Abstract
The bioactive lipid mediator leukotriene B4 (LTB4) greatly enhances phagocyte antimicrobial functions against a myriad of pathogens. In murine histoplasmosis, inhibition of the LT-generating enzyme 5-lypoxigenase (5-LO) increases the susceptibility of the host to infection. In this study, we investigated whether murine resistance or susceptibility to Histoplasma capsulatum infection is associated with leukotriene production and an enhancement of in vivo and/or in vitro antimicrobial effector function. We show that susceptible C57BL/6 mice exhibit a higher fungal burden in the lung and spleen, increased mortality, lower expression levels of 5-LO and leukotriene B4 receptor 1 (BLT1) and decreased LTB4 production compared to the resistant 129/Sv mice. Moreover, we demonstrate that endogenous and exogenous LTs are required for the optimal phagocytosis of H. capsulatum by macrophages from both murine strains, although C57BL/6 macrophages are more sensitive to the effects of LTB4 than 129/Sv macrophages. Therefore, our results provide novel evidence that LTB4 production and BLT1 signaling are required for a histoplasmosis-resistant phenotype.
Introduction
Histoplasmosis is caused by the dimorphic pathogenic fungus Histoplasma capsulatum, and infection occurs when microconidia are inhaled and transformed into yeast in the lung environment [1], [2]. Resistance to H. capsulatum infection requires cooperation between cells of the innate immune system, e.g., macrophages and dendritic cells, and cells of the adaptive immune system, e.g., CD4+ and CD8+ T cells. Interaction between the fungus and resident macrophages results in increased production of the proinflammatory cytokines IL-12, TNF-α, IL-1, IFN-γ and GM-CSF, which are essential for the development of a protective immune response [3], [4], [5]. Furthermore, production of leukotrienes (LTs), which are lipid mediators, is essential for the control of the host defense response during H. capsulatum infection [6], [7]. LTs are derived from the metabolism of arachidonic acid (AA) by the action of 5-lipoxygenase (5-LO) in association with 5-lipoxygenase-activating protein (FLAP). There are two major classes of LTs, namely LTB4 and cysteinyl-LTs (CysLTs), which include LTC4, LTD4 and LTE4 [8], [9], [10]; these two classes of mediators act by binding to the high-affinity G protein-coupled receptors BLT1 and CysLT1, respectively [11], [12]. LTB4 is predominantly synthesized by phagocytes in response to inflammatory or infectious stimuli and has a number of biological functions, including the stimulation of leukocyte migration, the activation of macrophages, eosinophils, neutrophils and T cells [13], [14], [15], opsonized and non-opsonized phagocytosis [7], [16], [17], [18], the production of antimicrobial mediators and microbial killing [19]. CysLTs elicit smooth muscle contraction, mucus secretion and edema during asthma and enhance microbial ingestion and killing [20]. Although both classes of LTs enhance macrophage antimicrobial killing, we have previously shown that LTB4 is approximately 10-fold more potent than CysLTs in enhancing the phagocytosis and killing of microbes [21]. The protective role of endogenous LTs has been demonstrated in several models of infection [6], [7], [18], [22], [23], [24], [25], [26], [27], and our group has a longstanding interest in investigating the role of LTs in pathogen infection, including histoplasmosis. Using pharmacologic and genetic approaches, we demonstrated that endogenous LTs augment the susceptibility of mice to primary and secondary H. capsulatum infections by amplifying fungal clearance and the production of the proinflammatory cytokines IL-12 and IFN-γ and the microbicidal molecule nitric oxide (NO). We have also shown that LTs are essential for enhancement of the activation and recruitment of effector and memory T cells to the site of infection [6], [7], [27].
The pattern of host resistance and susceptibility has been extensively studied in response to infection with Paracoccidioides brasiliensis [28], [29] and the protozoan parasite Leishmania major [25]. Although the production of proinflammatory cytokines is a crucial step in disease progression or resistance in most cases, whether lipids are also involved in these processes remains largely unknown. C57BL/6 mice infected with H. capsulatum are more susceptible to histoplasmosis than infected A/J mice [30], and spleen cells from susceptible C57BL/6 mice have been shown to produce lower amounts of IFN-γ than cells from resistant A/J animals. It has therefore been suggested that IFN-γ production is responsible for the differences observed in resistance/susceptibility during murine histoplasmosis. Nevertheless, because IFN-γ production and its effects can be dependent on LTB4 production as a model of toxoplasmosis, we speculated that LTB4 could be a missing link in the resistance to H. capsulatum infection. The data from our laboratory demonstrate that 129/Sv mice are more resistant to H. capsulatum infection compared to C57BL/6 mice [7], [27]. Therefore, we sought to investigate whether susceptibility and resistance to H. capsulatum infection are associated with differential LT production and/or action in these two animal strains.
