Abstract
The rupture of a metastatic mixed adenoneuroendocrine carcinoma (MANEC) has not been previously reported, although the neuroendocrine cell carcinoma is often associated with a high incidence of hepatic metastases. The patient was a 39-year-old male who presented with upper abdominal pain over 3 months. Computed tomography showed multiple tumors in both hepatic lobes, while lower gastrointestinal endoscopy revealed a tumor in the transverse colon. Histopathologic examination of the tumor revealed it to be a neuroendocrine cell carcinoma. After the resection of the primary tumor, hepatic metastases rapidly increased, and one of them in the left lateral segment was ruptured with significant hemorrhage. The rupture led us to undertake the emergency operation to stop the bleeding. Histology showed a high-grade large-cell neuroendocrine carcinoma associated with moderately differentiated tubular adenocarcinoma. The Ki-67 labeling index was 80% (G3). The diagnosis was mixed adenoneuroendocrine carcinoma according to the 2010 World Health Organization guidelines. Hepatic arterial infusion chemotherapy, systemic chemotherapy, and transcatheter arterial chemoembolization did not decrease the tumor progress, and the patient died on postoperative day 110. Reporting this highly malignant case, I hope all doctors can be interested in MANEC.
Key words: MANEC, Liver metastasis, Rupture
Lower gastrointestinal (GI) neuroendocrine cell carcinoma is a rare malignancy of the GI tract and has been reported as the incidence rate of approximately 0.2% in all colorectal cancer cases in Japan.1 The disease is famous for its poor prognosis due to high proliferative capability and early vascular invasion leading to multiple organ metastases.2 Neuroendocrine cell carcinoma is sometimes associated with adenocarcinoma, classified as “mixed adenoneuroendocrine carcinoma (MANEC)” in the 2010 World Health Organization (WHO) guidelines.3,4 Here, we report a case of neuroendocrine cell carcinoma of the colon with concurrent moderately differentiated tubular adenocarcinoma that progressed rapidly after hepatic rupture. This case report is accompanied with a literature review.
Case Report
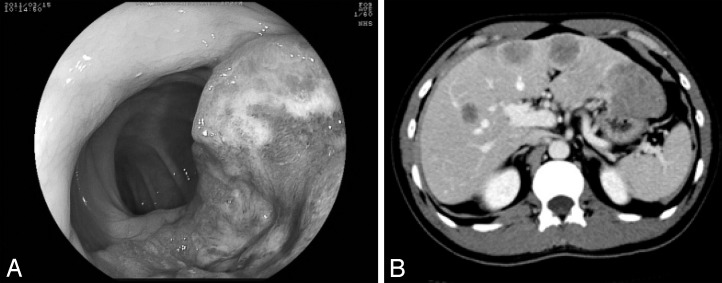
The patient is a 39-year-old male who presented to a local physician with a 3-month history of upper abdominal pain. A mass was palpable in the right hypochondrium and computed tomography (CT) showed multiple tumors in both hepatic lobes. The patient was hospitalized at our department for further investigations and treatment. Complete blood cell count showed increased levels of leucocytes (11,800/μL) with no anemia (hemoglobin, 14.2 g/dL). Laboratory findings showed an increase in lactate dehydrogenase level (385 IU/L) and decrease in prothrombin time (75.6%). Significantly high carcinoembryonic antigen (341.4 ng/mL) and carbohydrate antigen 19-9 (8648 U/mL) levels were noted. Colonoscopy showed that there was a semicircular tumor approximately 60 cm from the anus. Biopsy results of this lesion suggested a diagnosis of neuroendocrine cell carcinoma (Fig. 1A).
Fig. 1.
(A) There was a semicircular tumor in the transverse colon. (B) Multiple hypovascular masses were noted in both hepatic lobes.
