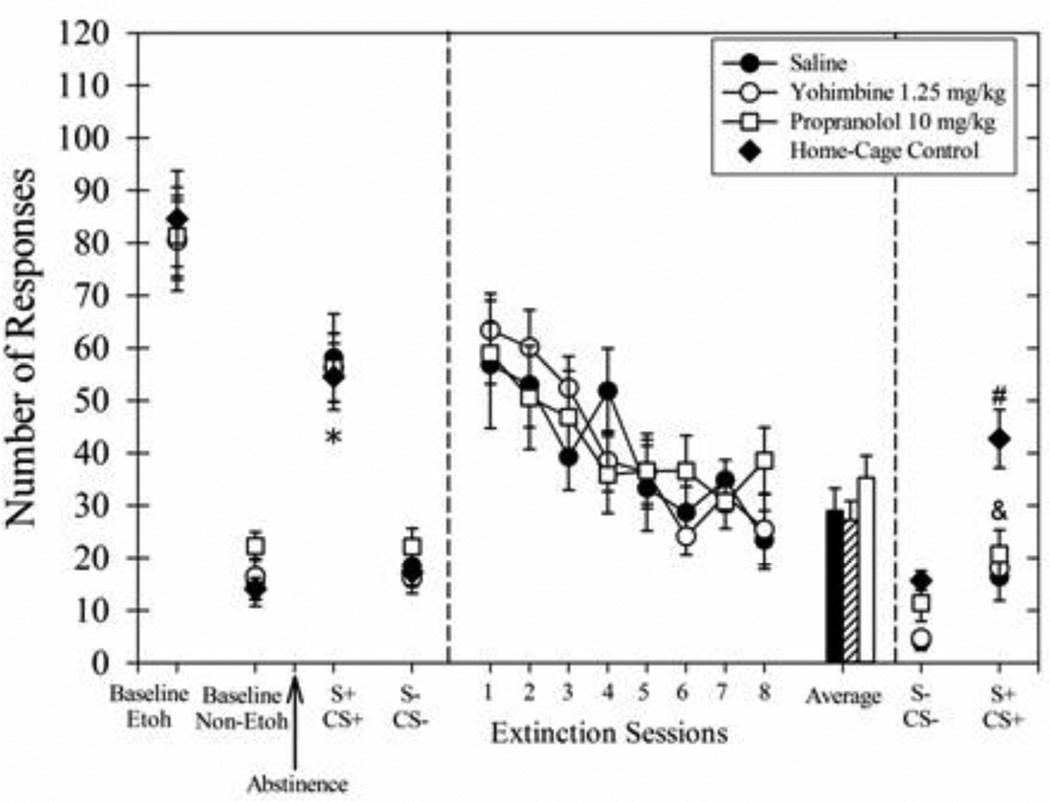
The baseline number of responses during the last 3 days of ethanol self-administration is shown in Fig 2 (left panel) which resulted in ethanol intakes of 0.93 ± 0.12 g/kg for the Saline group, 0.88 ± 0.09 g/kg for the Yohimbine group, 0.89 ± 0.07 g/kg for the Propranolol group and 0.91 ± 0.09 g/kg for the Home-Cage Control group. The average number of responses for ethanol was similar across all treatment groups. Responding during the S+/CS+ test session was greater than responding during the S−/CS− test session for all groups which demonstrates that the rats were discriminating between these stimulus conditions (main effect of cue type, F(1,24) = 142.23, p<0.001).
Fig. 2.
Average number of responses during multiple phases of Experiment 1 wherein long extinction sessions (45 min) were followed by post-session intraperitoneal injections of saline (filled circles), 1.25 mg/kg yohimbine (open circles), or 10 mg/kg propranolol (open squares). The home-cage group (filled diamonds) is also shown where appropriate. The left panel shows the average responding during the last 3 days of baseline ethanol (Etoh) and discrimination (Non-Etoh) days. The arrow indicates the 7-day abstinence period. To the right of the arrow is the responding during consecutive cue-induced responding sessions with Etoh cues (S+/CS+) and Non-Etoh cues (S−/CS−). The middle panel shows the responding during the 8 long-duration extinction sessions and the average of the last 3 cue extinction sessions for the rats receiving post-session saline (solid black bar), yohimbine (lined bar), or propranolol (solid white bar) immediately after each 45 min session. The symbols in the far right panel show the responding during cue-induced responding sessions on consecutive days after the 8-session extinction treatment sessions. Data for all groups (n=7) are shown as means ± SEM. *Indicates responding for all groups during S+/CS+ session greater than responding during S−/CS− session before the 8-session extinction treatment (p<0.05). #Indicates responding for home-cage group during final S+/CS+ session greater than responding for all other groups during the same session (p<0.05). &Indicates responding for all groups during final S+/CS+ session greater than responding during final S−/CS− session after the extinction treatment (p<0.05).
