Abstract
Objectives:
To test the hypothesis that daily acute intermittent hypoxia (dAIH) and dAIH combined with overground walking improve walking speed and endurance in persons with chronic incomplete spinal cord injury (iSCI).
Methods:
Nineteen subjects completed the randomized, double-blind, placebo-controlled, crossover study. Participants received 15, 90-second hypoxic exposures (dAIH, fraction of inspired oxygen [Fio2] = 0.09) or daily normoxia (dSHAM, Fio2 = 0.21) at 60-second normoxic intervals on 5 consecutive days; dAIH was given alone or combined with 30 minutes of overground walking 1 hour later. Walking speed and endurance were quantified using 10-Meter and 6-Minute Walk Tests. The trial is registered at ClinicalTrials.gov (NCT01272349).
Results:
dAIH improved walking speed and endurance. Ten-Meter Walk time improved with dAIH vs dSHAM after 1 day (mean difference [MD] 3.8 seconds, 95% confidence interval [CI] 1.1–6.5 seconds, p = 0.006) and 2 weeks (MD 3.8 seconds, 95% CI 0.9–6.7 seconds, p = 0.010). Six-Minute Walk distance increased with combined dAIH + walking vs dSHAM + walking after 5 days (MD 94.4 m, 95% CI 17.5–171.3 m, p = 0.017) and 1-week follow-up (MD 97.0 m, 95% CI 20.1–173.9 m, p = 0.014). dAIH + walking increased walking distance more than dAIH after 1 day (MD 67.7 m, 95% CI 1.3–134.1 m, p = 0.046), 5 days (MD 107.0 m, 95% CI 40.6–173.4 m, p = 0.002), and 1-week follow-up (MD 136.0 m, 95% CI 65.3–206.6 m, p < 0.001).
Conclusions:
dAIH ± walking improved walking speed and distance in persons with chronic iSCI. The impact of dAIH is enhanced by combination with walking, demonstrating that combinatorial therapies may promote greater functional benefits in persons with iSCI.
Classification of evidence:
This study provides Class I evidence that transient hypoxia (through measured breathing treatments), along with overground walking training, improves walking speed and endurance after iSCI.
Approximately 59% of all spinal injuries are incomplete,1 leaving spared pathways capable of plasticity.2,3 While spontaneous plasticity partially restores walking ability, these modest gains seldom enable return to overground walking.3–5 Thus, there is a need for strategies promoting plasticity in spared pathways to restore overground walking in persons with incomplete spinal cord injuries (iSCI).
Acute intermittent hypoxia (AIH) is a novel, noninvasive means to induce spinal plasticity, strengthening spared pathways to motoneurons after iSCI. In rodents, daily AIH (dAIH) activates carotid chemoafferents that stimulate episodic serotonin release,6,7 which triggers synthesis of brain-derived neurotrophic factor (BDNF) and activation of TrkB, subsequently strengthening synaptic input and motor output of respiratory and somatic motor nuclei.8–12 In humans with iSCI, a single AIH exposure increases ankle strength.13 However, it is unknown whether AIH-induced gains may restore overground walking in persons with iSCI.
Combinatorial therapies can augment plasticity after iSCI.2,14–16 In rats with iSCI, dAIH combined with ladder walking leads to near complete recovery of ladder walking ability.9 Thus, combining dAIH with overground walking may further enhance walking recovery.
In this study, we tested the hypothesis that dAIH augments walking speed and endurance, and that combined dAIH + walking enhances this effect. We conclude that dAIH ± walking are potentially effective strategies to improve overground walking in persons with chronic iSCI.
METHODS
Subjects.
Twenty-two adults with chronic (>1 year postinjury) iSCI enrolled in this study (table); 19 completed the study (figure 1). Based on means and variability of prior data,13 we estimated 2 groups of 10 would give approximately 85% power to detect differences in walking speed of 20% between dAIH and daily normoxia (dSHAM) at a 0.05 significance level. We included subjects with iSCI between levels C2 and T12, no joint contractures, some voluntary ankle, knee, and hip movements, ability to ambulate at least one step without human assistance, and ability to follow verbal and visual commands. We excluded subjects with progressive SCI, brain injury, cardiopulmonary complications such as obstructive sleep apnea, or other concurrent medical conditions. Subjects maintained prescribed medications and were not taking serotonin-related antidepressants. Subjects participated in experiments at Emory University or Rehabilitation Institute of Chicago.
Table.
