Abstract
The impact of cancer involving the peripancreatic soft tissue (PST), irrespective of margin status, following a resection of pancreatic adenocarcinoma is not known. The purpose of this study is to determine such an impact on a cohort of patients. Data from 274 patients who underwent pancreatic surgery by our team between 1998 and 2012 was reviewed. Of those 119 patients who had pancreatic resection for adenocarcinoma were retrospectively analyzed. Patients were categorized into 3 groups: Group 1 = R1 resection (N = 39), Group 2 = R0 with involved PST (N = 54), and Group 3 = R0 with uninvolved PST (N = 26). Demographics, operative data, tumor characteristics and overall survival (OS) were evaluated. Operations performed were: Whipple (N = 53), pylorus sparing Whipple (N = 41), total pancreatectomy (N = 11), and other (N = 14). Median OS for Groups 1, 2, and 3 were 8.5 months, 12 months, and 69.6 months respectively (P < 0.001). Tumor size (P = 0.016), margin status (P = 0.006), grade (P = 0.001), stage (P = 0.037), PST status (P < 0.001), complications (P = 0.046), transfusion history (P = 0.003) were all predictors of survival. Cox regression analysis demonstrated that grade (HR = 3.1), PST involvement (HR = 2.7), transfusion requirement (HR = 2.6) and margin status (HR = 2.0) were the only independent predictors of mortality. PST is a novel predictor of poor outcome for patients with resected pancreatic cancer.
Key words: Peripancreatic soft tissue, Novel predictor, pancreas, Malignancy, Outcome, Adenocarcinoma
Pancreatic cancer is the fourth most common cause of cancer death in the United States.1 This cancer has the highest mortality among all cancers.1 The 5-year overall survival (5-year-OS) of the involved patients is reported to be as low as 6%–18%.1,2 In patients with localized disease, complete surgical resection is the only curative treatment3 and the 5-year-OS can be as high as 25%.4–7 Due to the often late presentation of pancreatic cancer, only a minority of the patients (10%–20%) are considered to be a candidate for curative resection (CR). At the time of diagnosis, more than 50% of patients have already developed distant metastasis and 35% have locally advanced disease.8 Patients with locally advanced disease are believed to benefit from radical surgeries to achieve R-0 resection, where all post resection margins are tumor free. Although the majority of the previous studies have emphasized the importance of surgical margin status as a predictor of survival in these patients,3,5,7,9 others have not.10,11 However, the impact of an involved peripancreatic soft tissue (PST), irrespective of resection margin status, following a pancreatectomy is not known. We determined the impact of involved PST on a cohort of patients with pancreatic adenocarcinoma.
Material and Methods
Following IRB approval, we retrospectively reviewed data from 274 patients who underwent pancreatic surgery by our team between February 1998 and January 2012. After initial workup, which included routine blood tests and tumor markers (CEA, CA19-9), pancreas protocol computed tomography (CT), and either positron emission tomography (PET)/PET-CT scan, magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), or, more recently, endoscopic ultrasound scan (EUS), the patients were discussed by a multidisciplinary team of gastroenterologists, interventional radiologists, oncologists, oncologic surgeons, and hepato-pancreato-biliary (HPB) surgeons. Distant metastasis or major artery involvement (celiac or superior mesenteric artery) by tumor infiltrate was considered as a contraindication to CR in the patients in whom palliative procedures were performed if necessary. The involvement of the superior mesenteric vein (SMV) or portal vein (PV), if tumor thrombi were not present inside these vessels, was not considered as a contraindication for CR. In the current study we defined peripancreatic soft tissue (PST) involvement as the presence of tumor cells in the peripancreatic soft tissues, including the anterior and posterior plain of fat and fibrous tissues.
Malignant causes were present in 175 patients. Among them, 119 patients with adenocarcinoma underwent CR of the pancreas pathology. This group (119 patients) is the focus of the current study. These 119 patients were categorized into 3 groups: Group 1 = R1 resection (N = 39), Group 2 = R0 with involved PST (N = 54), and Group 3 = R0 with uninvolved PST (N = 26). Demographic data, duration of operation, use of hemodilution technique (when 1 or 2 bottles of blood is taken from the patient before the surgery and transfused back immediately after the operation), estimated blood loss (EBL), amount of blood transfusion (intra-operative and postoperative), length of hospital stay (LOS), rate and severity of complications (pancreatic leak, biliary leak, bleeding, gastroparesis, infection, etc.), peri-operative mortality, overall survival, and long-term outcome were evaluated.
In this study, peri-operative complication was defined as the presence of intra- or postoperative complications up to 30 days post surgery or the time of patient's discharge, whichever was longer. The severity of peri-operative complications were scored based on the revised Clavien-Dindo (CD) classification.12,13 When more than one complication occurred, the score of the most severe complication was considered as the complication score of the patient.