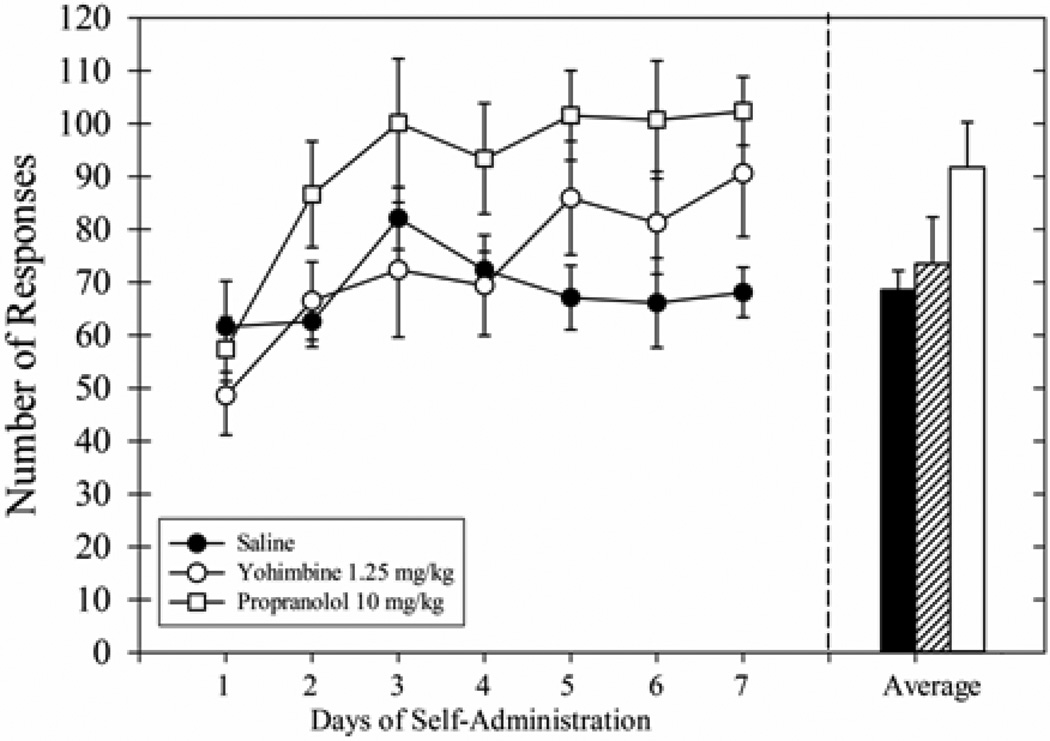
During the 8 extinction sessions (Fig 2, middle panel), responding was similar across groups (no main effect of treatment). Responding for all groups decreased across treatment (main effect of days, F(7,126) = 13.21, p<0.001) but the interaction effect was not significant. The responding appeared to stabilize during the last 3 sessions and the average of the responding during the last 3 sessions was similar across groups (no main effect of treatment).
After the 8-session extinction treatment period, ethanol cue-induced responding was tested again (Fig 2, right panel). The drug injections during the treatment period failed to differentially affect responding during ethanol cue-induced responding sessions. Responding during the final S+/CS+ session was not significantly different from responding during the S+/CS+ session prior to the cue exposure treatment [F(3,24) = 1.44, p=0.26]. However, the long extinction sessions decreased cue-induced responding across the treatment groups. Both the main effect of time [F(1,24) = 78.28, p<0.001] and interaction effect were significant [F(3,24) = 3.55, p<0.05]. Post-hoc tests revealed that the 3 extinction treatment groups decreased responding during the final S+/CS+ session (p<0.05). In contrast, rats that remained in their home cages during the treatment period emitted a similar number of responses during both the initial S+/CS+ session and the final S+/CS+ session and their responding during the final S+/CS+ session was higher than the responding of all other groups during this session (p<0.05). All rats continued to discriminate between the S−/CS− cues and the S+/CS+ cues presented on the following day; when comparing responding during these 2 sessions, we found a main effect of treatment [F(3,24) = 8.29, p<0.001], cue type [F(1,24) = 80.71, p<0.001], and an interaction effect [F(3,24) = 5.19, p<0.01]. All groups responded significantly more during the final S+/CS+ session compared to the within-group responding during the final S−/CS− session (p<0.05) even though the absolute number of responses was relatively low.
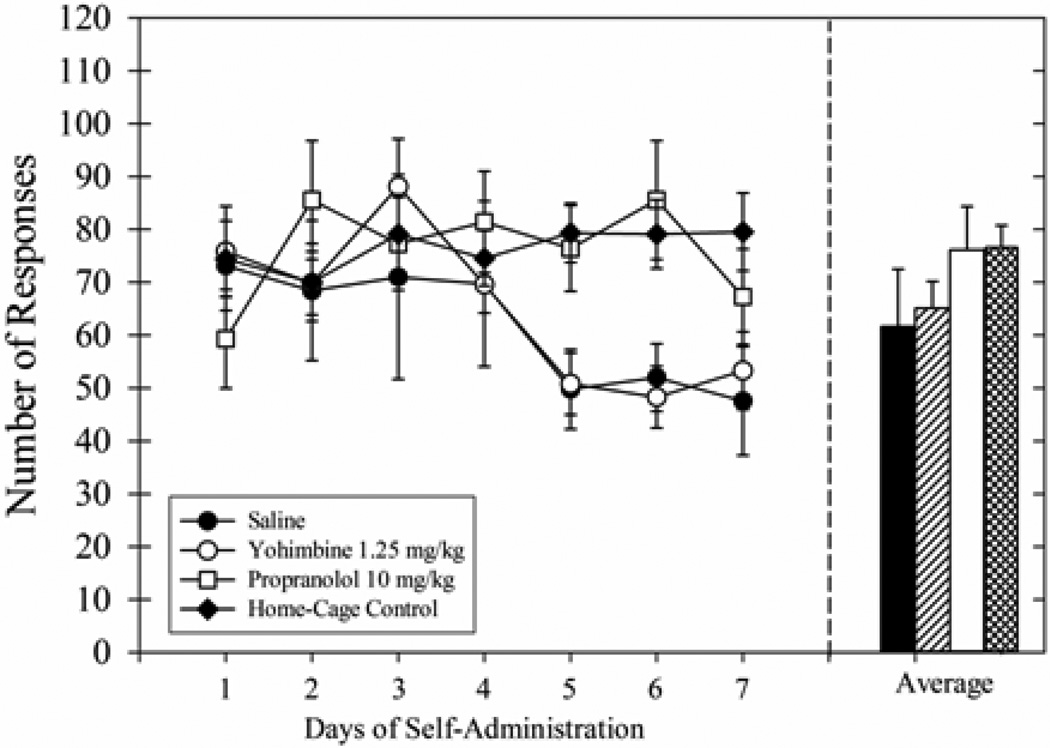
Operant ethanol self-administration was examined after the cue-induced responding sessions. Responding was stable across the 7 days for the Home-Cage Control group and the Propranolol group (Fig 3, right panel). However, responding appeared to decrease during the last 3 days for the Saline group and the Yohimbine group. Although the main effect of group was not significant, there were significant effects for time [F(6,144) = 4.48, p<0.001] and the interaction [F(18,144) = 3.15, p<0.001]. The post-hoc tests with Bonferroni Correction failed to show significant effects for planned comparisons between groups (e.g., Saline group vs. Home-Cage Control group). The 7-day average of ethanol responding (Fig 3, right panel) was not significantly different across groups. Overall, the repeated, long extinction sessions failed to decrease ethanol self-administration and the post-session injections had no subsequent effects on responding for ethanol.