Baseline characteristics of randomized subjects (N = 19) participating in study
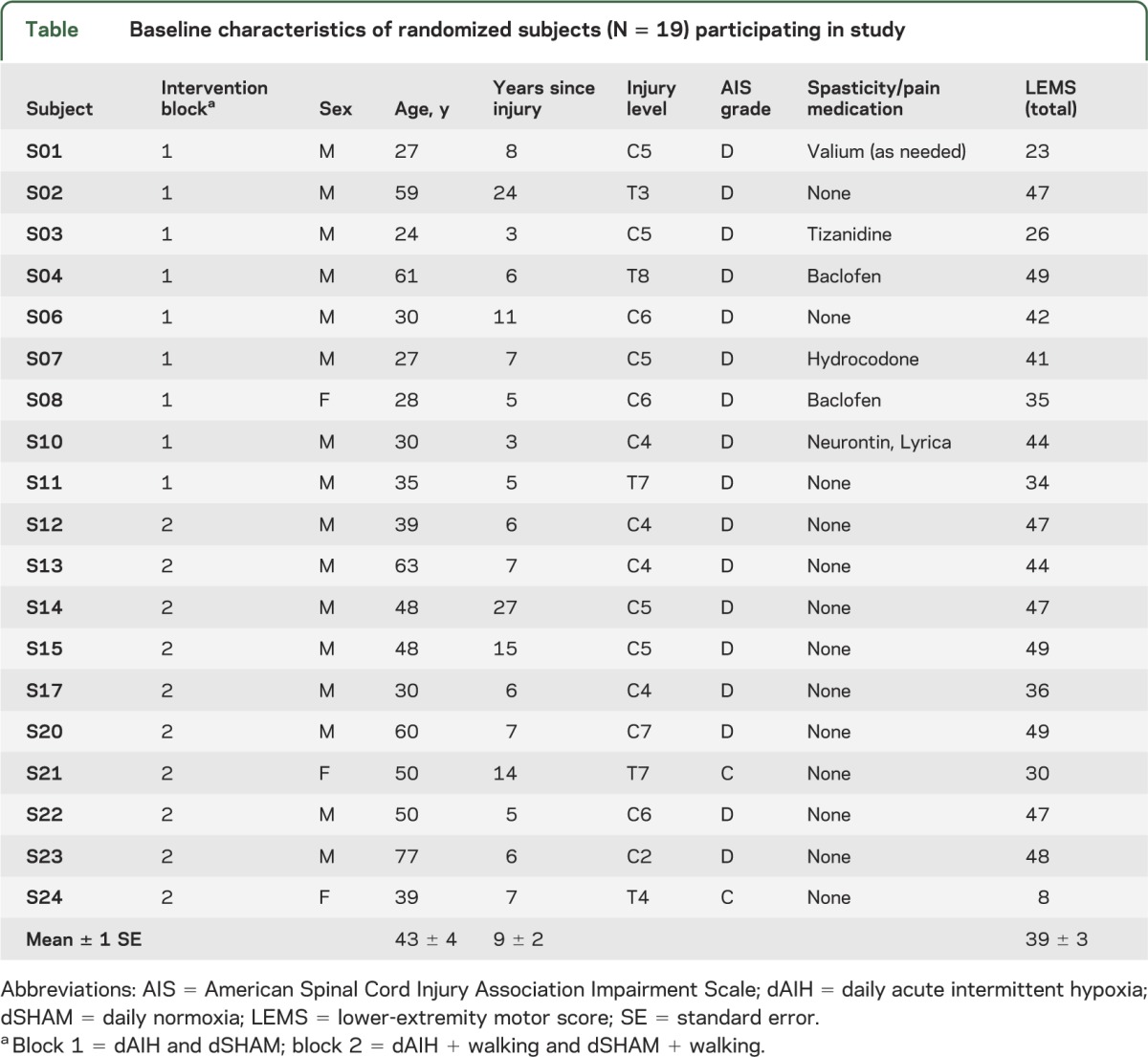
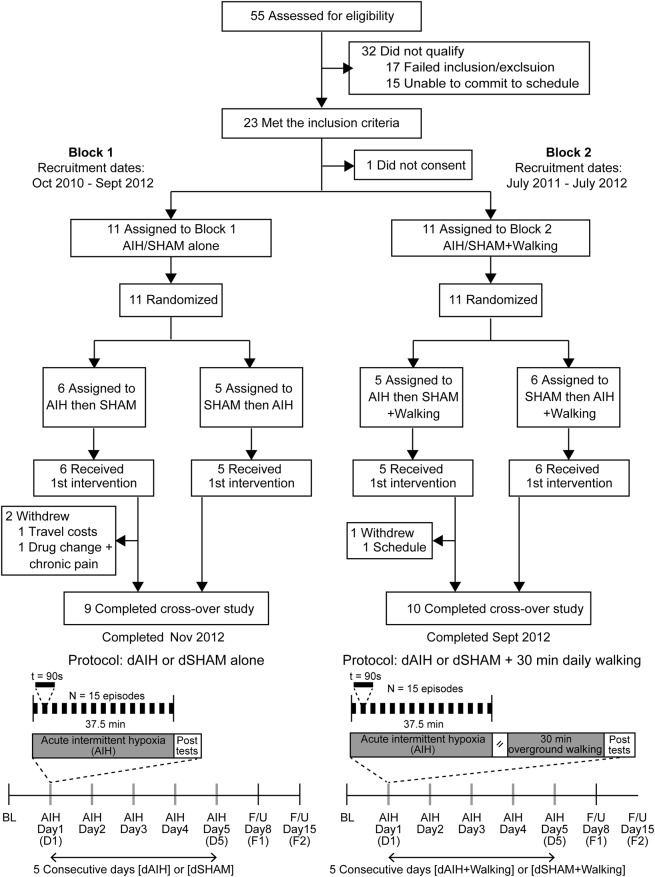
Figure 1. Trial recruitment flowchart and experimental protocol.