Results and Discussion
C57BL/6 mice are more susceptible to H. capsulatum infection than 129/Sv mice
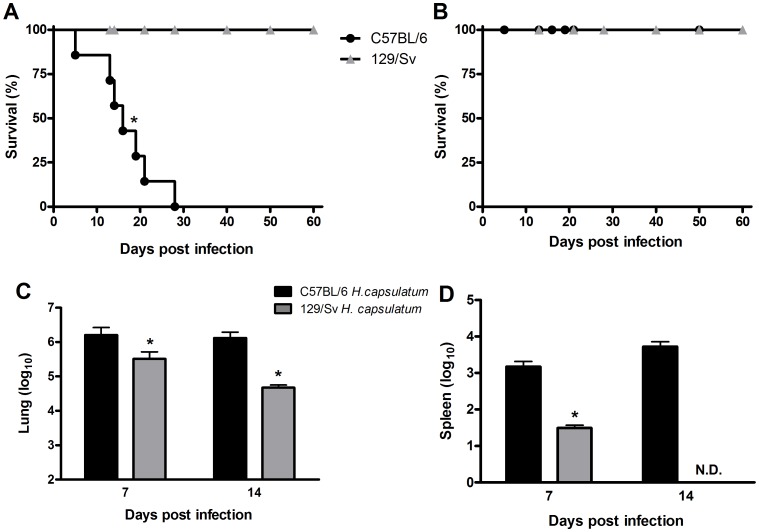
We first confirmed the differences in the H. capsulatum infection susceptibility of C57BL/6 and 129/Sv mice. Both strains were infected with 1×106 yeast cells; 100% of the 129/Sv mice survived up to 60 days after infection, whereas 100% of the C57BL/6 mice died during this period (Figure 1A). We then determined whether the increased mortality of the C57BL/6 mice correlated with impaired fungal clearance. We infected both strains of mice with a sub-lethal dose to evaluate the resolution of the infection. Although we observed a similar survival pattern (100%) in both strains throughout the study (Figure 1B), the fungal loads in the lungs and spleens of the C57BL/6 mice was significantly higher than those in the 129/Sv mice at 7 and 14 days post-infection (Figure 1C, D).
Figure 1. Survival rate and fungal burden in H. capsulatum-infected C57BL/6 and 129/Sv mice.
C57BL/6 and sv129 mice were infected i.t. with 1×106 (A) or 5×105 (B) yeast cells, and their survival was followed for 60 days (n = 6). The fungal loads in the lungs (C) and spleen (D) of mice infected with 5×105 yeast cells were evaluated at 7 and 14 days post-H. capsulatum infection. The data are expressed as the mean ± SEM from one representative experiment of a total of three experiments (n = 6/each experiment). *sv129 compared with C57BL/6. p<0.05 was considered significant.
C57BL/6 and 129/Sv mice exhibit distinct patterns of inflammatory cell recruitment to the lung
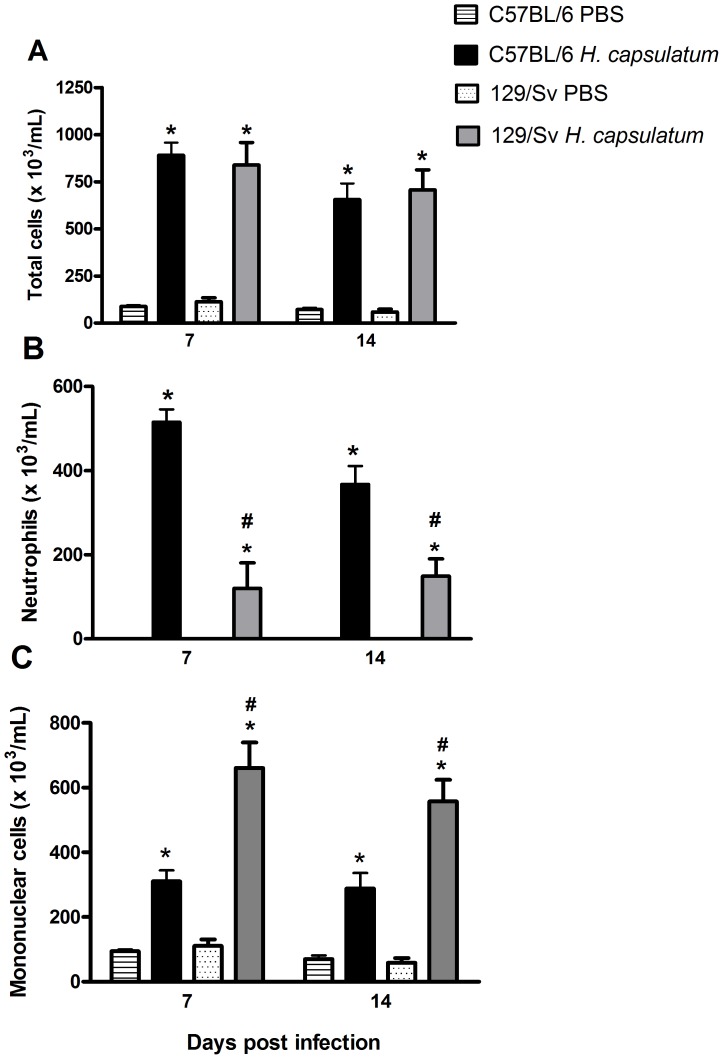
We next sought to investigate whether lung leukocyte recruitment is associated with murine susceptibility and resistance. We observed higher neutrophil recruitment to the bronchoalveolar space in susceptible C57BL/6 mice compared with resistant 129/Sv mice at 7 and 14 days post-infection (Figure 2A). In contrast, we observed higher mononuclear cell recruitment to the bronchoalveolar space throughout the course of infection in resistant 129/Sv mice compared with susceptible C57BL/6 mice (Figure 2B). Moreover, the intense influx of neutrophils in C57BL/6 mice was associated with high levels of TNF-α (Figure 3). We have previously show both 5-LO deficient mice and mice treated with the MK886 and infected with H. capsulatum also exhibits high production of TNF-a which is associated with increased neutrophil migration to the lung [6], [31] Although our groups and others have previously shown that neutrophils are the predominant cell type in the early inflammatory response against H. capsulatum in the lung [1], [7], [15], [32], the role of these cells in the host defense against this mycosis remains unclear. In vitro, neutrophils exhibit potent microbicidal capacity against the mycelial form