Fig. 3.
Average number of responses during the 7-day period of reacquisition of ethanol-reinforced responding (left panel) for Experiment 1. These rats previously received injections of saline (filled circles), 1.25 mg/kg yohimbine (open circles), or 10 mg/kg propranolol (open squares) following 45 min extinction sessions. The home-cage group (filled diamonds) is also shown. In the right panel, the average number of responses for all 7 days of ethanol self-administration sessions is shown for the same groups (saline = solid black bar, 1.25 mg/kg yohimbine = lined bar, 10 mg/kg propranolol = solid white bar, home-cage rats = cross-hatched bar). Data for all groups (n=7) are shown as means ± SEM.
3.2 Experiment 2: Repeated, short-duration extinction sessions with adrenergic manipulation
The baseline number of responses during the last 3 days of ethanol self-administration is shown in Fig 4 (left panel) which resulted in ethanol intakes of 0.92 ± 0.06 g/kg for the Saline group, 0.87 ± 0.09 g/kg for the Yohimbine group, and 0.85 ± 0.08 g/kg for the Propranolol group. In Experiment 2, the average number of responses for ethanol was similar across the groups. Responding during the S+/CS+ test session was greater than responding during the S−/CS− test session for all groups which shows that the rats were discriminating between stimulus conditions (main effect of cue type, F(1,21) = 31.58, p<0.001).
Fig. 4.
Average number of responses during multiple phases of Experiment 2 wherein short-duration extinction sessions (5 min) were followed by post-session intraperitoneal injections of saline (filled circles), 1.25 mg/kg yohimbine (open circles), or 10 mg/kg propranolol (open squares). The left panel shows the average responding during the last 3 days of baseline ethanol (Etoh) and discrimination (Non- Etoh) days. The arrow indicates the 7-day abstinence period. To the right of the arrow is the responding during consecutive cue-induced responding sessions with Etoh cues (S+/CS+) and Non-Etoh cues (S−/CS−). The middle panel shows the responding during the 8 short-duration extinction sessions and the average of the last 3 extinction sessions for the rats receiving post-session saline (solid black bar), yohimbine (lined bar), and propranolol (solid white bar) immediately after each 5 min session. The symbols in the far right panel show the responding during cue-induced responding sessions on consecutive days after the cue exposure/injection sessions. Data for all groups (n=8) are shown as means ± SEM. *Indicates responding for all groups during S+/CS+ sessions greater than responding during S−/CS− sessions before the 8-session extinction treatment (p<0.05). &Indicates responding for all groups during final S+/CS+ session greater than responding during final S−/CS− session after the extinction treatment (p<0.05).
During the 8 extinction sessions (Fig 4, middle panel), the responding was similar across groups (no main effect of treatment). However, responding for all groups decreased across the treatment even though each session lasted only 5 min (main effect of days, F(7,147) = 11.69, p<0.001). The interaction effect was not significant. The average responding during the last 3 sessions was similar across groups (no main effect of treatment).
After the 8-session extinction treatment period, ethanol cue-induced responding was tested again (Fig 4, right panel). The conditions during the treatment period failed to differentially affect responding during cue-induced responding. Responding during the final S+/CS+ session was not significantly different from responding during the S+/CS+ session prior to the cue exposure treatment [F(2,21) = 0.07, p=0.93]. However, the short-duration extinction treatment sessions decreased cue-conditioned reinstatement for all groups. The main effect of time was significant [F(1,24) = 78.28, p<0.001] but the interaction effect was not significant. Even though all groups decreased responding during the S+/CS+ session and responding was similar across groups (no main effect of treatment), all rats responded differentially in the presence of the S−/CS− cues and the S+/CS+ cues presented on the following day (main effect of cue type [F(1,21) = 4.93, p<0.05]). All groups responded significantly more during the final S+/CS+ session compared to the within-group responding during the final S−/CS− session (p<0.05). However, planned post-hoc tests were not conducted because the interaction effect was not significant [F(2,21) = 0.68, p=.52].