Flowchart showing recruitment, randomization, and data collection from subjects in blocks 1 and 2. In block 1, subjects received a breathing intervention consisting of 15 episodes of breathing mild hypoxia (fraction of inspired oxygen [Fio2] = 0.09) for 90 seconds alternating with 60-second normoxia (Fio2 = 0.21). This intervention was repeated once daily for 5 days. Alternatively, subjects received 5 days of continuous normoxia. Overground walking speed and endurance were quantified by the 10-Meter Walk Test and 6-Minute Walk Test, respectively, at baseline (BL), within 60 minutes after acute intermittent hypoxia (AIH) on the first (D1) and fifth (D5) exposure days, and in follow-ups (F/U) at 1 week (F1, i.e., D8) and 2 weeks (F2, i.e., D15). In block 2, subjects received a combinatorial intervention of daily AIH and overground walking (dAIH + walking). After the breathing intervention and a 35- to 45-minute rest period, subjects walked overground at maximal exertion for 30 minutes each day. Overground walking speed and endurance were quantified at BL, D1, D5, F1, and F2. dSHAM = daily normoxia.
Standard protocol approvals, registrations, and patient consents.
Institutional Review Boards at Emory University, Northwestern University, Rehabilitation Institute of Chicago, and Shepherd Center approved this study. Participants met inclusion/exclusion criteria and gave written informed consent. The study was conducted in accordance with the Declaration of Helsinki, registered with ClinicalTrials.gov (NCT01272349), and reported according to CONSORT 2010 guidelines.
Block design and randomization.
This study consisted of 2 experimental blocks (figure 1). In block 1, researchers used a list randomizer to assign 11 subjects to first receive either dAIH or dSHAM, and then the other intervention a minimum of 2 weeks later. In block 2, researchers randomized 11 subjects to first receive either dAIH + walking or dSHAM + walking, and then the other intervention a minimum of 2 weeks later. Randomization and 2-week washout period between interventions were intended to minimize order effect. No differences between baselines for either block were detected for primary outcome measures of walking speed or endurance (paired t tests, all p > 0.05). Participants were comparable in impairment score, assistive device, baseline strength scores, and male/female ratio (2-sample t tests, p > 0.05), but differed in age (p = 0.03). To ensure masking, we did not reveal protocols or intervention order to participants or clinical evaluators. Participants were unable to consistently distinguish between AIH and SHAM. Intervention allocation was recorded in a password-protected document to maintain blinding.
AIH delivery.
A gas mixing system (HYP-123; Hypoxico Inc., New York, NY) provided low inspired oxygen as reported previously.13 Subjects breathed via a nonrebreathing mask attached to a reservoir bag filled by the mixing system between hypoxic exposures. We monitored oxygen concentration to ensure delivery of fraction of inspired oxygen (Fio2) = 0.10 ± 0.02 (OM-25RME; Maxtec Inc., Salt Lake City, UT).
Safety monitoring.
We monitored for incidence of headaches, pain, lightheadedness, dizziness, altered vision, respiratory distress, cyanosis, spasms, autonomic dysreflexia, or change in function during and after treatments. We also monitored cardiovascular responses to treatment before, during, and immediately after treatment to ensure that participants did not exceed heart rate <40 bpm or >160 bpm, systolic blood pressure levels <85 mm Hg or >160 mm Hg, and oxyhemoglobin saturation levels <75%.
Protocols.
To determine effects of dAIH on changes in walking speed and endurance, 9 subjects were studied (block 1; figure 1). Subjects received dAIH (5 consecutive days), consisting of 15, 90-second hypoxic episodes (Fio2 = 0.09) with 60-second normoxic intervals (Fio2 = 0.21; figure 1). At a different time, the same subjects received dSHAM, consisting of 15, 90-second simulated normoxic episodes (Fio2 = 0.21) with 60-second normoxic intervals (Fio2 = 0.21). To reduce carryover effects, we randomized intervention order with a minimum of 2 weeks between dAIH and dSHAM exposures. Walking ability was measured at baseline, within 60 minutes post-AIH on the first (D1) and fifth (D5) exposure days, and in follow-ups at 1 week (F1, i.e., D8) and 2 weeks (F2, i.e., D15). We performed this experimental block at Emory University.
To determine combined effects of dAIH + walking on changes in overground walking speed and endurance, 10 subjects were studied (block 2; figure 1). Subjects received the dAIH and dSHAM protocols, followed within 60 minutes by walking overground at maximal sustainable exertion for 30 minutes. We used overground walking for its greater effects on walking speed and endurance vs treadmill training and its similarity to community ambulation.5 Subjects rested as needed, but resting time was not included in the 30 minutes. The sequence of dAIH + walking and SHAM + walking was randomized. We measured walking ability 60 minutes postwalking at baseline, D1, D5, and at follow-ups F1 and F2. We performed this experimental block at the Rehabilitation Institute of Chicago.
Walking assessment.
Clinical evaluators assessed walking ability at baseline, D1, D5, F1, and F2 using 2 primary outcome measures: 1) walking speed with the 10-Meter Walk Test (10MWT), and 2) walking endurance with the 6-Minute Walk Test (6MWT).17 These metrics have high interrater and intrarater reliability in persons with SCI. In the 10MWT, the evaluator asked subjects to walk 10 m as quickly but safely as possible for 3 trials. In the 6MWT, the evaluator asked subjects to walk as far as safely possible in 6 minutes. Subjects rested as needed but were instructed not to sit. Participants used minimum assistive devices for all testing. As secondary outcome measures, evaluators assessed walking quality using the Walking Index for Spinal Cord Injury and Spinal Cord Injury Functional Ambulation Inventory.