of H. capsulatum (25), whereas neutrophils exhibit only a microbiostatic effect against the yeast form in vivo. We have previously shown that the depletion of neutrophils by anti-Gr1 (RB6-8C5) antibody treatment restricts fungal growth in the early stages of infection and prevents the spread of yeast cells to other organs but does not modify the subsequent response [32]. In contrast, these results are in agreement with our previous results demonstrating that LT deficiency during H. capsulatum infection results in intense neutrophil recruitment and pulmonary injury, resulting in higher morbidity and mortality in mice [6], [7]. Increased neutrophil infiltration into the bronchoalveolar space after Paracoccidioides brasiliensis infection was also observed in susceptible B10.A mice compared with resistant A/J mice. In the referenced previous study, the antibody-mediated depletion of granulocytes in both strains decreased the survival of only the susceptible B10.A mice [33]. Although both mouse strains (B10.A and A/J) exhibited low neutrophil numbers associated with increased fungal recovery at the beginning of the infection, the spread of the fungus to other organs was observed in only the susceptible B10.A mice. We hypothesize that neutrophils may be important inflammatory cells involved in the control of fungal infection. However, granuloma formation in histoplasmosis is considered important for controlling the growth and dissemination of yeast and protecting against tissue injury [34]. Taken together, the persistence of neutrophil recruitment associated with decreased mononuclear cell recruitment into the lungs could be involved in the increased susceptibility of the C57BL/6 mice.
Figure 2. Differential leukocyte recruitment to the lung in resistant (129/Sv) and susceptible (C57BL/6) mice.
Cells were obtained from mice at 7 and 14 days after i.t. injection of PBS or 5×105 H. capsulatum yeast cells, as described in the Materials and Methods section. The total cells (A), neutrophils (B) and mononuclear cells (C) were enumerated and identified after Rosenfeld staining. The data are expressed as the mean ± SEM from one representative experiment of a total of three experiments (n = 6/each experiment). *C57BL/6 and sv129 compared with PBS; #C57BL/6 compared with sv129. p<0.05 was considered significant.
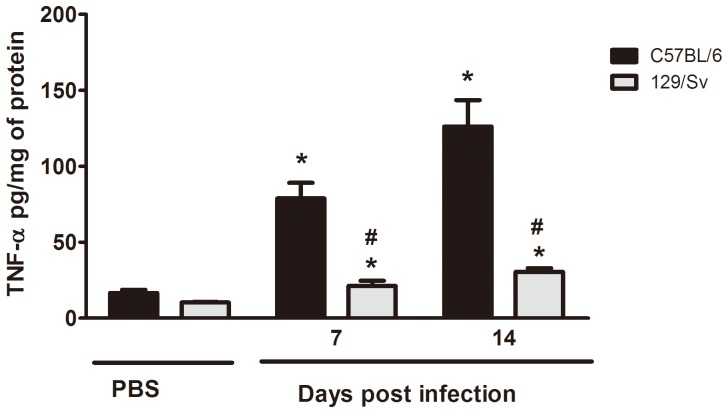
Figure 3. TNF-α production in lung of resistant (129/Sv) and susceptible (C57BL/6) mice.
Lungs were removed at 7 and 14 days after i.t. injection of PBS or 5×105 H. capsulatum yeast cells. TNF-α levels were determinate by ELISA. Data are presented as the mean ± SEM and are representative from one of two independent experiments (n = 6/each experiment). * 129/Sv compared with C57BL/6; #129/Sv H. capsulatum compared with C57BL/6 H. capsulatum. p<0.05 vs. PBS.