Final pathology reports were rereviewed by another pathologist blinded to patient's information for evaluation of the tumor characteristics (histology, size, location, grade, stage, lymph node, margin, and PST status). During follow up, if CA 19-9 was high before the surgery and declined after surgery, its level was checked regularly for early detection of recurrence. In addition, regular imaging including CT and/or PET/PET-CT was also used (every 3 months up to 1 year, then every 6 months for 2 more years and yearly up to 5 years thereafter) for detection of recurrence during the follow-ups. Patients were followed indefinitely. Descriptive statistics were used to summarize the data. Student's t-test, ANOVA, Chi-Square test, Kaplan-Meier method and log-rank test was used for statistical analysis. In a multivariate analysis using a Cox regression model, variables were assessed in search of independent risk factors predicting poor outcome in the patients. A P-value of < 0.05 was considered significant. All analysis was performed using the SAS system 9.2 (Gray, North Carolina).
Results
The mean age of the patients was 64.1 + 10.2 years. Fifty nine of the patients (49.6%) were female and 89 (74.8%) were Caucasian. Age, gender, and race were not significantly different between Group 1, Group 2, and Group 3 (P > 0.05 for all) (Table 1). Ninety-six percent of lesions (N = 114) were solid. The mean tumor size was 3.8 + 1.9 cm, and 59.7% of patients (N = 71) had lymph node involvement at the time of surgery. The grades were: 1 (4.3%), 2 (51.7%), 3 (41.5%), and 4 (2.5%). The stages were: 1A (4.2%), 1B (7.6%), IIA (26.0%), IIB (47.9%), III (10.9%), and IV (3.4%). Patients and tumor characteristics are depicted in Table 1, and the type of operation performed in these 119 patients is summarized in Table 2.
Table 1.
Comparison of patient's and tumor characteristics, hospital stay, and operative data in the three groups of patients
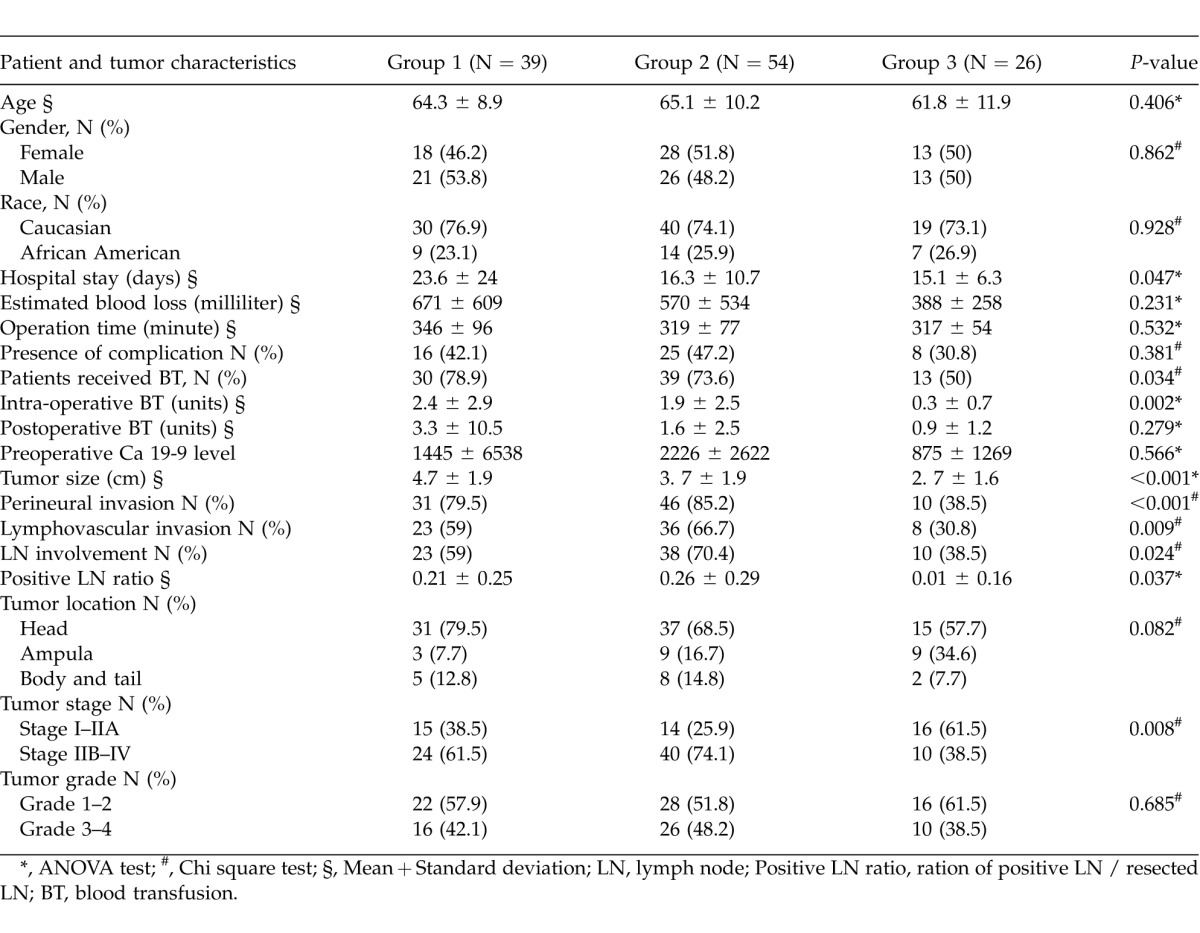
Table 2.