Operant ethanol self-administration was examined for several days after the cue-induced responding sessions. Responding was variable across groups (Fig 5, left panel). Although responding for the Saline group was stable across the entire reacquisition period, responding for the Yohimbine and Propranolol groups appeared to increase during the first few days and stabilize around day 5. Both the Yohimbine and Propranolol groups nearly doubled average responding within the first few days before reaching stability on days 5–7. Although the main effect of treatment was only trending toward significance [F(2,21) = 2.92, p=0.08], there were significant effects for time [F(6,126) = 13.78, p<0.001] and an interaction effect [F(12,126) = 3.15, p<0.005]. The post-hoc tests with Bonferroni Correction showed that the responding for Propranolol group was significantly greater than responding for the Saline group (p<0.05). The 7-day average of ethanol responding (Fig 5, right panel) was not significantly different across groups. Overall, the repeated, short extinction sessions had no effect on ethanol self-administration and the post-session injections failed to differentially alter subsequent responding for ethanol.
Fig. 5.
Average number of responses during the 7-day period of reacquisition of ethanol-reinforced responding (left panel) for Experiment 2. These rats previously received injections of saline (filled circles), 1.25 mg/kg yohimbine (open circles), or 10 mg/kg propranolol (open squares) following 5 min extinction sessions. In the right panel, the average number of responses for all 7 days of ethanol selfadministration sessions is shown for the same groups (saline = solid black bar, 1.25 mg/kg yohimbine = lined bar, 10 mg/kg propranolol = solid white bar). Data for all groups (n=8) are shown as means ± SEM.
3.3 Experiment 3: Repeated, long-duration extinction sessions with MK-801 pretreatment
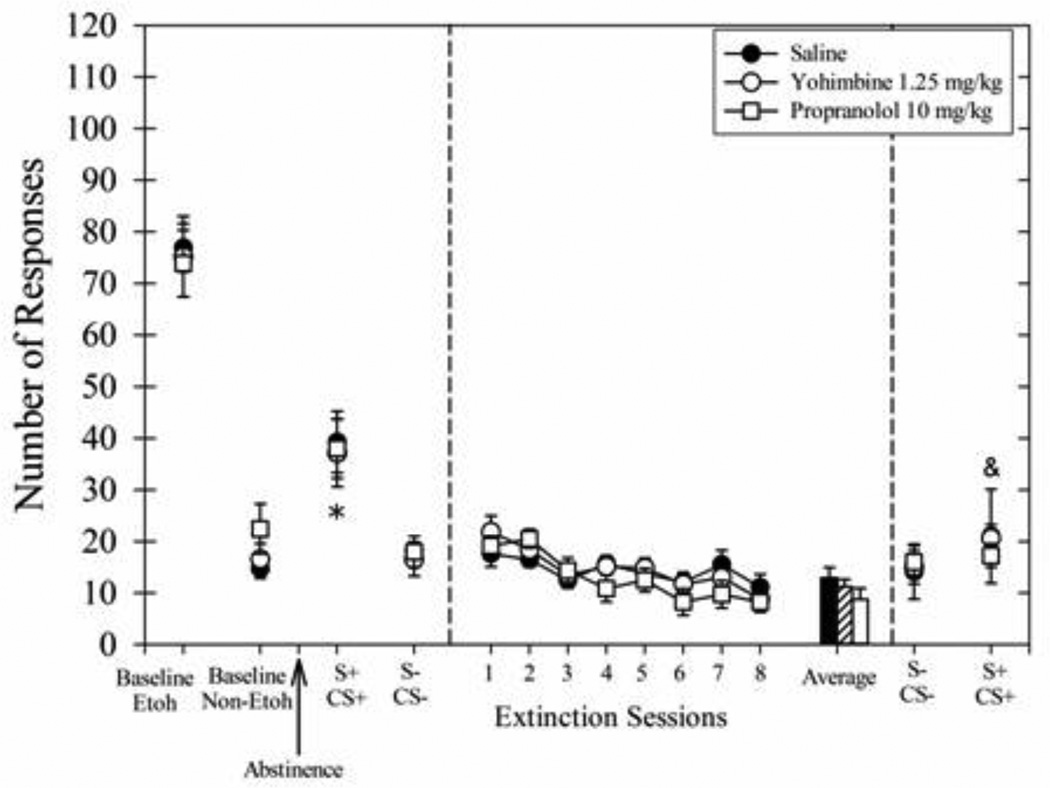
The baseline number of responses during the last 3 days of ethanol self-administration is shown in Fig 6 (left panel) which resulted in ethanol intakes of 0.74 ± 0.12 g/kg for the Saline group, 0.76 ± 0.13 g/kg for the MK-801 group, and 0.81 ± 0.15 g/kg for the Home-Cage MK-801 group. In Experiment 3, the average number of responses for ethanol was similar across groups. Responding during the S+/CS+ session was greater than responding during the S−/CS− session for all groups which shows that the rats were discriminating between stimulus conditions (main effect of cue type, F(1,18) = 45.53, p<0.001).
Fig. 6.