The LTB4 synthetic capacity is higher in resistant mice compared to susceptible animals
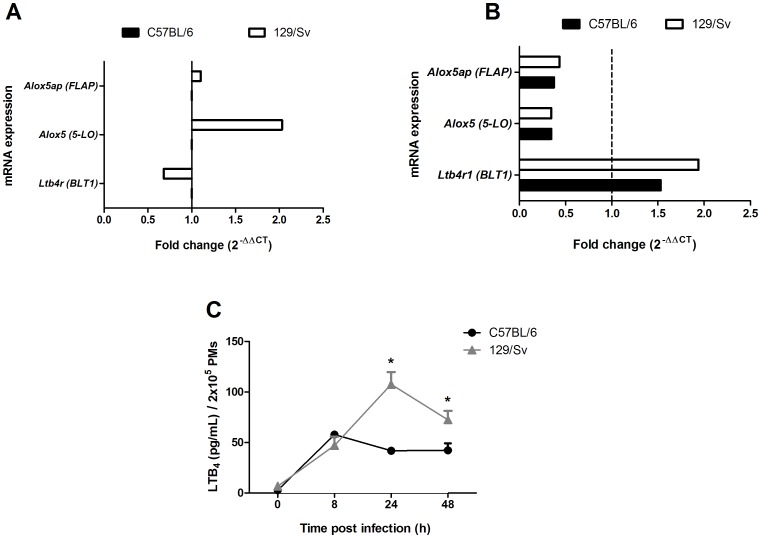
We previously demonstrated that LTs play a critical role in H. capsulatum infection by activating macrophage antimicrobial effector functions and recruiting T cells to the site of infection [6], [7], [27]. To evaluate whether 5-LO metabolites are involved in the susceptibility and resistance to H. capsulatum infection, we first analyzed the expression of the alox5 (5-LO) gene and alox5ap (FLAP) expression using quantitative real-time PCR. We observed that the resistant mice exhibited higher levels of alox5 (5-LO) gene expression but not alox5ap (FLAP) expression than PMs from the susceptible mice resident (Figure 4A). We did not observe any differences in the expression of Alox5 and Alox5ap mRNA in PMs from both strains infected with H. capsulatum. However, when PMs were infected with H. capsulatum, the expression of Ltb4r (BLT1) decreased ∼30% in PMs from resistant mice (Figure 4B). Moreover, 129/Sv PMs produced 157% (after 24 h) and 58% (after 48 h) more LTB4 than PMs from C57BL/6 mice upon H. capsulatum infection in vitro (Figure 4C). Higher LTB4 production in PMs from the resistant mice correlated with a marked increase in alox5 (5-LO) mRNA expression but not alox5ap (FLAP) expression when compared with to the PMs from the susceptible mice resident (Figure 4B). Our results suggest that the genetic background of the host influences its ability to express 5-LO, which is reflected in disease susceptibility or resistance. We have previously shown that macrophages from susceptible BALB/c mice challenged with Leishmania amazonensis produce lower amounts of LTB4 than resistant C3H/HePas mice. Our study provides novel information regarding the differential production of LTB4 by different murine strains.
Figure 4. Differential 5-LO enzyme expression and LTB4 production in C57BL/6 and 129/Sv mice.
The expression of alox5, aloxp5 and Ltbr1 mRNA in PMs from C57BL/6 and sv129 (A) and PMs infected with H. capsulatum (B) for 6 h as described in the Material and Methods. (B) LTB4 production by PMs from C57BL/6 and 129/Sv after in vitro infection with H. capsulatum (MOI = 1∶5) was measured by ELISA. The data are expressed as the mean ± SEM from one representative experiment of a total of two experiments (n = 3 to 5/each experiment). *sv129 compared with C57BL/6. p<0.05 was considered significant.
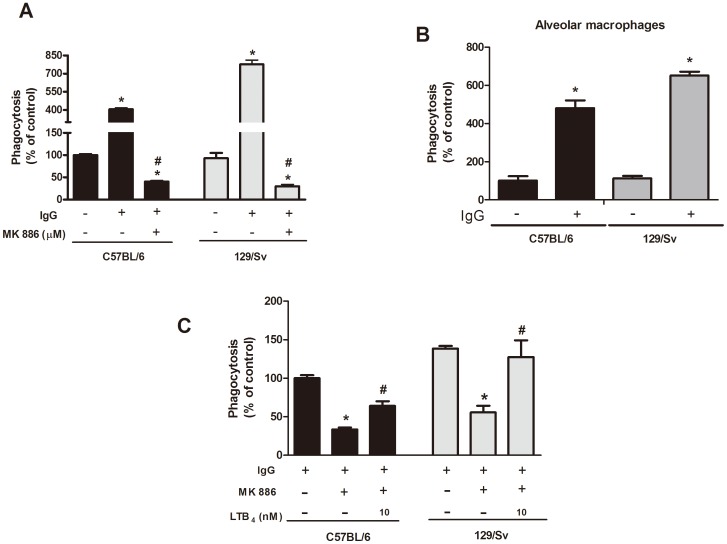
The differential requirement of LTB4 enhances opsonized and non-opsonized phagocytosis in resistant mice
We and other groups have demonstrated that LTB4 production is required for optimal macrophage effector mechanisms, such as phagocytosis and killing [7], [17], [18]. Because we observed that PMs from resistant mice produce higher amounts of LTB4 compared to PMs from susceptible mice, we sought to evaluate the importance of endogenous LTB4 in yeast ingestion by PMs from both mice strains. When PMs from resistant and susceptible mice were challenged with non-opsonized and IgG-opsonized yeast cells, no difference was observed in their abilities to phagocytose the non-opsonized yeast cells. However, as expected, coating the yeast cells with IgG increased the fungal uptake capacity of PMs from resistant mice by 50% compared with PMs from susceptible mice (Figure 5A). Similar phagocytic ingestion were observed in alveolar macrophages from resistant and susceptible mice (Figure 5B). Subsequently, we investigated whether the difference in the uptake of IgG-opsonized H. capsulatum by macrophages from susceptible and resistant mice could be explained by the LTB4/BLT1 signaling axis. The phagocytosis of opsonized yeast by macrophages from both strains was equally dependent on the endogenous synthesis of LTs because pretreatment of the cells with the FLAP inhibitor MK886 nearly abolished the uptake of the fungus by cells from both strains (Figure 5B). Interestingly, PMs from 129/Sv mice were more sensitive to MK886 compared to PMs from C57BL/6 mice, indicating a more profound LT dependency for ingestion in the resistant mice. Moreover, exogenous LTB4 partially restored phagocytosis of yeasts by PMs from susceptible mice whereas macrophages from resistant were total restored (Figure 5C).