Type of the operation, distribution and severity score of postoperative complications based on the revised Clavien-Dindo classification (CD grade) in the 119 patients
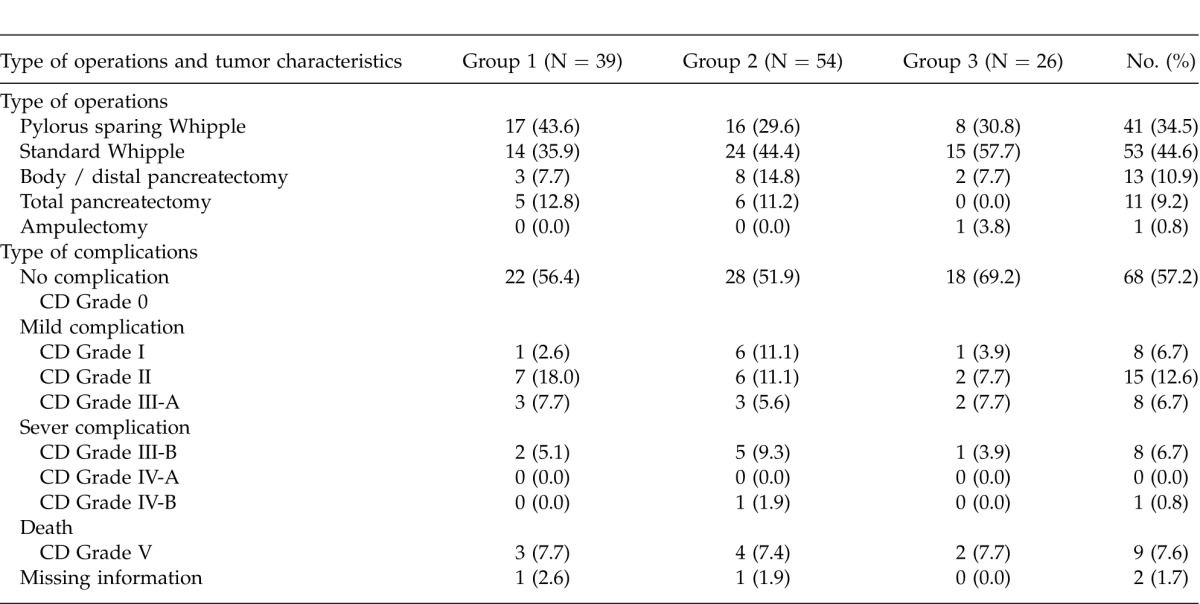
The mean OR time was 326.1 + 78.7 minutes (range = 150–520 minutes) and the mean EBL was 552.4 + 508.5 ml (range = 75–2500). Eighty-two patients (68.9%) received a blood transfusion during their hospital stay (intra-operative and postoperative), and the average amount of the transfusion was 3.7 + 7.1 units (range 0–57 units). Hemodilution was used in 30 patients (25.2%), and these patients required significantly less blood to be transfused during their hospital stay (1.4 + 2.1 units) compared with the other patients (4.5 + 8.0 units, P = 0.001). Pre-operation CA 19-9 serum levels > 200 U/mL were significantly associated with a higher risk of vascular involvement (PV and SMV) in the patients (22.45% versus 4.88%, P = 0.032). More than 57% of the patients (N = 68) did not have any complications in the peri-operative period. Table 2 summarizes the peri-operative complications in the patients based on their CD severity score.