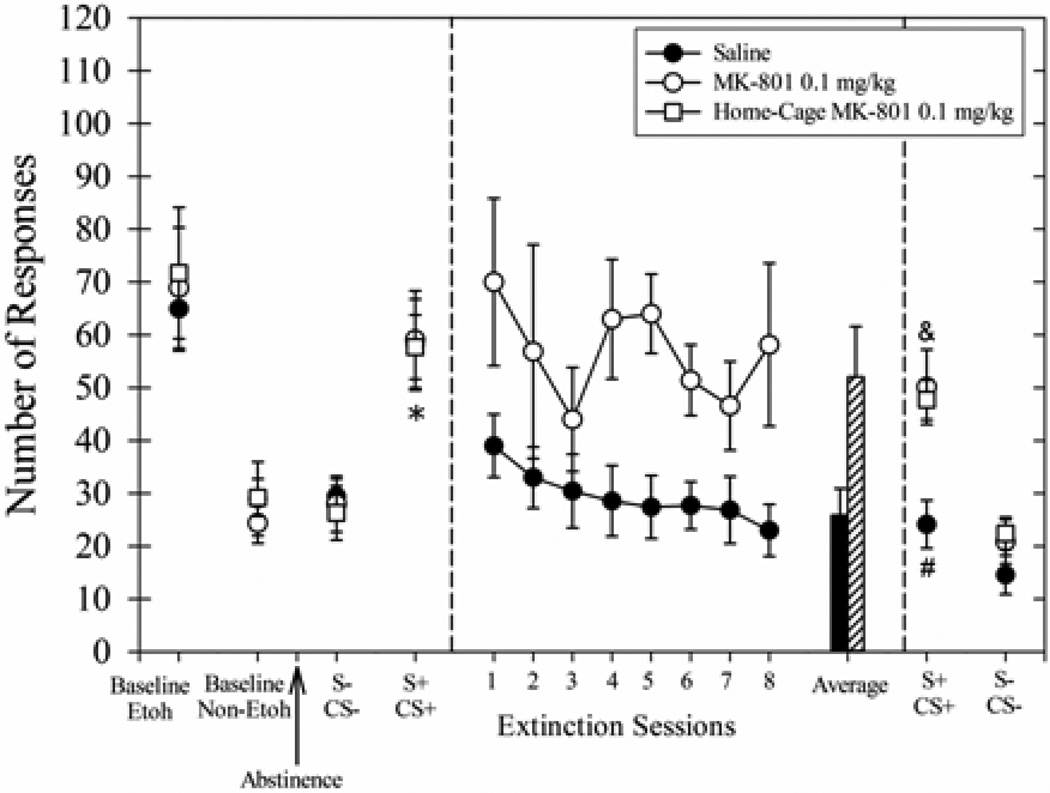
Average number of responses during multiple phases of Experiment 3 wherein long-duration extinction sessions (45 min) were preceded by 20 min pretreatment injections of saline (filled circles) or 0.1 mg/kg MK-801 (open circles). The home-cage group (open boxes) was not exposed to the extinction sessions but received injections of 0.1 mg/kg MK-801 and returned to the home-cage. The left panel shows the average responding during the last 3 days of baseline ethanol (Etoh) and discrimination (Non-Etoh) days. The arrow indicates the 7-day abstinence period. To the right of the arrow is the responding during consecutive cue-induced responding sessions with Non-Etoh cues (S−/CS−) and Etoh cues (S+/CS+). The middle panel shows the responding during the 8 long-duration extinction sessions and the average of the last 3 cue extinction sessions for the rats receiving saline (solid black bar) or MK-801 (lined bar) 20 min prior to the session. The symbols in the far right panel show the responding during cue-induced responding sessions on consecutive days after the 8-session extinction treatment sessions. Data for all groups (n=7) are shown as means ± SEM. *Indicates responding for all groups during S+/CS+ session greater than responding during S−/CS− session before the 8-session extinction treatment (p<0.05). #Indicates responding for MK-801 group and the home-cage MK-801 group during final S+/CS+ session greater than responding for saline group during the same session (p<0.05). &Indicates responding MK-801 group and the home-cage MK-801 group during final S+/CS+ session greater than responding during final S−/CS− session after the extinction treatment (p<0.05).
During the 8 long-duration extinction sessions (Fig 6, middle panel), the group receiving MK-801 pretreatments showed greater responding than the Saline group (main effect of treatment, F(1,12) = 5.40, p<0.05). The main effect of days was trending toward significance [F(7, 84) = 2.02, p=0.06)] which may have been affected by the lack of a systematic effect of MK-801 across treatment days. The interaction effect was not significant. The average responding during the last 3 sessions was greater for the MK-801 group as compared to the Saline group [t(12) = −2.44, p<0.05]. Hence, injections of 0.1 mg/kg MK-801 increased responding when given 20 min prior to long extinction sessions.
After the 8-session extinction treatment period, ethanol cue-induced responding was tested (Fig 6, right panel). Treatment conditions differentially affected responding during the ethanol cue-induced responding test. A comparison of the responding during the final S+/CS+ session to the responding during the S+/CS+ session prior to the cue exposure treatment showed a significant main effect of treatment [F(2,18) = 5.03, p<0.05], time [F(1,18) = 82.74, p<0.001], and a significant interaction effect [F(2,18) = 6.55, p<0.0.01]. The Saline group decreased responding during the final S+/CS+ session as compared to the initial S+/CS+ session (p<0.01). This finding is consistent with the evidence from Experiment 1 indicating that the repeated, long-duration extinction sessions decreased the effectiveness of the S+/CS+ to increase responding. Other planned post-hoc tests indicated that responding for both MK-801 groups was similar during the final S+/CS+ session and the initial S+/CS+ session prior to the extinction sessions (p<0.05). Responding for both MK-801 groups during the final S+/CS+ session was higher than the responding for the Saline group during this session (p<0.05).