Statistical analyses.
We implemented a repeated-measure, randomized, double-blind (subject and evaluator), crossover design. We used a linear mixed model with fixed effects18 and Bonferroni corrections to test the primary hypothesis that dAIH enhances overground walking speed (10MWT, time in seconds) and endurance (6MWT, distance in meters), with intervention and day as fixed main effects and either 10MWT or 6MWT as the repeated measure. We found no difference in variance between groups for both repeated measures (Levene test, p > 0.05).19 Differences from baseline were used for comparisons between and within interventions (dAIH vs dSHAM; dAIH + walking vs dSHAM + walking) at D1, D5, F1, and F2. We used a nonparametric Friedman test with post hoc signed-rank comparisons to examine the effects of dAIH ± walking on measures of mobility, walking quality, and spasticity between baseline and D1, D5, F1, and F2.
We used SPSS Statistics 20 (IBM Inc., Armonk, NY) for our statistical analyses. Results were considered significant at p < 0.05 and reported with 95% confidence intervals (CIs). We reported mean differences and percent changes relative to baseline as mean ± 1 standard error and identified data outliers using an outlier labeling multiplier of 2.2.20
Classification of evidence.
This study provides Class I evidence that dAIH and dAIH + walking can elicit significant improvements in overground walking speed and endurance in persons with iSCI.
RESULTS
Subjects exposed to dAIH (n = 9) and dAIH + walking (n = 10) improved overground walking ability. dAIH induced significant improvements in walking speed (10MWT). While improvements also were seen in walking endurance (6MWT), they were not significantly greater than with dSHAM. When dAIH was combined with overground walking, the impact was greater on walking endurance than walking speed. dAIH + walking induced significant improvements in walking endurance (6MWT), but not walking speed (10MWT).
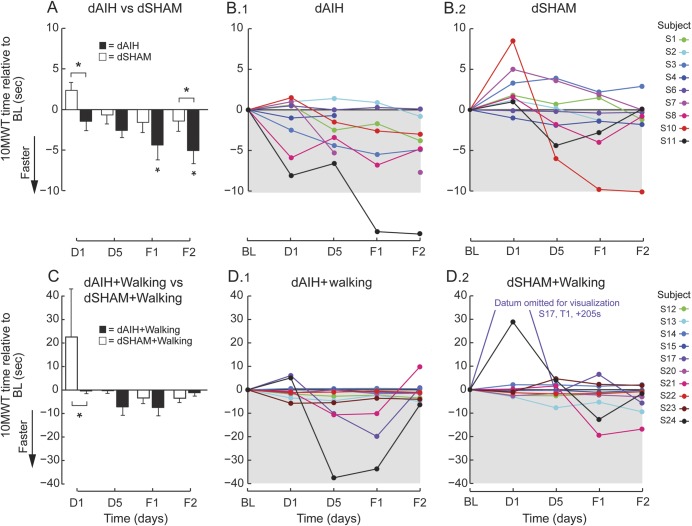
Subjects receiving dAIH showed significant increases in overground walking speed, expressed as decreases in 10MWT time vs baseline. Decreases in time were greater for dAIH compared with dSHAM after only one AIH exposure (figure 2, A and B; 95% CI 1.1–6.5 seconds, p = 0.006) and this difference persisted at F2 (95% CI 0.9–6.7 seconds, p = 0.010). After 5 days of dAIH, 10MWT time also was faster compared with baseline at F1 (95% CI 0.1–8.5 seconds, p = 0.040) and F2 (95% CI 1.0–9.1 seconds, p = 0.005). The improvement relative to baseline is partially driven by the exceedingly large response of S11 to dAIH, because removing this outlier reduces the significance (F1 p = 0.534; F2 p = 0.056). The differences between interventions are robust against this outlier implying a group-wide effect.
Figure 2. dAIH-induced increases in overground walking speed in persons with chronic incomplete spinal cord injury.
(A) Bars represent mean ± 1 standard error changes in 10-Meter Walk Test (10MWT) times (seconds) across all subjects at each time point for daily acute intermittent hypoxia (dAIH) (black) and daily normoxia (dSHAM) (white). Asterisks indicate significance relative to baseline (BL) (repeated-measures linear mixed model, p < 0.05) and brackets with asterisks indicate significant differences between interventions dAIH and dSHAM (repeated-measures linear mixed model, p < 0.05). (B) Subject changes in 10MWT times (seconds) relative to BL across days 1 (D1) and 5 (D5) and follow-ups 1 (F1) and 2 (F2) during dAIH intervention (B.1) and during dSHAM intervention (B.2). Decreases in time represent increases in walking speed. (C) Same mean comparisons as in panel A for dAIH + walking (black) and dSHAM + walking (white). (D) Same subject trends as in panel B for dAIH + walking (D.1) and dSHAM + walking (D.2).