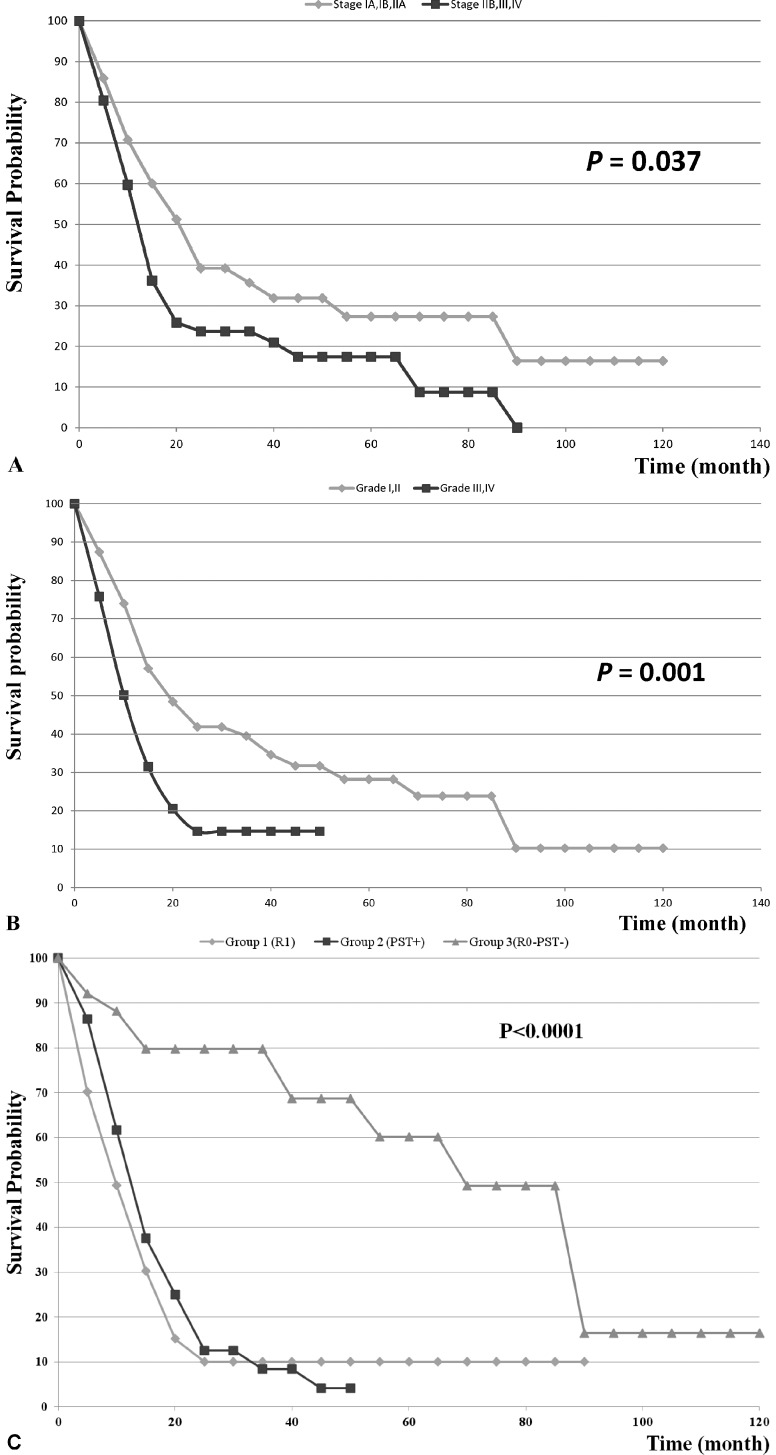
The 5-year-OS for the entire group of patients was 20.6%. Stages IIB–IV had a significantly lower median overall survival than stages I-IIA (P = 0.037). Fig. 1A shows the overall survival of the patients based on the tumor stage in the patients. Patients with tumor grades of I–II had a significantly higher median overall survival compared with patients with tumor grade III–IV (P = 0.001). Figure-1B shows the overall survival of the patients based on the tumor grade in the patients.
Fig. 1.
(A) Overall survival of the 119 patients with pancreatic adenocarcinoma based on their tumor stage. (B) Overall survival of the 119 patients with pancreatic adenocarcinoma based on their tumor grade. (C) Overall survival in the three groups of patients with pancreatic adenocarcinoma. Group 1 = R1 resection (N = 39), Group 2 = R0 with involved PST (N = 54), and Group 3 = R0 with uninvolved PST (N = 26).
