Abstract
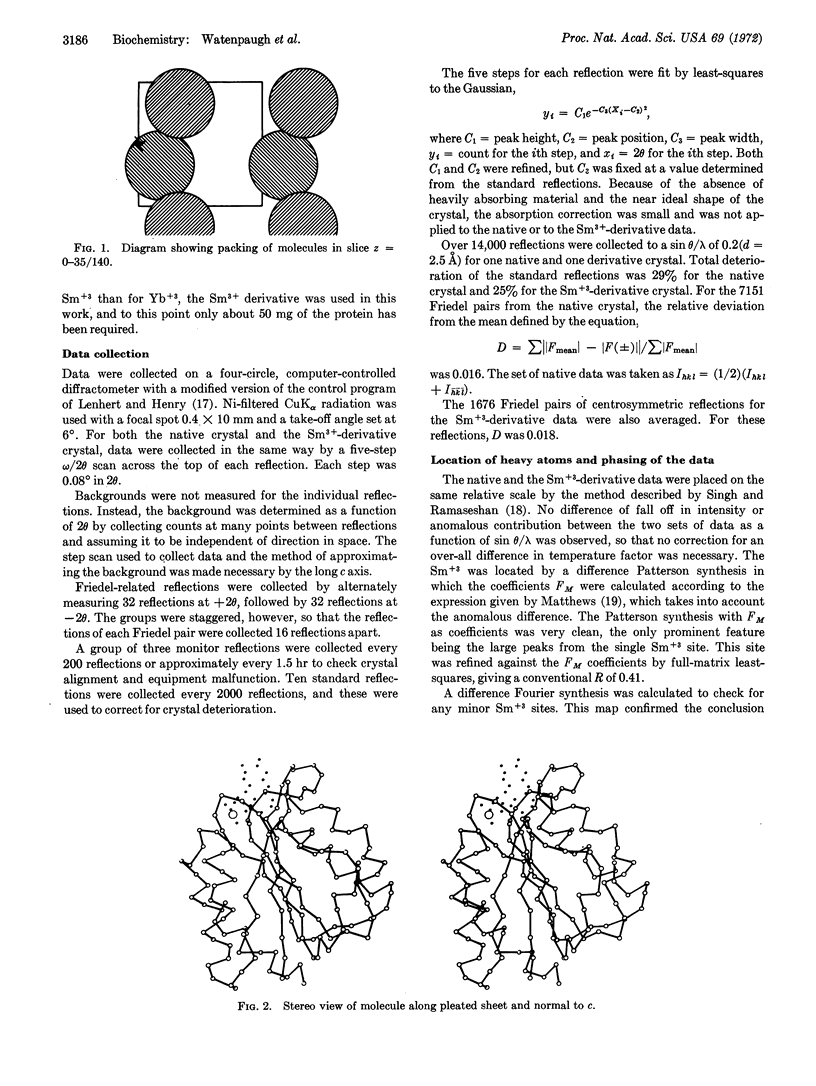
Flavodoxin from Desulfovibrio vulgaris crystallizes in the oxidized form as well-formed, tetragonal bipyramids, space group P43212, unit-cell parameters, a = b = 51.6 Å, c = 139.6 Å, 8 molecules per unit cell.
The structure has been determined at 2.5-Å resolution with phases based on a single isomorphous derivative. The phase ambiguity of a single derivative was resolved by use of anomalous scattering from the single-site Sm+3.
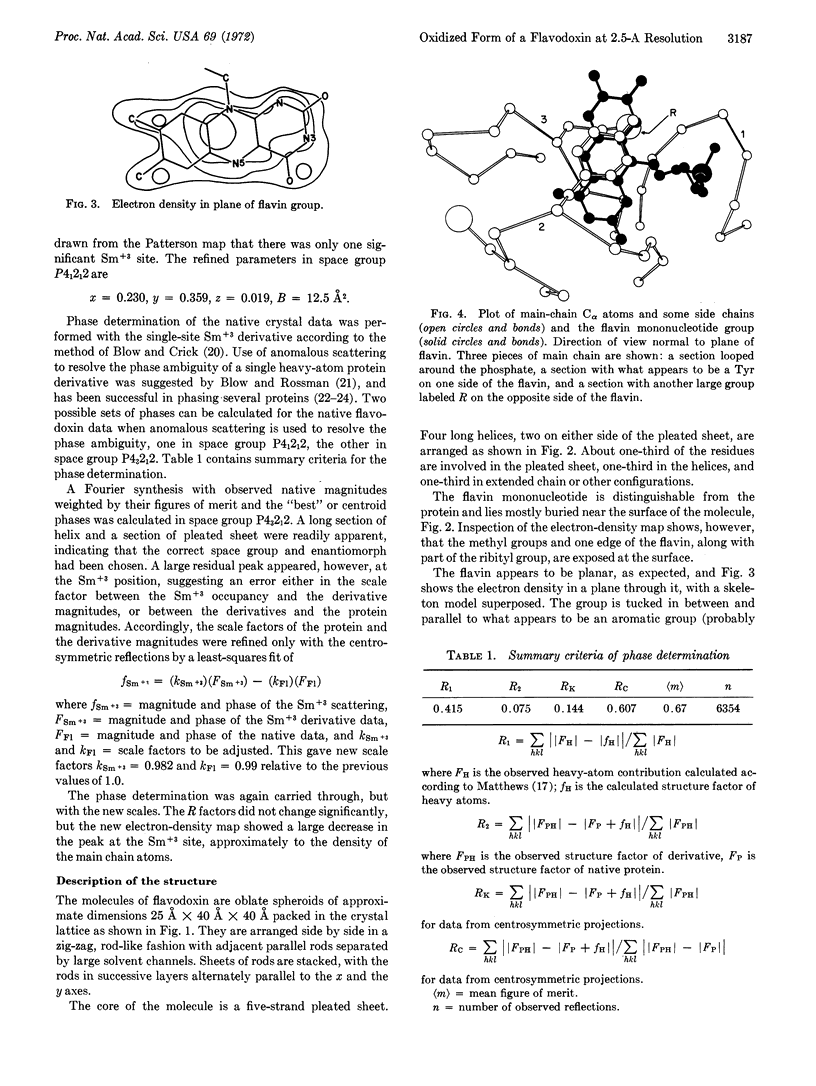
The molecule has a five-strand pleated sheet core with two long helices on either side of the sheet. The flavin mononucleotide lies mostly buried on one side of the molecule, but the methyl groups, one edge of the flavin, and part of the ribityl are exposed at the surface.