Figure 5. Effect of endogenous and exogenous LTB4 on the phagocytosis of H. capsulatum by C57BL/6 and 129/Sv macrophages.
PMs from C57BL/6 and 129/Sv mice were incubated for 2 h with IgG-opsonized or non-opsonized yeast cells at a yeast-to-cell ratio of 1∶5. (A) PMs were pretreated with the LT synthesis inhibitor MK886 (1 µM) for 20 min. (B) AMs from C57BL/6 and 129/Sv mice were incubated for 2 h with IgG-opsonized or non-opsonized yeast cells at a yeast-to-cell ratio of 1∶5. (C) PMs were pretreated with the LT synthesis inhibitor MK886 (1 µM) for 20 min, followed by challenge with LTB4 (10 nm) for 5 min before the infection (C). The data are expressed as the mean ± SEM from one representative experiment of a total of two experiments (n = 3 to 5). *sv129 compared with C57BL/6. #sv129 and C57BL/6 compared with MK886/LTB4 treatment. p<0.05 was considered significant.
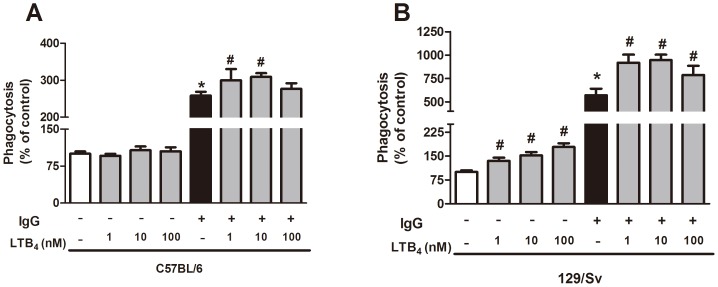
We then investigated the effects of exogenous LTB4 on the phagocytosis capacity of both strains. Interestingly, LTB4 treatment enhanced H. capsulatum phagocytosis by PMs from 129/Sv mice. Compared with the opsonized fungus, LTB4 enhanced fungal ingestion by cells from both strains, but PMs from 129/Sv mice were more responsive to LTB4 than PMs from C57BL/6 mice (Figure 6A, B).
Figure 6. Effect of exogenous LTB4 on the phagocytosis of yeast by C57BL/6 and 129/Sv macrophages.
PMs from C57BL/6 (A) and 129/Sv (B) mice were incubated for 2 h with IgG-opsonized or non-opsonized yeast at a yeast-to-cell ratio of 1∶5 in the presence or absence of exogenous LTB4. The data are expressed as the mean ± SEM from one representative experiment of a total of two experiments (n = 3 to 5). *sv129 compared with C57BL/6; #129/Sv and C57BL/6 compared to LTB4 treatment. p<0.05 was considered significant.
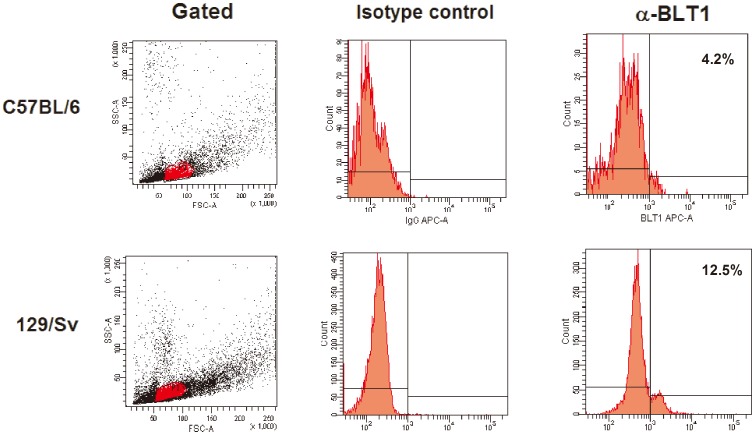
H. capsulatum resistance is associated with a high level of BLT1 expression in the plasma membrane
Because 129/Sv PMs are more sensitive to LTB4 than PMs from C57BL/6 mice, we assessed BLT1 expression in these mice. We observed that 12.5% of macrophages from resistant mice expressed the receptor, whereas only 4.2% of PMs from susceptible animals expressed the receptor (Figure 7). The roles of LTB4 and BLT1 in cell activation have been demonstrated by several investigators [17], [18], [35]. Talvani et al. [36] have shown that LTB4 increases NO production, phagocytosis and microbicidal activity in macrophages infected with T. cruzi. Furthermore, Serezani et al. [37] have demonstrated that LTB4/BLT1 signaling in macrophages is essential for activation of the MyD88/NF-κB pathway and the production of proinflammatory cytokines. The role of LTB4 in enhancing the phagocytosis of non-opsonized pathogens is controversial. However, Okamoto et al. [16] demonstrated that BLT1-deficient macrophages could ingest zymosan. Morato-Marques et al. [18] showed that LTB4 enhances the phagocytosis of C. albicans. Our results provide an answer for this discrepancy: whereas Okomoto et al. [16] used macrophages from C57BL/6 mice, which are less responsive to LTB4, we used macrophages from 129/Sv mice, which are highly responsive to LTB4. Moreover, Campos et al. [38] observed that the increased phagocytosis of IgG-opsonized targets by macrophages treated with LTB4 was dependent on the activation of signaling molecules, such as PKC-α/δ, ERK1/2 and PI3K, and that all of these events were dependent on the interaction between LTB4 and BLT1. Thus, we hypothesize that the decreased effect of LTB4 on yeast phagocytosis by PMs from susceptible mice may be related to a deficiency in BLT1 expression and, consequently, in the activation of the protein kinases involved in this process. In summary, our study further advances the knowledge in the field of murine histoplasmosis by dissecting the differential production of LTB4 and the subsequent responses in two mouse strains.