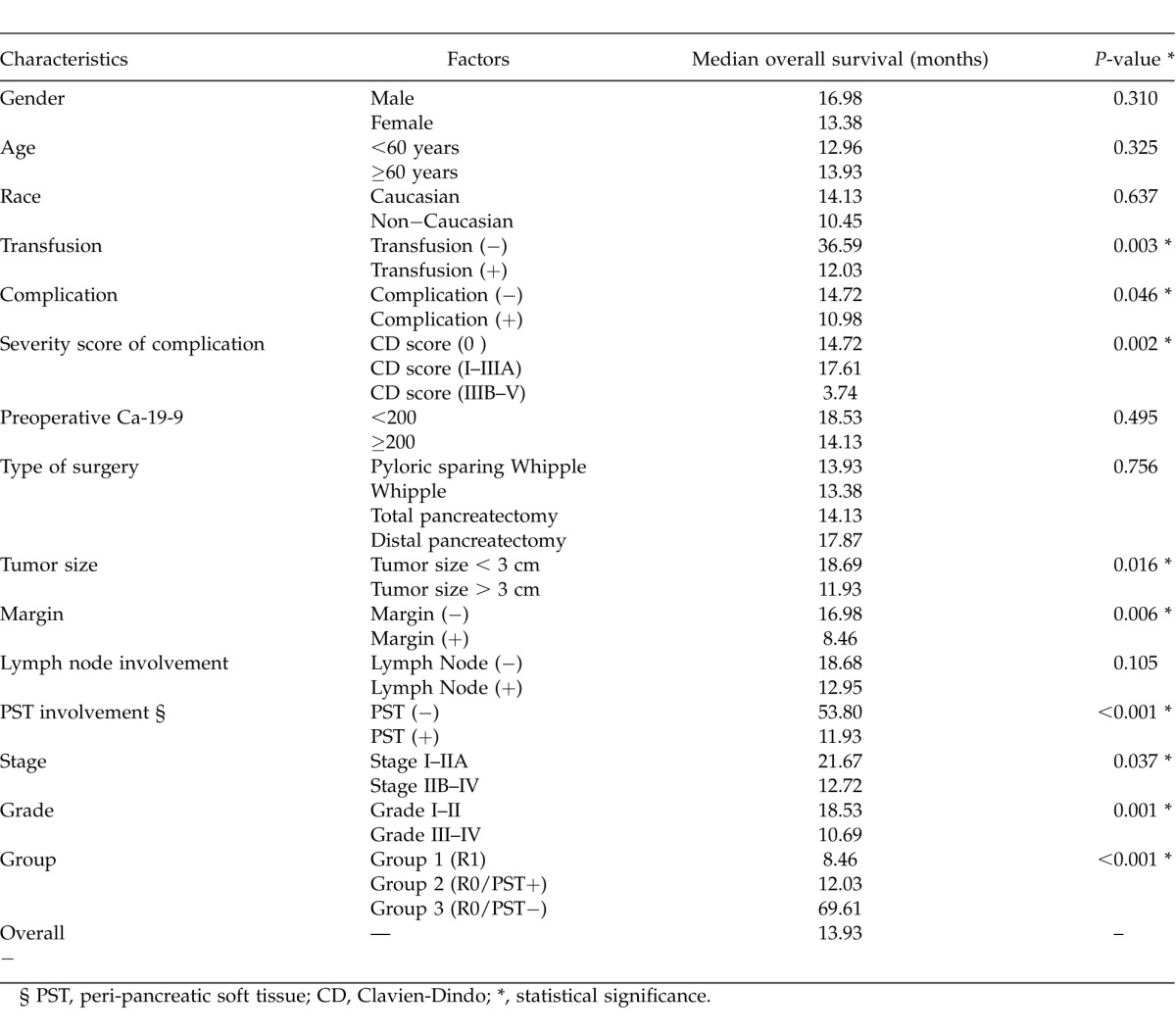
Median OS for Groups 1, 2, and 3 were 8.5 months, 12 months, and 69.6 months respectively (P < 0.001). The overall survival of the patients in the three groups has been compared in Fig. 1C. Tumor size (P = 0.016), margin status (P = 0.006), grade (P = 0.001), stage (P = 0.037), PST status (P < 0.001), presence of complications (P = 0.046), severity of complications (P = 0.002) and transfusion history (P = 0.003) were all predictors of survival. Patient and tumor characteristics and univariate survival analysis in the 119 patients are listed in Table 3.
Table 3.