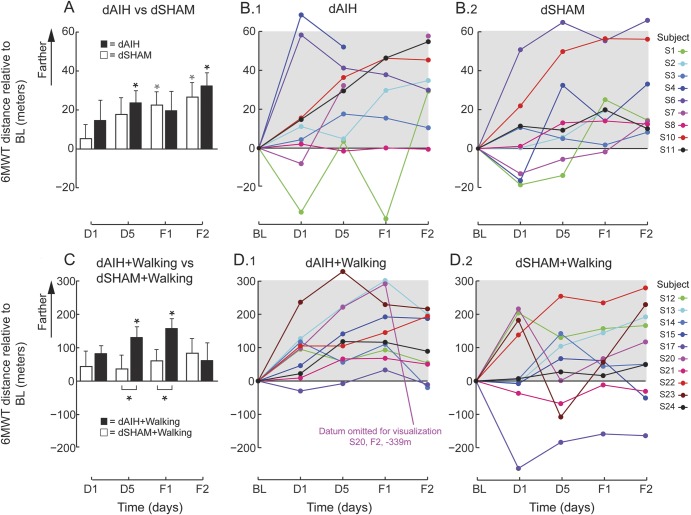
Walking endurance increased after dAIH alone, expressed as increases in 6MWT distance vs baseline (figure 3, A and B; 95% CI 2.4–45.4 m, p = 0.019). These changes persisted at F2 (95% CI 11.4–55.9 m, p < 0.001). There was no difference in 6MWT between dAIH vs dSHAM (p = 0.25), because both yielded increased 6MWT distances (dSHAM: F1 95% CI 1.2–44.3 m, p = 0.031; F2 95% CI 4.2–48.7, p = 0.009), suggesting that repeated 6MWT alone in naive subjects may improve walking endurance.
Figure 3. dAIH-induced sustained increases in overground walking endurance in persons with chronic incomplete spinal cord injury.
(A) Bars represent mean ± 1 standard error changes in 6-Minute Walk Test (6MWT) distances (meters) across all subjects at each time point for either daily acute intermittent hypoxia (dAIH) (black) or daily normoxia (dSHAM) (white). Asterisks indicate significance relative to baseline (BL) (repeated-measures linear mixed model, p < 0.05) and brackets with asterisks indicate significant differences between interventions dAIH and dSHAM (repeated-measures linear mixed model, p < 0.05). (B) Subject changes in 6MWT distances (meters) relative to baseline (BL) across days 1 (D1) and 5 (D5) and follow-ups 1 (F1) and 2 (F2) during dAIH intervention (dAIH, B.1) and during dSHAM intervention (dSHAM, B.2). (C) Same mean comparisons as in panel A for dAIH with daily overground walking (dAIH + walking, black) and dSHAM + walking (white). (D) Same subject trends as in panel B for dAIH + walking (D.1) and dSHAM + walking (D.2).
Subjects receiving dAIH + walking (n = 10) walked farther on the 6MWT vs baseline after 5 days of combined intervention (figure 3, C and D; 95% CI 19.5–242.3 m, p = 0.011). These changes persisted at F1 (95% CI 46.4–269.2 m, p = 0.001), but were no longer significant at F2. dAIH + walking also increased walking endurance more than dSHAM + walking at D5 (95% CI 17.5–171.3 m, p = 0.017) and F1 (95% CI 20.1–173.9 m, p = 0.014).
Although 10MWT times remained unchanged from baseline after AIH + walking, the decrease was greater than dSHAM + walking after one exposure (figure 2, C and D; 95% CI 4.9–41.1 seconds, p = 0.013). A few of the large responses of S17, S21, and S24 with both dSHAM + walking and dAIH + walking were statistical outliers. These subjects exhibited fatigue during D1 testing with dSHAM + walking and then large improvements in 10MWT times with dAIH + walking, suggesting dAIH may have countered fatigue. However, removing these outliers shifted significance between interventions from D1 to D5 (p = 0.005) and added significance relative to baseline at D5 with dAIH + walking (p = 0.025).
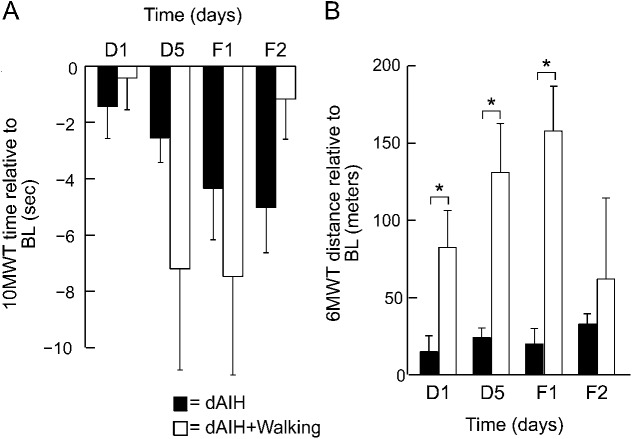
Although the block design limits the ability to directly compare the intervention groups, the data suggest that dAIH + walking may exert greater effects than dAIH alone, particularly on walking endurance. Combined dAIH + walking increased walking distance more than dAIH alone at D1 (95% CI 1.3–134.1 m, p = 0.046), D5 (95% CI 40.6–173.4 m, p = 0.002), and F1 (95% CI 65.3–206.6 m, p = 0.001). After 5 days of dAIH + walking, walking distance increased 30.5% ± 29.1% above baseline, continued to increase at F1 (36.6% ± 26.3%), and was still present at F2 (17.8% ± 31.7%); these changes were more than 2-fold greater than dAIH alone at D5 (15.5% ± 13.6%) and F1 (16.8% ± 24.7%). In contrast, combined dAIH + walking did not improve speed more than dAIH alone (figure 4).