Figure 7. BLT1 expression on C57BL/6 (A) and 129/Sv (B) macrophages.
The resident cells were obtained as described in the Material and Methods, and the expression of the higher affinity receptor for LTB4 was evaluated by flow cytometry. The mononuclear population was gated using the forward/side scatters and analyzed to determine the fluorescence intensity on the cells. The numbers in the histograms indicate the percentage of cells expressing the BLT1 receptor. The results shown are from one experiment and are representative of two independent experiments.
Materials and Methods
Animals
C57BL/6 and 129/Sv mice (6–8 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and were bred in the Faculdade de Ciências Farmacêuticas de Ribeirão Preto (Universidade de São Paulo, Brazil). All of the experiments were approved and conducted in accordance with the guidelines of the Animal Care Committee of Prefeitura of Campus of Ribeirão Preto (PCARP) of the University of São Paulo (Permit Number 08.1.390.53.3). Infected animals were maintained in biohazard facilities and housed in cages within a laminar flow safety enclosure under standard conditions.
Preparation of H. capsulatum and infection
H. capsulatum was recovered from a patient at the Hospital das Clínicas at the Faculdade de Medicina de Ribeirão Preto of the Universidade de São Paulo, Brazil. Yeast cells were obtained by fungal culture at 37°C on BHI blood agar for 7–10 days and used once their viability reached ≥90%, as measured using fluorescein diacetate and ethidium bromide. The infection was performed as described previously [7]. Briefly, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), restrained on a small board and intratracheally (i.t.) infected with 5×105 or 1×106 viable H. capsulatum yeast cells in 100 µl of PBS. The control animals received 100 µl of phosphate-buffered saline (PBS) i.t.
Culture of organ-infecting H. capsulatum
The recovery of H. capsulatum from the lungs and spleens of the infected mice was performed as previously described by Secatto et al. [7]. Three serial dilutions were performed, and 0.2 ml each serial-dilution was plated on a BHI-agar-blood agar. The number of yeast cells was counted after incubation at 37°C for 21 days and used to express the fungal burden in the tissues. The results are expressed as the number of colony forming units (CFUs) per lung and spleen.
Bronchoalveolar lavage fluid (BALF) analysis and differential cell counts
On days 7 and 14 after infection, the animals were euthanized in a carbon dioxide chamber. The cells present in the bronchoalveolar space were enumerated by differentially counting the neutrophils and mononuclear cells in the BALF, as previously described [6]. The numbers of neutrophils and mononuclear cells were determined using a Neubauer chamber, and the cells were identified by Cytospin and panoptic staining [15].
Measurement of TNF-α and total protein
Lungs were removed on days 7 and 14 post-infection to measure TNF-α. Briefly, tissue was homogenized (Mixer Homogenizer, Labortechnik, Germany) in 2 ml of RPMI 1640, centrifuged and stored at −70°C until assayd. A specific enzyme immunoassay was used according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). The sensitivity of the assay was <10 ng/mL.
Concentration total proteins were quantified in the lungs homogenate of mice by Coomassie protein assay reagent (Rockford, USA), according to the manufacturer's instructions. Data was expressed concentration of TNF-α (pg/ml)/total protein (mg/ml).
Isolation of peritoneal macrophages (PMs) and alveolar macrophages (AMs) and cell culture
PMs were obtained from naive C57BL/6 and 129/Sv mice, as described previously [7]. AMs were obtained from naive C57BL/6 and 129/Sv mice by lung lavage as described previously [39]. The percentage of macrophages was determined microscopically with a panoptic stain, and the purity of the cells was higher than 95%. Cells were cultured overnight in RPMI containing 10% fetal bovine serum and 1% gentamicin. After 24 h, the cells were washed twice with warm serum-free medium to remove all of the non-adherent cells, and the adherent cells were used to assess the degree of fungal phagocytosis.
Measurement of LTB4
LTB4 production was measured in the supernatant of the macrophage culture according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, Michigan, USA).