Univariate analysis of overall survival by patient and tumor characteristics
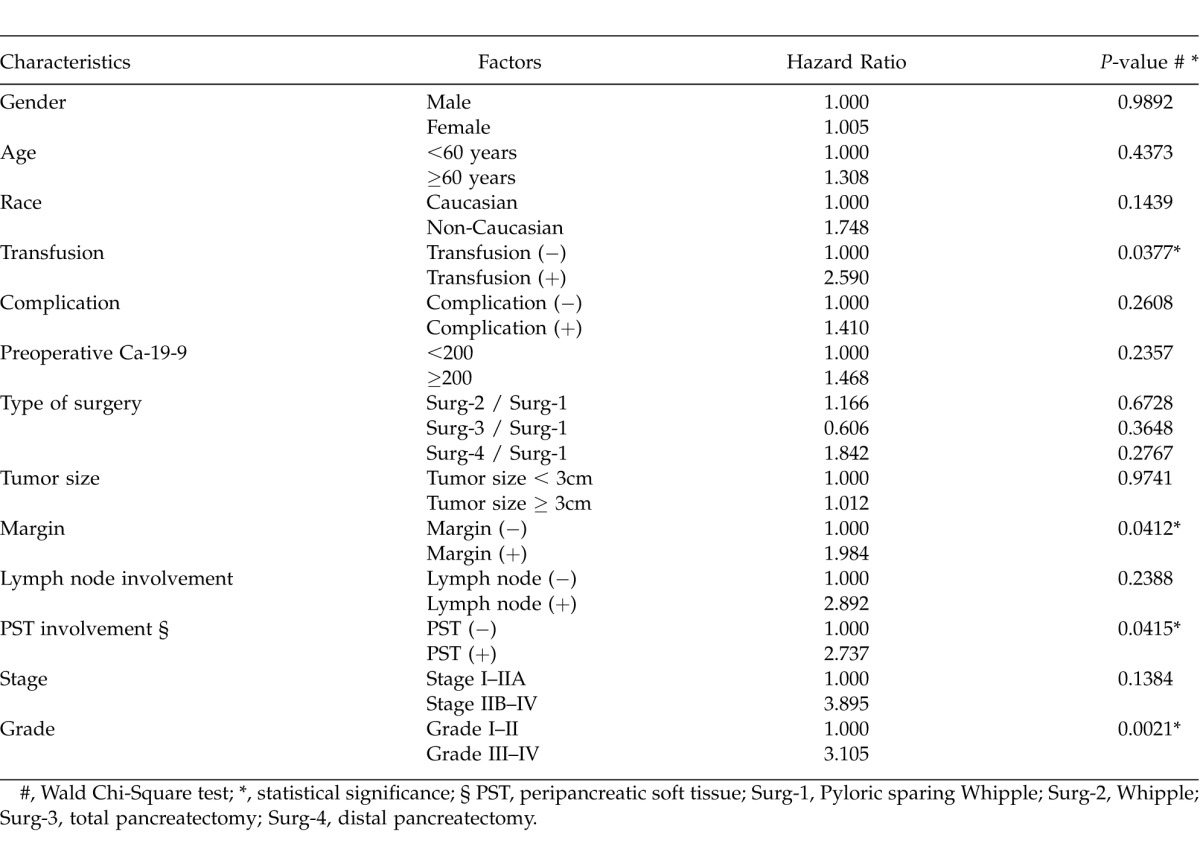
Cox regression analysis demonstrated that grade (hazard ratio = 3.1, P = 0.002), PST involvement (HR = 2.7, P = 0.041), transfusion requirement (HR = 2.6, P = 0.038), and margin status (HR = 2.0, P = 0.041) were the only independent predictors of mortality (Table 4). The mean overall survival for the patients was 18.1 months and 40 (33.6%) patients are currently alive.
Table 4.
Multivariate analysis of overall survival by patient and tumor characteristics
Discussion
Although improvement in surgical techniques and expertise, patient selection, and peri-operative care, along with the implementation of multidisciplinary approach to patient's care, has led to reduced morbidity and an improved 5-year-OS in high volume pancreatic centers in some reports,5,8,14–16 pancreatic cancer is still one of the exceptional cancers for which survival has not improved significantly for nearly 40 years.1 More accurate imaging modalities such as PET-CT, EUS, and new generations of CT or MRI/MRCP have all contributed to earlier detection of pancreatic cancers and improvement in their preoperative staging. This has led to a better patient selection and an increase in curative resectability rate in patients suffering from a pancreatic malignancy.4,8 All of these factors have to some extent played a role in improving long-term patient survival worldwide. In spite of these advances, a majority of patients with pancreatic cancer are still diagnosed late. As many as 50% of these patients are found to have metastasis at the time of diagnosis, and curative surgery is not an option. Furthermore, those patients who have locally advanced disease, even in the form of large venous involvement (SMV/PV), may benefit from a more aggressive surgery.2,17,18
There has been a wide variation in the rate of R1 reported in the literature, which ranges from figures below 20% to more than 75%.19 The previous studies have also not been consistent in showing the impact of surgical margin status on the prediction of survival in patients with pancreatic cancer. Although the majority of the studies have emphasized the importance of surgical margin status as a predictor of survival in these patients,3,5,7,9 other studies have failed to show a significant difference of outcome based on the margin status.10,11 There have also been a variety of definitions between different studies as to what is called R1. Furthermore, there has been a historic controversy regarding the definition of microscopic margin involvement between United States, Canadian, and European centers. In the United States and Canada R1 is considered by many pathologists as the presence of pancreatic tumor at the surface of resection, but in Europe the presence of a tumor within 1 mm of the margin is called R1.20
The lack of standardized protocol for postsurgical pathologic evaluations of tissues, problems in distinguishing distal bile duct cancers from pancreatic and ampullary cancers, and confusion in the nomenclature might have all contributed towards these variations in the reported results and outcomes, which will preclude any comparison of data between different studies.20 It also confirms the unreliability of the margin status concept in its current definition, the “presence of residual tumor after treatment” according to the International Union against Cancer Classification (renamed Union for International Cancer Control) (UICC),21 as a predictor of survival and performance measure guide for surgeons and reporting pathologists.19 This has led to a call for redefining this terminology in an internationally recognized fashion.19