Figure 4. dAIH with daily overground walking produces greater improvements in walking compared with dAIH alone.
(A) Bars represent mean ± 1 standard error changes in 10-Meter Walk Test (10MWT) times (seconds) across all subjects at days 1 (D1) and 5 (D5) and follow-ups 1 (F1) and 2 (F2) for daily acute intermittent hypoxia (dAIH) (black) or the combinatorial intervention of dAIH with daily overground walking (white). (B) Same trends as in panel A for mean ± 1 standard error changes in 6-Minute Walk Test (6MWT) distances (meters). Brackets with asterisks indicate significant differences between interventions dAIH and dAIH + walking (repeated-measures linear mixed model, p < 0.05). BL = baseline.
For both primary outcome measures, 10MWT and 6MWT, the absolute risk reduction ratio was estimated as the number of subjects who did not improve with dSHAM (or dSHAM + walking) at D5 minus the number of subjects who did not improve with dAIH (or dAIH + walking) at D5 out of the total number of subjects. Number need to treat (NNT) was estimated as 1/absolute risk reduction. NNT with dAIH is 5 for 10MWT and 9 for 6MWT. NNT with dAIH + walking is 3 for 10MWT and 5 for 6MWT, indicating greater efficacy.
dAIH was well tolerated by all subjects, and no adverse events were reported or observed. Participants did not report any discomfort before, during, and after treatments. Cardiovascular responses remained consistent after dAIH exposures. We recorded oxyhemoglobin saturation and heart rate at the finger (GE Dash 4000 Monitor, GE Healthcare or Autocorr Plus, Smith Medical Inc.) at 2-second intervals. Oxyhemoglobin saturation reached 78.0 ± 1.5 during hypoxic episodes for dAIH, which was slightly lower than 81.9 ± 1.0 seen for dAIH + walking (2-sample t test; p = 0.381). The mean change in heart rate was 5.7 ± 0.4 for dAIH alone and 4.4 ± 0.8 for dAIH + walking; these responses did not differ (p = 0.165). We recorded blood pressure at baseline and post-AIH. With dAIH alone, we found no change in mean arterial blood pressure before (71 ± 4 mm Hg) vs after (74 ± 3 mm Hg) D1 (paired t test; p = 0.22), or before (74 ± 4 mm Hg) vs after (73 ± 3 mm Hg) D5 (p = 0.62). There also were no differences between D1 and D5 (paired t test; p = 0.30).
We found no change in motor or cognitive function levels before and after dAIH. Specifically, we considered changes in 1) spasms using Spinal Cord Assessment Tool for Spasticity, 2) strength using lower-extremity motor score from the American Spinal Injury Association Impairment Scale, 3) self-care using Spinal Cord Independence Measure, and 4) cognition using Mini-Mental State Examination. Motor and cognitive function levels did not differ from baseline for all clinical assessments (all p > 0.200), despite anecdotal cases of improved lifestyle. These results complement previous studies showing mild doses of AIH to be safe in humans.13,21
DISCUSSION
Our data provide Class I level of evidence that dAIH alone or combined with overground walking holds promise as a safe, effective intervention to restore function in persons with chronic iSCI. With dAIH alone, the primary benefit was increased speed, whereas the combined intervention of dAIH + walking had greater impact on walking endurance. All subjects improved walking ability on the 10MWT or 6MWT, and improvements persisted for more than 3 days in 18 of 19 subjects. With a single AIH exposure, subjects improved 10MWT times by 17.6% over baseline, and 15% after dAIH + walking. Subjects improved 6MWT distance by 24% with dAIH alone, and 36.6% with the combined intervention of dAIH + walking.
The magnitude and duration of dAIH effects is surprising given the limited treatment duration. Contemporary walking therapies for persons with iSCI are typically 4 to 12 weeks in duration, but effect sizes and clinically meaningful improvements are limited. A recent study examined the effects of 4 types of walking therapy applied for 60 sessions (12 weeks), all showing small to moderate effect sizes. The most effective, overground walking training, improved walking speed by 0.09 m/s and endurance by 14.2 m.5 In comparison, dAIH achieved nearly double the effect size for speed and equivalent for endurance, while the combination of dAIH + walking achieved more than double the effect size for speed and endurance. dAIH + walking resulted in increased walking speed of 0.09 m/s and endurance of >100 m, which are comparable to or greater than those seen with much longer training studies.5,22,23 More than 30% of all subjects achieved a clinically meaningful change in walking speed (≥0.13 m/s) and more than 70% achieved a clinically meaningful change in walking endurance (≥50 m).22,24 Thus, dAIH may be most potent, as a plasticity-promoting primer, when coupled with task-specific training to bolster training outcomes.