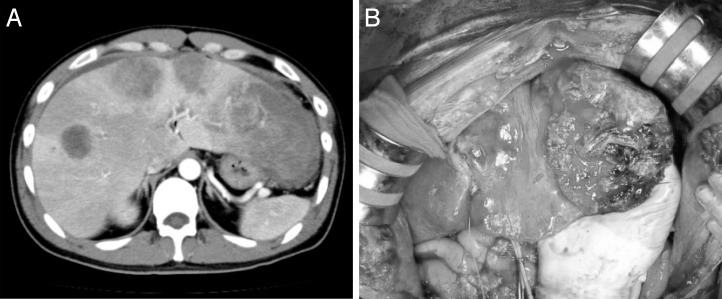
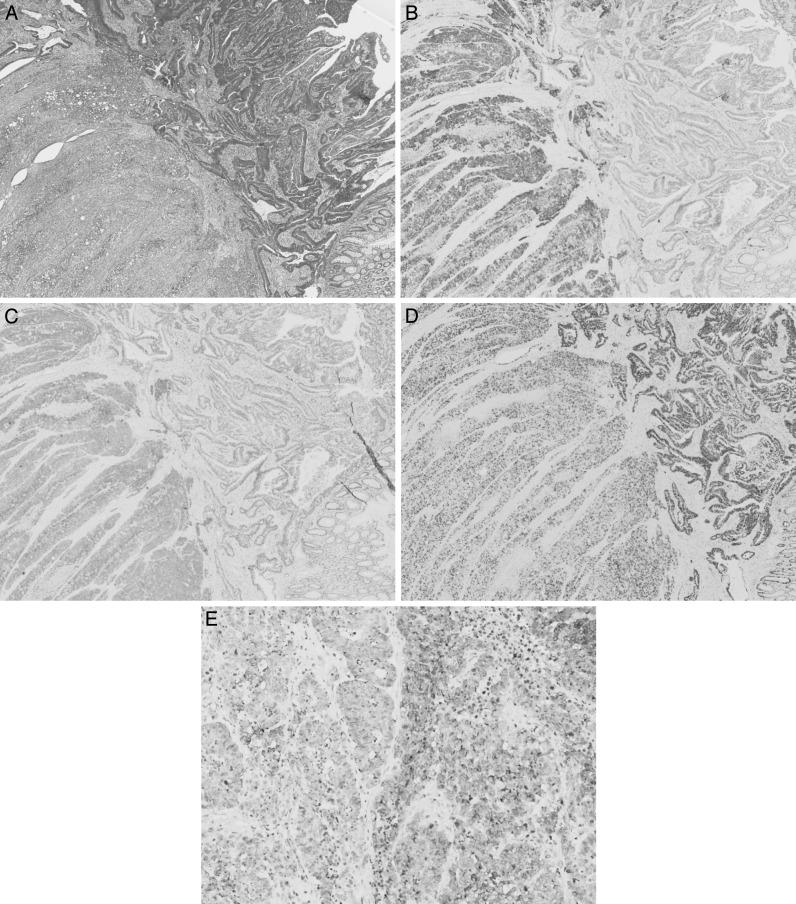
Abdominal CT revealed that there were multiple hypovascular masses noted in both hepatic lobes with maximum diameters of 5 cm (Fig. 1B). Localized intestinal wall thickening, a high concentration of adipose tissue, and peripheral nodules indicative of lymphatic metastases were seen in the transverse colon. The diagnoses were neuroendocrine carcinoma of the colon and multiple hepatic metastases. The transverse colon was partially resected with extensive lymph node dissection as primary surgery. Subsequent chemotherapy and hepatic resection were planned after the primary surgery. However, continued leaking of chylous ascites after surgery led us to leave the drainage tube for further observations. Anemia gradually increased from postoperative day (POD) 9, and a sanguineous exudate was noted to be draining on POD 11. A contrast-enhanced CT showed rapid enlargement of the tumor and fluid collection in the left abdominal cavity, indicating tumor rupture. An emergency laparotomy was performed on POD 12 (Fig. 2A). In the hepatic left lateral segment, soft and necrotic tumor caused the free rupture with massive bleeding (Fig. 2B). Effective hemostasis was achieved by partial hepatectomy involving the necrotic part of the tumor as much as possible and applying a hemostatic agent. The surgery lasted for 2 hours and 59 minutes, and the intraoperative hemorrhage volume was 700 mL. Histopathologic examination results showed that the main part of the primary lesion (colon) comprised cells forming solid nests with irregularly enlarged nuclei and clear nucleoli that were CD56 (+), synaptophysin (+), and chromogranin (+), which correspond to high-grade large-cell–type endocrine cell carcinoma. However, more than 30% of the tumor comprised a moderately differentiated adenocarcinoma with tall columnar cells forming acinar fusion in the area between the lamina propria and submucosal layer. The main part of the liver metastases comprised neuroendocrine carcinoma cell, while some regions were mixed with components equivalent to moderately differentiated tubular adenocarcinoma. In both lesions, the Ki-67 labeling index was high (80%) with KRAS mutation (–) and a somatostatin receptor subtype 2A (SSTR2A) score of 3 (Fig. 3A–E). As judged by the high Ki-67 index (enough to predict the poor prognosis of the patients), we started additional treatment as soon as possible. Hepatic arterial infusion chemotherapy with 5-fluorouracil was initiated from POD 23 of the initial surgery. However, the size of the tumor increased and was rated as a progressive disease based on Response Evaluation Criteria in Solid Tumors (RECIST). Tumor markers continued to increase despite chemotherapy with cetuximab + mFOLFOX6 (folinic acid, fluorouracil, and oxaliplatin) combined with octreotide. Further chemotherapy with cisplatin + camptothecin-11 (CDDP + CPT11) and transcatheter arterial chemoembolization (TACE) were initiated, but treatment was unsuccessful and the patient died on POD 110.