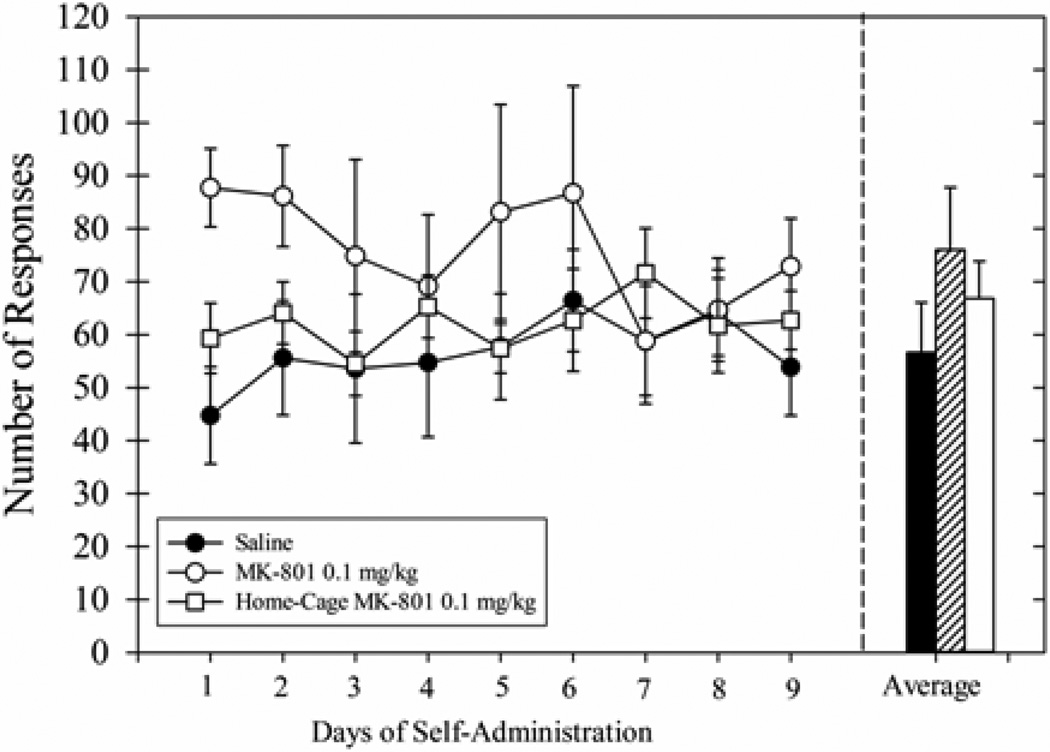
Operant ethanol self-administration was examined during the several days after the cue-induced responding sessions. Responding was variable across groups (Fig 7, left panel). Although responding for the Saline group was relatively stable across the entire reacquisition period, responding for the group that received MK-801 injections prior to extinction sessions appeared to increase during the first few days. The average responding for this group during the first day of re-exposure to ethanol self-administration was twice that for the Saline group. However, the responding across groups was similar by the third day of the 9-day period. The statistical analyses indicated the main effects for treatment and time were not significant, the interaction effect was trending toward significance [F(16,144) = 1.53, p=0.10]. The 9-day average of ethanol responding (Fig 7, right panel) was not significantly different across groups. Consistent with the data from Experiment 1, these data show that the repeated, long-duration extinction sessions failed to decrease ethanol self-administration.
Fig. 7.
Average number of responses during the 9-day period of reacquisition of ethanol-reinforced responding (left panel) for Experiment 3 wherein long-duration extinction sessions (45 min) were preceded by intraperitoneal injections of saline (filled circles) or 0.1 mg/kg MK-801(open circles). The group that received MK-801 in the home-cage (open squares) is also shown. In the right panel, the average number of responses for all 9 days of ethanol self-administration sessions is shown for the same groups (saline = solid black bar, 0.1 mg/kg MK-801 = lined bar, home-cage 0.1 mg/kg MK-801 = solid white bar). Data for all groups (n=7) are shown as means ± SEM.
4. Discussion
The main findings of these experiments show that cue exposure during extinction sessions of long or short duration failed to interact with post-session adrenergic manipulations via yohimbine and propranolol to influence ethanol cue-induced responding or subsequent reacquisition of ethanol self-administration. MK-801 blocked the effect of long extinction sessions on cue-induced responding but had no effect on self-administration. Overall, the data suggest that manipulation of the NMDA system in combination with alcohol cue exposure therapy during extinction-like sessions may be more effective than manipulation of the adrenergic system in reducing the strength of alcohol-cue associations in this specific model of alcohol relapse.
Our main hypothesis was that cue exposure during long extinction sessions would stimulate the neurochemical mechanisms associated with extinction-like processes (consolidation) while cue exposure during short sessions would stimulate mechanisms associated with activational processes (reconsolidation). If adrenergic activity is required for this consolidation, then elevating adrenergic activity with post-session yohimbine should further stimulate these processes while blocking adrenergic activity with propranolol should attenuate the neurochemical processes involved in consolidation. Contrary to our hypotheses, ethanol cue-induced responding was not altered by cue exposure during long or short extinction sessions. One possible explanation for the general lack of effects is that the drug doses chosen were inactive. This explanation is unlikely since pre-session injections of the same yohimbine dose dramatically increased reinstatement responding for food (Nair et al., 2006), heroin (Banna et al., 2010) and ethanol (Le et al., 2005) in rats. Propranolol, in our study, was administered in the same dose, route, and post-session timing that disrupted cocaine CPP (Bernardi et al., 2006) and fear conditioning (Debiec and LeDoux, 2004). A second possible explanation is that the extinction-like processes stimulated by the 8 sessions may have dominated the effects on behavior regardless of adrenergic postsession treatment. For example, all groups in Experiment 1 emitted a substantial number of unreinforced responses. These unreinforced responses may have stimulated extinction-like processes that were too powerful for post-session drug manipulations to modify. However, the duration of time spent in 8 extinction-like sessions and the amount of unreinforced responses emitted during the longer sessions in Experiment 1 did not appear to have a greater extinction-like effect on cue-induced responding compared to shorter sessions in Experiment 2. Although the amount of responding was similar during long and short extinction sessions, we expected the duration of session to be especially important. In the context of conditioning, the term “reactivation” has been used to refer to extinction sessions that elicit the processes of reconsolidation for sessions lasting 5 min (von der Goltz et al., 2009). A slightly longer 30 min session may serve as an “extinction” trial (Janak and Corbit, 2011; LaLumiere et al., 2010). Although the boundary between reactivation (reconsolidation) and extinction (consolidation) may depend upon session duration (Tronson and Taylor, 2007), that boundary may change for different reinforcers (e.g., cocaine, sucrose, ethanol) and different paradigms (e.g., CPP, operant). The third, and most plausible, explanation for our findings is that adrenergic processes may not modulate the associative processes linking ethanol and ethanol-associated cues. This explanation is consistent with published literature. For example, the role of the adrenergic system in ethanol CPP has been explored using post-session injections of propranolol (Font and Cunningham, 2012). In that study, a single post-retrieval injection of propranolol following a reactivation session failed to affect reconsolidation and that repeated post-retrieval injections of propranolol failed to alter the time course of extinction (consolidation). Together, our results suggest that the adrenergic system is not involved in consolidation or reconsolidation regarding ethanol and ethanol-paired cues.