Conventionally, the resection margins have included the bile duct resection margin, pancreatic neck transection margin, uncinate process margin (medial resection margin), and gastric/pyloric or duodenal resection margins. However, it is not a common practice by pathologists to look at the tissue around the posterior surface of the pancreas head, the surface of the head that faces the superior mesenteric vessels and as a misnomer is called “retroperitoneal surface,” and the anterior surface of the pancreas head, which is not a true resection margin.20 We are lacking a general consensus as to which parts of the pancreatic head constitutes the circumferential resection margin.
A comparison between the survival of the patients with equivocal margin involvement (tumor involvement within 1 mm of, but not directly reaching, one or more resection margins) and unequivocal margin involvement (tumor directly reaching one or more resection margins) confirmed that even equivocal margin involvement should be considered as incomplete resection of the pancreatic cancer.22
In the current study, we went further and explored the impact of PST tumor involvement compared with unequivocal margin involvement on the overall survival of patients with pancreatic cancer. In the current study we defined PST involvement as the presence of tumor cells in the peripancreatic soft tissues including the anterior and posterior plain of fat and fibrous tissues. The impact of an involved PST, irrespective of resection margin status, following a pancreatectomy has not been investigated before.
In our study PST tumor involvement independently predicted a poor outcome in patients who underwent a pancreatectomy for malignancy. The overall survival in patients with PST involvement even in the presence of a negative resection margin was similar to those with a positive margin. Due to the fact that there is no current consensus on the definition of either resection margin or R1 resection, we propose that this novel predictor of outcome (PST tumor involvement), which is quite practical and simple to report, be added to the definition of positive margin status or, interchangeably, be used as R1 resection.
This retrospective study, like others, has shown that the most important factor in achieving long-term survival in patients with resectable pancreatic cancer is obtaining an R-0 resection. In contrast to the previous studies, we would like to emphasize that the presence of R-0 resection in its current definition does not indicate the best outcome if there is PST tumor involvement. Only in the presence of negative PST would R-0 resection count as a strong predictor of favorable outcome and improved survival in pancreatic cancer patients.
In the previous studies, age of the patient,3,23,24 stage of the disease,24,25 tumor grade,3,5,24 lymph-node status,3,5,7,24–26 resection margin status,3,5,7,9 presence of complications,23,27,28 size of the tumor,5,9 and transfusion requirement9,23 have all been proposed as factors predicting outcome in the patients with pancreatic cancer. While in our experience tumor size, stage, grade, presence and severity of complications, margin status, PST involvement, and transfusion requirement were all important predictors of mortality in the patients with pancreatic adenocarcinoma, grade of the tumor, PST involvement, margin status, and transfusion requirement were the only independent factors predicting the outcome in this group of patients.
Conclusion
In conclusion, better patient selection, intra- and peri-operative care, center volume, and surgeon experience have all contributed to recent improvements in the outcomes of major pancreatic surgeries. Involvement of peripancreatic soft tissue independently predicts a poor outcome in patients who had pancreatectomy for pancreatic adenocarcinoma. Our study demonstrates that survival in patients with peripancreatic soft tissue tumor involvement even in the presence of a negative resection margin is similar to those with a positive margin. In our experience the most important factors in predicting long-term survival were grade of the tumor, PST involvement, margin status, and transfusion requirement.