Pundits in SCI rehabilitation champion the need for combinatorial approaches to amplify small improvements of single interventions, leading to improved quality of life.2,14,25 Whereas combined cellular therapies are sometimes successful in animal iSCI models, such therapies have seldom been combined with walking training in humans because of risks of systemic drug administration.2,15,26 AIH noninvasively induces endogenous spinal plasticity, making it a promising combinatorial adjuvant to rehabilitation training.
dAIH may elicit improved walking ability in humans with iSCI via a serotonin- and BDNF-dependent mechanism similar to that shown in rats.8,9 Immunohistochemistry in animal models shows that dAIH induces plasticity through increased expression of BDNF and phosphorylated TrkB via serotonin-dependent mechanisms in respiratory and nonrespiratory motor nuclei.9 Available evidence suggests that AIH-induced recovery of breathing and walking function after spinal injury is a result of increased synaptic strength and/or motor excitability.9,12,27 Although facilitative respiratory plasticity may partially contribute to improved walking endurance by increasing cardiorespiratory reserve, it is likely accompanied by changes in somatic motor areas more closely related to locomotor control. Serotonin and BDNF have been linked to spinal locomotor recovery after SCI,15,28,29 consistent with the hypothesis that dAIH elicits walking improvements in persons with iSCI through similar mechanisms.10 In animal models, BDNF is more effective when combined with locomotor training and its benefits are primarily seen on the trained task.9,15,30,31 Similarly, the largest improvements in overground walking occurred with combined dAIH + walking and were greatest on the trained task of walking endurance.
Although prolonged intermittent hypoxia causes serious morbidity, considerable data demonstrate that low-dose dAIH, as used here, elicits meaningful functional benefits without inducing pathology.21,32–34 The severity of hypoxemia, number of dAIH episodes, and exposure duration in this protocol were low (15 episodes per day, 5 days) compared with the chronic intermittent hypoxia experienced in obstructive sleep apnea (80–400 episodes per day for years). Similar protocols in rats show no evidence for hypertension, weight loss,35 or hippocampal gliosis or cell death.9 In this and our prior study,13 we observed no adverse events with dAIH. Because serotonin and BDNF have been linked to increased spasticity,36–38 there was a possibility that dAIH could increase spasticity or autonomic dysreflexia. However, we found no evidence that dAIH or dAIH + walking increased spasticity or caused prolonged elevations in heart rate or blood pressure. This evidence suggests that dAIH is a safe and effective means to restore motor function with chronic iSCI. Variations in dAIH protocols (number of exposures and duration) may yield greater safety and efficacy in humans. Optimizing training after dAIH also could increase gains of the combinatorial interventions. Future study is warranted to determine protocols that maximize AIH-induced functional gains and sustainability without invoking maladaptive plasticity and adverse consequences.
Despite careful design, our study has limitations. The study's small sample size, along with heterogeneity of participants' functional capabilities and injury level, may compromise the ability to identify relationships between injury severity and AIH-induced improvements. Although our randomization and crossover design reduced confounding covariates because subjects acted as their own controls, sequential interventions may result in carryover effects. For instance, carryover from dAIH-induced walking improvements in the first sequence may cause greater dSHAM-induced walking performance in the second sequence. Future large-scale trials should consider stratified populations according to function or injury level without crossover to limit confounds.
We anticipate that this approach will provide new directions for neurology aimed at understanding mechanisms of neural plasticity and, subsequently, improve function in persons with SCI. Evidence is now emerging that low-dose dAIH may be a safe, effective treatment to promote meaningful recovery after iSCI and, possibly, motor deficits of other clinical disorders.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Mike Jones, PhD, and Keith Tansey, MD, PhD, for assistance with allocating personnel and participant recruitment from the Shepherd Center, Atlanta, GA; Leslie VanHeil, PT, DScPT, Lauren McCollough, DPT, Karly Bishop, DO, for their assistance with subject recruitment, screening, and blind rating; Ian Cooke, Matthew Freeman, Victoria Stahl, PhD, and Sean Deeny, PhD, for assistance with data collection; and Kirk Easley, MS, for assistance with statistical analyses. The authors thank members of the Data Safety and Monitoring Board, particularly David Burke, MD, and Dale Strasser, MD. The authors extend special thanks to all study participants.
GLOSSARY
- AIH
acute intermittent hypoxia
- BDNF
brain-derived neurotrophic factor
- CI
confidence interval
- dAIH
daily acute intermittent hypoxia
- dSHAM
daily normoxia
- Fio2
fraction of inspired oxygen
- iSCI
incomplete spinal cord injury
- MD
mean difference
- NNT
number need to treat
- 6MWT
6-Minute Walk Test
- 10MWT
10-Meter Walk Test
Footnotes
Editorial, page 98
AUTHOR CONTRIBUTIONS
Heather B. Hayes, PhD, and Arun Jayaraman, PT, PhD, contributed to study design, participant recruitment, data collection, analyses, and interpretation. Dr. Hayes also performed statistical analyses (with consultation from biostatistician Kirk Easley, Masters of Applied Statistics), wrote the first draft of manuscript, and contributed to manuscript revisions and approval of final submission. Megan Herrmann, DPT, contributed to participant recruitment, screening, blind rating, data collection and analyses, as well as approval of final submission. Gordon S. Mitchell, PhD, and William Z. Rymer, MD, PhD, contributed to development of study concept and design, as well as data interpretation, literature search, figures, and manuscript revisions and approval of final submission. Randy D. Trumbower, PT, PhD, was the principal investigator for this study. He contributed to development of study concept and design, as well as data analyses and interpretation, literature search, figures, and manuscript revisions. As corresponding author, he had full access to all data and final responsibility for the decision to submit for publication.
STUDY FUNDING
Support for this work was provided by the US Department of Defense Spinal Cord Injury Research Program grant SC090355P2.