Reacquisition of ethanol self-administration was not systematically affected by extinction session duration alone or when combined with post-session adrenergic treatment. We hypothesized that the long extinction sessions experienced by the Saline group in Experiment 1 would decrease reacquisition responding below that of the Home-Cage Control group. We also hypothesized that post-session adrenergic manipulation would alter consolidation of new learning during extinction and drive responding during reacquisition up or down depending upon the post-session drug administered. However, the pattern of responding during reacquisition failed to support these predictions. The reacquisition responding during Experiment 2 was equally uninformative; responding appeared to increase across the reacquisition period regardless of drug experience.
The role of adrenergic mechanisms in memory is complex and may be involved in more emotionally arousing memories. The effects of propranolol and yohimbine on memory are often observed in fear conditioning paradigms and may involve the amygdala (e.g., Cain et al., 2004; Debiec and LeDoux, 2004). The drug effects on extinction may differ based on the arousal-inducing properties of the paradigm. For example, yohimbine failed to affect extinction of a cocaine CPP (Davis et al., 2008), but it facilitated the extinction of fear conditioning (Morris and Bouton, 2007). The emotional arousal during drug cue-association paradigms like CPP or cue-induced responding may be relatively weak compared to arousal during fear conditioning. Drug-paired memories may not trigger the glucocorticoid release that interacts synergistically with adrenaline to enhance memory processes (Roozendaal et al., 2006). The rats in our experiments were drinking moderate amounts of ethanol during short operant sessions. However, the intake levels were not enough to produce dependence and thus, the emotional arousal or amygdala activation associated with ethanol withdrawal and the aversive properties of ethanol were not likely to interact with adrenergic drug manipulation. To our knowledge, ethanol-seeking behavior and post-session propranolol has been explored in only one other study (Wouda et al., 2010). They used a paradigm very similar to the current experiments and found that repeated post-session propranolol injections decreased ethanol seeking during a subsequent test session. Importantly, our experiments extended the number of propranolol treatments and examined the interaction of session duration but we found no effect of repeated post-session propranolol on ethanol-seeking behavior. Other ethanol-seeking paradigms may be more useful for examining the utility of drugs targeting the adrenergic system. For example, exposure to stress induces ethanol-seeking behavior in rats (Le et al. 1998) and more recent evidence indicates that neurobiological stress systems involving corticotropin-releasing factor mediate the effects of adrenergic compounds such as yohimbine on ethanol-seeking behavior (Le et al., 2013). Thus, the cue-induced reinstatement used in the current experiments may not be as sensitive to adrenergic manipulation compared to other reinstatement procedures such as those involving stress.
The final experiment with the NMDA antagonist MK-801 demonstrated that our experimental procedures were sensitive to manipulation of other neurobiological systems involved in memory processes. In this experiment, 0.1 mg/kg MK-801 was given prior to long extinction sessions. MK-801 allowed responding to remain high during a subsequent cue-induced responding test. MK-801 may have been interfering with the acquisition of new memory formation during the extinction sessions. NMDA receptor antagonists have been shown to impair memory in animals tested in a variety of tasks (for review, see Castellano et al., 2001). The drug dose used in our experiment, 0.1 mg/kg, has been suggested to act as a “cognitive impairer” without causing sensory, locomotor, or tocixcological side effects in rats and appears to be effective when given 10 – 30 min prior to the task (van der Staay et al., 2011). This same drug dose was shown to impair effects of reactivation in a CPP paradigm (Sadler et al., 2007) and attenuate reconsdolidation of a CS-ethanol memory in a Pavlovian Intsrumental Transfer paradigm (Milton et al., 2012). Our findings are congruent with these published reports. Thus, pharmacological tools such as MK-801 may be more useful than adrenergic compounds in experiments designed to alter ethanol drug-cue associations and subsequent ethanol responding.
Overall, our findings indicate that cue exposure during extinction sessions of long or short duration failed to interact with post-session adrenergic manipulations to alter cue-induced responding for ethanol or ethanol self-administration in beneficial directions. This finding indicates that this approach may not be useful as an adjunct in cue exposure or extinction therapy for treating alcoholism. These findings provide beneficial information regarding experimental conditions in which post-session adrenergic manipulation is ineffective and highlight an alternative neurobiological mechanism the potential of investigating NMDA mechanisms using our paradigm. This basic experimental design may be expanded upon for further exploration as an animal model of cue exposure or extinction treatment.
Highlights.