Acknowledgments
The authors would like to thank Mr. John Cyrus and Ms. Talicia Tarver who provided us editorial assistance. This research is supported in part by the Malcolm Feist Chair in Transplantation and by the Donnie and Gail Juneau Endowed Chair in Transplantation. There are no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures. 2010 Available at: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. Accessed June 17, 2013. [Google Scholar]
- 2.Ramacciato G, Mercantini P, Petrucciani N, Ravaioli M, Cucchetti A, Del Gaudio M, et al. Does portal-superior mesenteric vein invasion still indicate irresectability for pancreatic carcinoma? Ann Surg Oncol. 2009;16(4):817–825. doi: 10.1245/s10434-008-0281-8. [DOI] [PubMed] [Google Scholar]
- 3.Jarufe NP, Coldham C, Mayer AD, Mirza DF, Buckels JA, Bramhall SR. Favourable prognostic factors in a large UK experience of adenocarcinoma of the head of the pancreas and periampullary region. Dig Surg. 2004;21(3):202–209. doi: 10.1159/000079346. [DOI] [PubMed] [Google Scholar]
- 4.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91(5):586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 5.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10(9):1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommerville CA, Limongelli P, Pai M, Ahmad R, Stamp G, Habib NA, et al. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: an analysis of clinicopathological factors. J Surg Oncol. 2009;100(8):651–656. doi: 10.1002/jso.21390. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Russell RC, Bramhall S, Theis B. Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg. 1997;84(10):1370–1376. [PubMed] [Google Scholar]
- 9.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221(6):721–733. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143(1):75–83. doi: 10.1001/archsurg.2007.17. discussion 83. [DOI] [PubMed] [Google Scholar]
- 11.Jamieson NB, Foulis AK, Oien KA, Going JJ, Glen P, Dickson EJ, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010;251(6):1003–1010. doi: 10.1097/SLA.0b013e3181d77369. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 14.Weitz J, Koch M, Friess H, Büchler MW. Impact of volume and specialization for cancer surgery. Dig Surg. 2004;21(4):253–261. doi: 10.1159/000080198. [DOI] [PubMed] [Google Scholar]
- 15.Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242(4):540–544. doi: 10.1097/01.sla.0000184190.20289.4b. discussion 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simunovic M, To T, Theriault M, Langer B. Relation between hospital surgical volume and outcome for pancreatic resection for neoplasm in a publicly funded health care system. CMAJ. 1999;160(5):643–648. [PMC free article] [PubMed] [Google Scholar]
- 17.Howard TJ, Villamustre N, Moore SA, DeWitt J, LeBlanc J, Maglinte D, et al. Efficacy of venous reconstruction in patients with adenocarcinoma of the pancreatic head. J Gastrointest Surg. 2003;7(8):1089–1095. doi: 10.1016/j.gassur.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Pancreaticoduodenectomy with en bloc portal vein resection for pancreatic carcinoma with suspected portal vein involvement. World J Surg. 2004;28(6):602–608. doi: 10.1007/s00268-004-7250-6. [DOI] [PubMed] [Google Scholar]
- 19.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB (Oxford) 2009;11(4):282–289. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbeke CS. Resection margins and R1 rates in pancreatic cancer–are we there yet? Histopathology. 2008;52(7):787–796. doi: 10.1111/j.1365-2559.2007.02935.x. [DOI] [PubMed] [Google Scholar]
- 21.Sobin LH, Wittekind C. International Union Against Cancer TNM classification of malignant tumours. 6th ed. New York, NY: Wiley-Liss; 2002. [Google Scholar]
- 22.Campbell F, Smith RA, Whelan P, Sutton R, Raraty M, Neoptolemos JP, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology. 2009;55(3):277–283. doi: 10.1111/j.1365-2559.2009.03376.x. [DOI] [PubMed] [Google Scholar]
- 23.de la Fuente SG, Bennett KM, Pappas TN, Scarborough JE. Pre- and intraoperative variables affecting early outcomes in elderly patients undergoing pancreaticoduodenectomy. HPB (Oxford) 2011;13(12):887–892. doi: 10.1111/j.1477-2574.2011.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasif N, Ko CY, Farrell J, Wainberg Z, Hines OJ, Rober H, Tomlinson JS. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17(9):2312–2320. doi: 10.1245/s10434-010-1071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz-Tovar J, Martín-Pérez E, Fernández-Contreras ME, Reguero-Callejas ME, Gamallo-Amat C. Identification of prognostic factors in pancreatic cancer. Cir Cir. 2011;79(4):313–322. [PubMed] [Google Scholar]
- 26.House MG, Gönen M, Jarnagin WR, D'Angelica M, DeMatteo RP, Fong Y, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11(11):1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 27.Kamphues C, Bova R, Schricke D, Hippler-Benscheidt M, Klauschen F, Stenzinger A, et al. Postoperative complications deteriorate long-term outcome in pancreatic cancer patients. Ann Surg Oncol. 2012;19(3):856–863. doi: 10.1245/s10434-011-2041-4. [DOI] [PubMed] [Google Scholar]
- 28.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]