Phagocytosis assays
The degree of yeast phagocytosis was assessed using the protocol described by Secatto et al. [7]. Briefly, H. capsulatum yeast cells were labeled with FITC (Amresco,Solon,OH) for 1 h at 37°C, as described by Serezani et al. [40]. The FITC-labeled H. capsulatum cells were further diluted in RPMI and incubated with PMs at a ratio of 1∶5 (yeast cells∶macrophages). After 2 h of incubation in the dark (37°C, 5% CO2), uningested yeast cells were removed by washing with PBS, and the residual extracellular FITC was quenched with trypan blue (250 mg/ml; Molecular Probes) for 1 min. Fluorescence was determined using a microplate fluorometer (485 nm excitation/535 nm emission, SPECTRAFluor Plus; Tecan, Research Triangle Park, NC). In some experiments, PMs were pretreated for 5 min with LTB4 (Cayman Chemical, Ann Arbor, Michigan) or MK888 (FLAP inhibitor; Merck Frosst, Canada) diluted in RPMI prior to being co-cultured with FITC-labeled H. capsulatum cells.
Flow cytometric analysis
PMs from control C57BL/6 and 129/Sv mice were obtained as decribed above and adjusted to concentration of 1×106 cells/100 µL. FcγRs were blocked by addition of unlabeled anti-CD16/32 for 40 min. After that, the cells were incubated with an anti-BLT1 mAb (Alexa647) (AbD Serotec) and isotype controls for 30 min at 4°C. The gate was perfomed using SSC/FSC parameters in FACSCanto (BD Biosciences) and FACS Diva software.
RNA isolation and quantitative real-time PCR (qRT-PCR)
The expression of mRNA was evaluated by qRT-PCR after 6 h of infection cells with H.capsulatum or medium. mRNA was obtained using an RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. The levels of alox5ap, alox5 and Blt1 mRNA were normalized to the mRNA levels of Actb and Gapdh using RT2 Profiler PCR Array kit (Qiagen). The results were analyzed using the 2−ΔΔCt method, and the level of gene expression in C57BL/6 cells was set at 1.
Statistical analysis
The data are presented as the mean ± SEM. Comparisons were performed using ANOVA, followed by the Newman-Keuls test. All of the statistical analyses were performed using the Prism 5.0 statistical program (GraphPad Software, San Diego, CA, USA). Any differences in survival were analyzed using the log-rank test. Differences with a p-value of less than 0.05 were considered statistically significant.
Funding Statement
This work was supported by Fundação de Amparo a Pesquisas do Estado de São Paulo (FAPESP, Brazil), the Conselho Nacional de Desenvolvimento Científico e Tecnológico 302097/2010-4 (CNPq, Brazil) and National Institutes of Health (NIH) HL103777-04. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Deepe GS Jr (2000) Immune response to early and late Histoplasma capsulatum infections. Curr Opin Microbiol 3: 359–362. [DOI] [PubMed] [Google Scholar]
- 2. Newman SL (2005) Interaction of Histoplasma capsulatum with human macrophages, dendritic cells, and neutrophils. Methods Mol Med 118: 181–191. [DOI] [PubMed] [Google Scholar]
- 3. Deepe GS Jr, Romani L, Calich VL, Huffnagle G, Arruda C, et al. (2000) Knockout mice as experimental models of virulence. Med Mycol 38 Suppl 1: 87–98. [PubMed] [Google Scholar]
- 4. Zhou P, Sieve MC, Bennett J, Kwon-Chung KJ, Tewari RP, et al. (1995) IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-gamma. J Immunol 155: 785–795. [PubMed] [Google Scholar]
- 5. Deepe GS Jr, McGuinness M (2006) Interleukin-1 and host control of pulmonary histoplasmosis. J Infect Dis 194: 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medeiros AI, Sa-Nunes A, Soares EG, Peres CM, Silva CL, et al. (2004) Blockade of endogenous leukotrienes exacerbates pulmonary histoplasmosis. Infect Immun 72: 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Secatto A, Rodrigues LC, Serezani CH, Ramos SG, Dias-Baruffi M, et al. (2012) 5-lipoxygenase deficiency impairs innate and adaptive immune responses during fungal infection. PLoS One [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samuelsson B (1983) Leukotrienes: a new class of mediators of immediate hypersensitivity reactions and inflammation. Adv Prostaglandin Thromboxane Leukot Res 11: 1–13. [PubMed] [Google Scholar]
- 9. Funk CD (2011) Leukotriene inflammatory mediators meet their match. Sci Transl Med 3: 66ps63. [DOI] [PubMed] [Google Scholar]
- 10. Peters-Golden M, Canetti C, Mancuso P, Coffey MJ (2005) Leukotrienes: underappreciated mediators of innate immune responses. J Immunol 174: 589–594. [DOI] [PubMed] [Google Scholar]
- 11. Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T (1997) A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 387: 620–624. [DOI] [PubMed] [Google Scholar]
- 12. Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, et al. (1999) Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 399: 789–793. [DOI] [PubMed] [Google Scholar]
- 13. Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ (1980) Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 286: 264–265. [DOI] [PubMed] [Google Scholar]
- 14. Faccioli LH, Nourshargh S, Moqbel R, Williams FM, Sehmi R, et al. (1991) The accumulation of 111In-eosinophils induced by inflammatory mediators, in vivo. Immunology 73: 222–227. [PMC free article] [PubMed] [Google Scholar]