Fig. 2.
(A) Multiple hypovascular masses were noted in both hepatic lobes. The white arrow showed external fluid due to bleeding. (B) The tumor was necrotic and ruptured.
Fig. 3.
(A) The tumor was composed of high-grade large-cell endocrine cell carcinoma with moderately differentiated tubular adenocarcinoma. Colon: HE (×40). (B) Chromogranin A (+). Colon: chromogranin A (×40). (C) Synaptophysin (+). Colon: synaptophysin (×40). (D) The Ki-67 labeling index was high in both lesion (80%). Colon: Ki-67 (×40). (E) The tumor in the liver was also composed of high-grade large-cell endocrine cell carcinoma with moderately differentiated tubular adenocarcinoma. Liver: chromogranin A (×200).
Discussion
This is the first report of a gastrointestinal–neuroendocrine tumor (GI-NET) with a high Ki-67 value caused hepatic rupture of liver metastatic tumor. Neuroendocrine cell carcinoma has been reported as a rapidly progressive, highly proliferative, and malignant carcinoma.2 The histopathologic characteristics include structural endocrine cells that are rich in chromatin with a high nuclear to cytoplasmic ratio and small to large nuclei in round to fusiform shapes and numerous nuclear divisions. It is also characterized by fibrocapillary interstitial tissue with necrotic foci, large solid nodules, or sheet-like nests with pseudorosette structures.5 Immunohistochemical staining with various markers is effective for identification of the tumor. Synaptophysin can be used for diagnosis even in poorly differentiated states. Chromogranin A is a neuroendocrine secretary granule known as a highly specific marker, with low sensitivity. In our patient, most regions of the primary and metastatic lesions were positive for synaptophysin and chromogranin A, and their characteristics corresponded to a neuroendocrine cell carcinoma of the large-cell type. Meanwhile, the other regions of the tumors were found to be composed of moderately differentiated adenocarcinoma cells exhibiting typical ductal structures. This form of mixed tumors with both neuroendocrine cell carcinoma and epithelial carcinoma cells has been previously classified into a collision tumor (coll), which appears as if 2 different elements are colliding into each other: a composite tumor (comp), which has both elements mixed together; and an amphicrine tumor (amph), which contains cells with characteristics of neuroendocrine and epithelial carcinomas.6 In our case, the tumor was classified into the first category, as there was a clear distinction between endocrine cell carcinoma and adenocarcinoma. The WHO defined and reclassified this carcinoma in 2010 as MANEC, morphologically recognizable as both gland-forming epithelial (rarely squamous cell component) and neuroendocrine cells (at least 30% of either component should be identified to qualify for this definition), and defined as a carcinoma since both components are malignant and should be graded.4 This definition is applicable to our case, and MANEC was considered to be a compatible diagnosis. The reason why these 2 types of carcinomas with different characteristics coexist in 1 tumor is extremely intriguing. Rosa et al7 recently indicated that neuroendocrine cells also occur in adenomas with no neoplastic transformation and suggested a new term for this category as “mixed adenoneuroendocrine tumors (MANETs).” Mondolfi et al8 studied relatively common MANEC of the gall bladder and suggested that precursor cells with overlapping features of neuroendocrine and glandular differentiation develop into MANEC during neoplastic transformation. In general, a highly malignant GI-NET is difficult to treat, and the only curative treatment option for neuroendocrine cell carcinoma is surgical resection. Diagnoses are usually made at a late stage of the disease and usually require more complex management including chemotherapy. Major treatment guidelines were created by the European Neuroendocrine Tumor Society (ENETS) in 2004 and by the National Comprehensive Cancer Network (NCCN) in 2011. In the ENETS guidelines, cisplatin + etoposide are considered effective for treatment of poorly differentiated GI-NET derived from the midgut or hindgut.9 In the NCCN guidelines, carboplatin + etoposide or cisplatin + irinotecan are recommended, which was derived from the regimen for small-cell carcinoma of the lung.10 Recently, others have reported using the treatment guidelines for colorectal cancer as a reference. Oshima et al11 reported a case in which CPT11 or mFOLFOX6 was effective for metachronous hepatic metastasis after resecting the primary lesion of endocrine cell carcinoma of the colon. There was also a case report of a patient who had a tumor with both neuroendocrine cell carcinoma and tubulovillous adenoma (including highly differentiated adenocarcinoma components) with hepatic metastases. This patient had died just 3 months after the resection of the primary lesion, despite undergoing therapy with bevacizumab + FOLFOX6.12 Another report concluded that TACE using doxorubicin was effective for hepatic metastases, which occur frequently.13 Other adjunctive medications have been suggested, including cisplatin, streptozocin, fluorouracil, and mitomycin C; however, the success rate varies between 25% and 80% and thus cannot be considered a standard line of therapy. We used cetuximab + FOLFOX combined with octreotide, based on the fact that the tumor was a moderately differentiated tubular adenocarcinoma with KRAS mutation (−) and endocrine cell carcinoma with SSTR2A (+).