Long- and short-duration extinction sessions decreased cue-induced responding
Adrenergic manipulation during extinction did not alter cue-induced responding
Repeated extinction sessions did not affect reacquisition of responding for alcohol
MK-801 blocked the effect of extinction sessions on cue-induced responding
NMDA antagonists may be better than adrenergic drugs at enhancing exposure therapy
Acknowledgments
This study was supported by grant 7 R15 AA015147-01 from the National Institute on Alcohol Abuse and Alcoholism. We thank Julie Broadbent, Gail Winger, and Jim Woods for feedback on the experimental design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Blakey R, Baker R. An exposure approach to alcohol abuse. Behav Res Ther. 1980;18:319–325. doi: 10.1016/0005-7967(80)90090-x. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C, Cestari V, Ciamei A. NMDA receptors and learning and memory processes. Curr Drug Targets. 2001;2:273–283. doi: 10.2174/1389450013348515. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of m1 or d opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Davis AR, Shields AD, Brigman JL, Norcross M, McElligott ZA, Holmes A, et al. Yohimbine impairs extinction of cocaine-conditioned place preference in an alpha2-adrenergic receptor independent process. Learn Mem. 2008;15:667–676. doi: 10.1101/lm.1079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J Consult Clin Psychol. 1994;62:809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Ubaldi M, Lourdusamy A, Soverchia L, Hardiman G, et al. Role of cannabinoidergic mechanisms in ethanol self-administration and ethanol seeking in rat adult offspring following perinatal exposure to Delta9-tetrahydrocannabinol. Toxicol Appl Pharmacol. 2007;223:73–85. doi: 10.1016/j.taap.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Font L, Cunningham CL. Post-retrieval propranolol treatment does not modulate reconsolidation or extinction of ethanol-induced conditioned place preference. Pharmacol Biochem Behav. 2012;101:222–230. doi: 10.1016/j.pbb.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Greeley J. Cue exposure in the treatment of drug dependence: the potential of a new method for preventing relapse. Drug Alcohol Rev. 1990;9:155–168. doi: 10.1080/09595239000185211. [DOI] [PubMed] [Google Scholar]
- Janak PH, Corbit LH. Deepened extinction following compound stimulus presentation: noradrenergic modulation. Learn Mem. 2011;18:1–10. doi: 10.1101/lm.1923211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement in alcohol seeking rats. Addiction Biol. 2013;18:488–451. doi: 10.1111/j.1369-1600.2011.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Pscyhopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learn Mem. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- Milton AL, Schramm MJ, Wawrzynski JR, Gore F, Oikonomou-Mpegeti F, Wang NQ, et al. Antagonism at NMDA receptors, but not beta-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology (Berl) 2012;219:751–761. doi: 10.1007/s00213-011-2399-9. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gray SM, Ghitza UE. Role of food type in yohimbine- and pellet-priming-induced reinstatement of food seeking. Physiol Behav. 2006;88:559–566. doi: 10.1016/j.physbeh.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering C, Liljequist S. Cue-induced behavioural activation: a novel model of alcohol craving? Psychopharmacology (Berl) 2003;168:307–313. doi: 10.1007/s00213-003-1454-6. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Fertig J, Baker L, Cooney N. Reactivity to alcohol cues in alcoholics and non-alcoholics: implications for a stimulus control analysis of drinking. Addict Behav. 1983;8:1–10. doi: 10.1016/0306-4603(83)90048-5. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JA, Otto MW, Sanders C, Emmelkamp PM. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. J Anxiety Disord. 2009;23:350–356. doi: 10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin H, Hodgson R, Stockwell T. Cue exposure and response prevention with alcoholics: a controlled trial. Behav Res Ther. 1983;21:435–446. doi: 10.1016/0005-7967(83)90013-x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Central but not peripheral beta-adrenergic antagonism blocks reconsolidation for a morphine place preference. Behav Brain Res. 2007;182:129–134. doi: 10.1016/j.bbr.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Reconsolidation of a morphine place preference: impact of the strength and age of memory on disruption by propranolol and midazolam. Behav Brain Res. 2010;213:201–207. doi: 10.1016/j.bbr.2010.04.056. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, et al. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96:1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, et al. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler R, Herzig V, Schmidt WJ. Repeated treatment with the NMDA antagonist MK-801 disrupts reconsolidation of memory for amphetamine-conditioned place preference. Behav Pharmacol. 2007;18:699–703. doi: 10.1097/FBP.0b013e3282effb81. [DOI] [PubMed] [Google Scholar]
- Sitharthan T, Sitharthan G, Hough MJ, Kavanagh DJ. Cue exposure in moderation drinking: a comparison with cognitive-behavior therapy. J Consult Clin Psychol. 1997;65:878–882. doi: 10.1037//0022-006x.65.5.878. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Noradrenergic enhancement of associative fear memory in humans. Neurobiol Learn Mem. 2011;96:263–271. doi: 10.1016/j.nlm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Turkkan JS, McCaul ME, Stitzer ML. Psychophysiological effects of alcohol-related stimuli: II. Enhancement with alcohol availability. Alcohol Clin Exp Res. 1989;13:392–398. doi: 10.1111/j.1530-0277.1989.tb00341.x. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res. 2011;220:215–229. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, et al. Cue-induced alcohol-seeking behaviour is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology (Berl) 2009;205:389–397. doi: 10.1007/s00213-009-1544-1. [DOI] [PubMed] [Google Scholar]
- Wouda JA, Diergaarde L, Riga D, van Mourik Y, Schoffelmeer AN, De Vries TJ. Disruption of Long-Term Alcohol-Related Memory Reconsolidation: Role of beta-Adrenoceptors and NMDA Receptors. Front Behav Neurosci. 2010;4:179. doi: 10.3389/fnbeh.2010.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]