- 15. Medeiros AI, Silva CL, Malheiro A, Maffei CM, Faccioli LH (1999) Leukotrienes are involved in leukocyte recruitment induced by live Histoplasma capsulatum or by the beta-glucan present in their cell wall. Br J Pharmacol 128: 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okamoto F, Saeki K, Sumimoto H, Yamasaki S, Yokomizo T (2010) Leukotriene B4 augments and restores Fc gammaRs-dependent phagocytosis in macrophages. J Biol Chem 285: 41113–41121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M (2005) Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 106: 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morato-Marques M, Campos MR, Kane S, Rangel AP, Lewis C, et al. (2011) Leukotrienes target F-actin/cofilin-1 to enhance alveolar macrophage anti-fungal activity. J Biol Chem 286: 28902–28913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flamand L, Tremblay MJ, Borgeat P (2007) Leukotriene B4 triggers the in vitro and in vivo release of potent antimicrobial agents. J Immunol 178: 8036–8045. [DOI] [PubMed] [Google Scholar]
- 20. Wan KS, Wu WF (2007) Eicosanoids in asthma. Acta Paediatr Taiwan 48: 299–304. [PubMed] [Google Scholar]
- 21. Lee SP, Serezani CH, Medeiros AI, Ballinger MN, Peters-Golden M (2009) Crosstalk between prostaglandin E2 and leukotriene B4 regulates phagocytosis in alveolar macrophages via combinatorial effects on cyclic AMP. J Immunol 182: 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peres CM, de Paula L, Medeiros AI, Sorgi CA, Soares EG, et al. (2007) Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis. Microbes Infect 9: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, et al. (1996) Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol 157: 5221–5224. [PubMed] [Google Scholar]
- 24. Machado ER, Ueta MT, Lourenco EV, Anibal FF, Sorgi CA, et al. (2005) Leukotrienes play a role in the control of parasite burden in murine strongyloidiasis. J Immunol 175: 3892–3899. [DOI] [PubMed] [Google Scholar]
- 25. Serezani CH, Perrela JH, Russo M, Peters-Golden M, Jancar S (2006) Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. J Immunol 177: 3201–3208. [DOI] [PubMed] [Google Scholar]
- 26. Chen N, Restivo A, Reiss CS (2001) Leukotrienes play protective roles early during experimental VSV encephalitis. J Neuroimmunol 120: 94–102. [DOI] [PubMed] [Google Scholar]
- 27. Medeiros AI, Sa-Nunes A, Turato WM, Secatto A, Frantz FG, et al. (2008) Leukotrienes are potent adjuvant during fungal infection: effects on memory T cells. J Immunol 181: 8544–8551. [DOI] [PubMed] [Google Scholar]
- 28. Calich VL, da Costa TA, Felonato M, Arruda C, Bernardino S, et al. (2008) Innate immunity to Paracoccidioides brasiliensis infection. Mycopathologia 165: 223–236. [DOI] [PubMed] [Google Scholar]
- 29. Pina A, Bernardino S, Calich VL (2008) Alveolar macrophages from susceptible mice are more competent than those of resistant mice to control initial Paracoccidioides brasiliensis infection. J Leukoc Biol 83: 1088–1099. [DOI] [PubMed] [Google Scholar]
- 30. Wu-Hsieh B (1989) Relative susceptibilities of inbred mouse strains C57BL/6 and A/J to infection with Histoplasma capsulatum. Infect Immun 57: 3788–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Secatto A, Rodrigues LC, Serezani CH, Ramos SG, Dias-Baruffi M, et al. (2012) 5-Lipoxygenase deficiency impairs innate and adaptive immune responses during fungal infection. PLoS One 7: e31701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sa-Nunes A, Medeiros AI, Sorgi CA, Soares EG, Maffei CM, et al. (2007) Gr-1+ cells play an essential role in an experimental model of disseminated histoplasmosis. Microbes Infect 9: 1393–1401. [DOI] [PubMed] [Google Scholar]
- 33. Pina A, Saldiva PH, Restrepo LE, Calich VL (2006) Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J Leukoc Biol 79: 1202–1213. [DOI] [PubMed] [Google Scholar]
- 34. Heninger E, Hogan LH, Karman J, Macvilay S, Hill B, et al. (2006) Characterization of the Histoplasma capsulatum-induced granuloma. J Immunol 177: 3303–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, et al. (2003) Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol 4: 982–990. [DOI] [PubMed] [Google Scholar]
- 36. Talvani A, Machado FS, Santana GC, Klein A, Barcelos L, et al. (2002) Leukotriene B(4) induces nitric oxide synthesis in Trypanosoma cruzi-infected murine macrophages and mediates resistance to infection. Infect Immun 70: 4247–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Serezani CH, Lewis C, Jancar S, Peters-Golden M (2011) Leukotriene B4 amplifies NF-kappaB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J Clin Invest 121: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campos MR, Serezani CH, Peters-Golden M, Jancar S (2009) Differential kinase requirement for enhancement of Fc gammaR-mediated phagocytosis in alveolar macrophages by leukotriene B4 vs. D4. Mol Immunol 46: 1204–1211. [DOI] [PubMed] [Google Scholar]
- 39. Mancuso P, Standiford TJ, Marshall T, Peters-Golden M (1998) 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun 66: 5140–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Serezani CH, Kane S, Medeiros AI, Cornett AM, Kim SH, et al. (2012) PTEN directly activates the actin depolymerization factor cofilin-1 during PGE2-mediated inhibition of phagocytosis of fungi. Sci Signal 5: ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]