As mentioned above, neuroendocrine carcinoma of the colon is highly aggressive. From recent reports, large-cell–type lymphatic involvement around the tumor, CD117(−), absence of vascular invasion, and unstable microsatellites owing to methylation have been found to improve the prognoses of patients with neuroendocrine carcinoma.14
In conclusion, this is the first report of a NET with a high Ki-67 value caused necrosis leading to the hepatic rupture. We anticipate the new molecular targeting drugs such as everolimus and sunitinib to see whether they improve the treatment outcome and overall prognosis.
References
- 1.Nishimura Y, Sekine T, Kobayashi T, Amikura K, Sakamoto Y, Tanaka Y. Clinicopathological study of rare histological types of colorectal cancer—Multi-Institutional Questionnaire Study [in Japanese with English abstract] Nippon Daicho Komonbyo Gakkai Zasshi (J JPN Soc Coloprotocol) 2004;57:132–140. [Google Scholar]
- 2.Otsuka M, Kato Y. Clinicopathological study of poorly differentiated and undifferentiated carcinoma of the large intestine: classification and special reference to the endocrine cell carcinoma [in Japanese with English abstract] Nihon Syokakigeka Gakkaizasshi (Jpn J Gastroenterol Surg) 1992;25(5):1248–1256. [Google Scholar]
- 3.Sano T. Pathology of carcinoid tumors [in Japanese] Nihon Rinsho. 2011;69(2):639–642. [PubMed] [Google Scholar]
- 4.Rindi G, Klimstra DS, Arnold R, Kloppel G, Bosman FT, Komminoth P, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hurban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Lyon, France: IRAC Press; 2010. pp. 13–14. In. eds. [Google Scholar]
- 5.Teturo H, Kenichi S. Diagnosis and treatment of colorectal neuroendocrine cell tumor [in Japanese with English abstract] Journal of Japan Surgical Society. 2008;109:152–156. [Google Scholar]
- 6.Lewin K. Carcinoid tumors and mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11((suppl 1)):71–86. doi: 10.1097/00000478-198700111-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rosa SL, Marando A, Sessa F, Capella C. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers. 2012;4:11–30. doi: 10.3390/cancers4010011. Available at doi.org/10.3390/cancers4010011"10.3390/cancers4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondolfi AEP, Slova D, Fan W, Attiyeh FF, Afithinos J, Reidy J, et al. Mixed adenoendocrine carcinoma (MANEC) of the gallbladder: a possible stem cell tumor? Pathol Int. 2011;61(10):608–614. doi: 10.1111/j.1440-1827.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 9.Plockinger U, Rindi G, Arnold R, Eriksson B, Krenning EP, de Herder WW, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumors: a consensus statement on behalf of the European Neuroendocrine Tumor Society (ENETS) Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Neuroendocrine Tumors. 2012 doi: 10.6004/jnccn.2009.0050. Version 1. Available at: http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed December 15, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Oshima Y, Isaka N, Takeuchi T, Arita S, Tanaka H, Koike N. A case of long-term survival after chemotherapy for liver metastases from endocrine cell carcinoma of colon [in Japanese with English abstract] J Jpn Surg Assoc. 2008;69(9):2331–2336. [Google Scholar]
- 12.Kuratate S, Inoue S, Cikakiyo M, Kaneda Y, Harino Y, Hirose T, et al. Coexistent poorly-differentiated neuroendocrine cell carcinoma and non-invasive well-differentiated adenocarcinoma in tubulovillous adenoma of the rectum: report of a case. J Med Invest. 2010;57(3–4):338–344. doi: 10.2152/jmi.57.338. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi S, Uetake H, Kikuchi A, Ono H, Matsuyama T, Kato S, et al. A long-term survival case of neuroendocrine tumor of the sigmoid colon with multiple liver metastases [in Japanese] Gan To Kagaku Ryoho. 2011;38(12):2271–2273. [PubMed] [Google Scholar]
- 14.Rosa SL, Marando A, Furlan D, Sahnane N, Capella C. Colorectal poorly differentiated neuroendocrine carcinomas and mixed adenoneuroendocrine carcinomas: insights into the diagnostic immunophenotype, assessment of methylation profile, and search for prognostic markers. Am J Surg Pathol. 2012;36(4):601–611. doi: 10.1097/PAS.0b013e318242e21c. [DOI] [PubMed] [Google Scholar]