DISCLOSURE
H. Hayes reports no disclosures. A. Jayaraman received research support by Ottobock Corporation, a rehabilitation technology company, USAMRAA Department of Defense W81XWH-10-C-1055, the NIH 2R44HD044271, and US Department of Education NIDRR H133E120010. M. Herrmann reports no disclosures. G. Mitchell is a study section member for the NIH(RIBT). He is a member of the editorial boards of the Journal of Applied Physiology, Comprehensive Physiology, and Frontiers in Physiology. He is funded by the NIH (HL69064, HL80209, HL111598) and the Department of Defense (SC090355P2). W. Rymer serves as a member of the scientific advisory board of Hocoma International, a rehabilitation technology company, and the Neilsen Foundation, a spinal cord injury not-for-profit. Dr. Rymer has served as a consultant for Allergan, a pharmaceutical company. He is a member of the editorial board of the Journal of NeuroEngineering and Rehabilitation. R. Trumbower received research support by the Craig H. Neilsen Foundation, the Department of Defense SC090355P2, and the NIH K12 HD055931. Go to Neurology.org for full disclosures.
REFERENCES
- 1.National Spinal Cord Injury Statistical Center Facts and Figures at a Glance. Birmingham: University of Alabama at Birmingham; 2013 [Google Scholar]
- 2.Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP, Roy RR. Rehabilitative therapies after spinal cord injury. J Neurotrauma 2006;23:560–570 [DOI] [PubMed] [Google Scholar]
- 3.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2001;2:263–273 [DOI] [PubMed] [Google Scholar]
- 4.van Hedel HJ. Gait speed in relation to categories of functional ambulation after spinal cord injury. Neurorehabil Neural Repair 2009;23:343–350 [DOI] [PubMed] [Google Scholar]
- 5.Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther 2011;91:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 2009;587:5469–5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 2000;121:135–146 [DOI] [PubMed] [Google Scholar]
- 8.Baker-Herman TL, Fuller DD, Bavis RW, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 2004;7:48–55 [DOI] [PubMed] [Google Scholar]
- 9.Lovett-Barr MR, Satriotomo I, Muir GD, et al. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 2012;32:3591–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 2012;237:103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 2003;23:2993–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 2005;25:2925–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 2012;26:163–172 [DOI] [PubMed] [Google Scholar]
- 14.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 2006;7:628–643 [DOI] [PubMed] [Google Scholar]
- 15.Weishaupt N, Li S, Di Pardo A, Sipione S, Fouad K. Synergistic effects of BDNF and rehabilitative training on recovery after cervical spinal cord injury. Behav Brain Res 2013;239:31–42 [DOI] [PubMed] [Google Scholar]
- 16.Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 2001;24:807–843 [DOI] [PubMed] [Google Scholar]
- 17.van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil 2005;86:190–196 [DOI] [PubMed] [Google Scholar]
- 18.Cleophas TJ, Zwinderman AH, van Ouwerkerk B. Clinical research: a novel approach to the analysis of repeated measures. Am J Ther 2012;19:e1–e7 [DOI] [PubMed] [Google Scholar]
- 19.Levene H. Robust tests for equality of variances. In: Olkin I, editor. Contributions to Probability and Statistics. Palo Alto, CA: Stanford University Press; 1960 [Google Scholar]
- 20.Hoaglin DC, Iglewicz B, Tukey JW. Performance of some resistant rules for outlier labeling. J Am Stat Assoc 1986;81:991–999 [Google Scholar]
- 21.Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med 2008;233:627–650 [DOI] [PubMed] [Google Scholar]
- 22.Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev 2012;11:CD006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wirz M, Zemon DH, Rupp R, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil 2005;86:672–680 [DOI] [PubMed] [Google Scholar]
- 24.Lam T, Wirz M, Lunenburger L, Dietz V. Swing phase resistance enhances flexor muscle activity during treadmill locomotion in incomplete spinal cord injury. Neurorehabil Neural Repair 2008;22:438–446 [DOI] [PubMed] [Google Scholar]
- 25.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001;82:818–824 [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm JC, Xu M, Cucoranu D, et al. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci 2012;32:5002–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol 2009;169:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol 2002;88:2187–2195 [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Gutin PH, Noble LJ, Nathan D, Yu JS, Nockels RP. Treatment with genetically engineered fibroblasts producing NGF or BDNF can accelerate recovery from traumatic spinal cord injury in the adult rat. Neuroreport 1996;7:2221–2225 [DOI] [PubMed] [Google Scholar]
- 30.Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain 2006;129:1534–1545 [DOI] [PubMed] [Google Scholar]
- 31.Lu P, Tuszynski MH. Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol 2008;209:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serebrovskaya TV. Intermittent hypoxia research in the former Soviet Union and the Commonwealth of Independent States: history and review of the concept and selected applications. High Alt Med Biol 2002;3:205–221 [DOI] [PubMed] [Google Scholar]
- 33.Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem 2004;385:217–221 [DOI] [PubMed] [Google Scholar]
- 34.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 2008;177:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 2009;217:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulenguez P, Liabeuf S, Bos R, et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 2010;16:302–307 [DOI] [PubMed] [Google Scholar]
- 37.Bos R, Sadlaoud K, Boulenguez P, et al. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci USA 2013;110:348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiatry 1990;53:754–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.