Keywords: protein structure, flavin mononucleotide, single isomorphous derivative, x-ray diffraction
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A., Bier C. J., Cotton F. A., Day V. W., Hazen E. E., Jr, Richardson D. C., Yonath A., Richardson J. S. A high resolution structure of an inhibitor complex of the extracellular nuclease of Staphylococcus aureus. I. Experimental procedures and chain tracing. J Biol Chem. 1971 Apr 10;246(7):2302–2316. [PubMed] [Google Scholar]
- Cusanovich M. A., Edmondson D. E. The isolation and characterization of Rhodospirillum rubrum flavodoxin. Biochem Biophys Res Commun. 1971 Oct 15;45(2):327–336. doi: 10.1016/0006-291x(71)90822-9. [DOI] [PubMed] [Google Scholar]
- Dubourdieu M., Le Gall J. Chemical study of two flavodoxins extracted from sulfate reducing bacteria. Biochem Biophys Res Commun. 1970 Mar 12;38(5):965–972. doi: 10.1016/0006-291x(70)90816-8. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., Tollin G. Flavin-protein interactions and the redox properties of the Shethna flavoprotein. Biochemistry. 1971 Jan 5;10(1):133–145. doi: 10.1021/bi00777a020. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C., Le Gall J., Bruschi M., Dubourdieu M. Regulation of the reduction of sulfite and thiosulfate by ferredoxin, flavodoxin and cytochrome cc' 3 in extracts of the sulfate reducer Desulfovibrio gigas. Biochim Biophys Acta. 1972 Mar 8;258(3):701–708. doi: 10.1016/0005-2744(72)90171-4. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C., Le Gall J. Etude du métabolisme des acides dicarboxyliques et du pyruvate chez les bactéries sulfato-réductrices. II. Transport des électrons; accepteurs finaux. Ann Inst Pasteur (Paris) 1970 Mar;118(3):288–301. [PubMed] [Google Scholar]
- Herriott J. R., Sieker L. C., Jensen L. H., Lovenberg W. Structure of rubredoxin: an x-ray study to 2.5 A resolution. J Mol Biol. 1970 Jun 14;50(2):391–406. doi: 10.1016/0022-2836(70)90200-7. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, D'Eustachio A. J., Hardy R. W. Flavodoxin: a flavoprotein with ferredoxin activity from Clostrium pasteurianum. Biochim Biophys Acta. 1966 Mar 7;113(3):626–628. doi: 10.1016/s0926-6593(66)80025-5. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Hardy R. W. Flavodoxin. Chemical and biological properties. J Biol Chem. 1967 Apr 10;242(7):1370–1374. [PubMed] [Google Scholar]
- Knight E., Jr, Hardy R. W. Isolation and characteristics of flavodoxin from nitrogen-fixing Clostridium pasteurianum. J Biol Chem. 1966 Jun 25;241(12):2752–2756. [PubMed] [Google Scholar]
- Le Gall J., Hatchikian E. C. Purification et propriétés d'une flavoprotéine intervenant dans la réduction du sulfite par Desulvovibrio gigas. C R Acad Sci Hebd Seances Acad Sci D. 1967 May 29;264(22):2580–2583. [PubMed] [Google Scholar]
- Ludwig M. L., Andersen R. D., Apgar P. A., Burnett R. M., LeQuesne M. E., Mayhew S. G. The structure of a clostridial flavodoxin, an electron-transferring flavoprotein. 3. An interpretation of an electron-density map at a nominal resolution of 3.25 Angstrom. Cold Spring Harb Symp Quant Biol. 1972;36:369–380. doi: 10.1101/sqb.1972.036.01.048. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Massey V. Purification and characterization of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):794–802. [PubMed] [Google Scholar]
- Mayhew S. G. Properties of two clostridial flavodoxins. Biochim Biophys Acta. 1971 May 12;235(2):276–288. doi: 10.1016/0005-2744(71)90206-3. [DOI] [PubMed] [Google Scholar]
- Sieker L. C., Adman E., Jensen L. H. Structure of the Fe-S complex in a bacterial ferredoxin. Nature. 1972 Jan 7;235(5332):40–42. doi: 10.1038/235040a0. [DOI] [PubMed] [Google Scholar]
- Van Lin B., Bothe H. Flavodoxin from Azotobacter vinelandii. Arch Mikrobiol. 1972;82(2):155–172. doi: 10.1007/BF01890407. [DOI] [PubMed] [Google Scholar]
- Vetter H., Jr, Knappe J. Flavodoxin and ferredoxin of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):433–446. doi: 10.1515/bchm2.1971.352.1.433. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Spiller H. Characterization of a flavodoxin from the green alga Chlorella. Biochem Biophys Res Commun. 1971 Oct 1;45(1):112–118. doi: 10.1016/0006-291x(71)90057-x. [DOI] [PubMed] [Google Scholar]



