Abstract
Background
Newly formed platelets are associated with increased aggregation and adverse outcomes in patients with coronary artery disease (CAD). The mechanisms involved in the regulation of platelet turnover in patients with CAD are largely unknown.
Aim
To investigate associations between platelet turnover parameters, thrombopoietin and markers of low-grade inflammation in patients with stable CAD. Furthermore, to explore the relationship between platelet turnover parameters and type 2 diabetes, prior myocardial infarction, smoking, age, gender and renal insufficiency.
Methods
We studied 581 stable CAD patients. Platelet turnover parameters (immature platelet fraction, immature platelet count, mean platelet volume, platelet distribution width and platelet large cell-ratio) were determined using automated flow cytometry (Sysmex XE-2100). Furthermore, we measured thrombopoietin and evaluated low-grade inflammation by measurement of high-sensitive CRP and interleukin-6.
Results
We found strong associations between the immature platelet fraction, immature platelet count, mean platelet volume, platelet distribution width and platelet large cell ratio (r = 0.61–0.99, p<0.0001). Thrombopoietin levels were inversely related to all of the platelet turnover parameters (r = −0.17–−0.25, p<0.0001). Moreover, thrombopoietin levels were significantly increased in patients with diabetes (p = 0.03) and in smokers (p = 0.003). Low-grade inflammation evaluated by high-sensitive CRP correlated significantly, yet weakly, with immature platelet count (r = 0.10, p = 0.03) and thrombopoietin (r = 0.16, p<0.001). Also interleukin-6 correlated with thrombopoietin (r = 0.10, p = 0.02).
Conclusion
In stable CAD patients, thrombopoietin was inversely associated with platelet turnover parameters. Furthermore, thrombopoietin levels were increased in patients with diabetes and in smokers. However, low-grade inflammation did not seem to have a substantial impact on platelet turnover parameters.
Introduction
Platelets are key players in the development of coronary atherothrombosis, which is the main cause of acute coronary syndromes. Within an individual, platelets are heterogeneous in both size and density. The circulating pool of platelets is held in an equilibrium, which is balanced by platelet production and consumption. In patients with increased platelet turnover, a larger population of young platelets in peripheral blood can now be identified and quantified by staining for messenger ribonucleic acid (mRNA) using either manual techniques [1] or automated flow cytometry [2]. These newly formed platelets, often referred to as “reticulated” or “immature” platelets, lack genomic DNA but contain megakaryocyte-derived mRNA and thus have the translational capacity necessary for protein synthesis [3]. Moreover, immature platelets are characterized by a higher number of dense granules and an increased platelet volume than older platelets [1]. Finally, larger platelets have been shown to be enzymatically and metabolically more active and to have a higher thrombotic potential than smaller platelets [4]–[6].
Mean platelet volume (MPV) has been used as a surrogate marker of platelet turnover and has been shown to be increased in the acute phase of myocardial infarction [7] and also to be a predictor of adverse cardiovascular outcomes in healthy subjects [8] and in patients with previous myocardial infarction [9]. Furthermore, MPV has been reported to be increased in patients with cardiovascular risk factors such as diabetes mellitus [10], smoking [11] and obesity [12].
Increased platelet consumption has been described in patients with coronary atherosclerosis and may be explained by a pathophysiological interaction between platelets and atherosclerotic vessels [13]. Several studies have reported increased levels of immature platelets in patients with acute coronary syndrome [7], [14]–[17] and in patients with previous stent thrombosis [18], [19]. Moreover, high levels of immature platelets are associated with increased residual platelet aggregation in stable patients with coronary artery disease (CAD) receiving antiplatelet therapy [18], [20], [21]. Finally, immature platelets have been shown to be independent predictors of cardiovascular death in patients with acute coronary syndrome [22].
Some studies have investigated platelet volume indices in stable CAD patients [10], [16], [17], [20]. Only a previous study from our group [20] has included the multitude of platelet turnover parameters as evaluated in the present study. Still, gaps of knowledge exist regarding platelet turnover parameters in stable CAD patients. Thrombopoietin and interleukin-6 (IL-6) have been suggested as important regulators of platelet production, yet the mechanisms involved in platelet production and increased turnover are largely unknown [7], [23]. Furthermore, only sparse data exists about the impact of thrombopoietin and low-grade inflammation on platelet turnover in stable CAD patients.
In this hypothesis-generating study, we investigated associations between platelet turnover parameters, thrombopoietin and markers of low-grade inflammation in stable, high-risk CAD patients receiving low-dose aspirin as mono antiplatelet therapy. Furthermore, we explored if platelet turnover parameters were related to the presence of type 2 diabetes, prior myocardial infarction, current smoking, age, gender or renal insufficiency.
Methods
Study Population
We performed a cross-sectional study including 581 stable patients with angiographically documented CAD. In addition, all patients had either prior myocardial infarction (at least 12 months ago), type 2 diabetes mellitus or both. Patients were recruited from the Western Denmark Heart Registry [24] and enrolled from February 2009 to January 2011.
All diabetic patients were diagnosed with type 2 diabetes and treated with oral antidiabetic drugs and/or insulin. All non-diabetic patients had fasting plasma glucose levels <7.0 mmol/L at the time of inclusion. Smokers were defined as active smokers at the time of blood sampling and non-smokers consisted of never smokers and previous smokers. All patients included in the study were treated with 75 mg non-enteric coated aspirin once daily as mono antiplatelet therapy. Patients with platelet counts <120×109/L or >450×109/L were excluded. The in- and exclusion criteria have previously been described in detail [25].
Ethics Statement
The study was conducted in agreement with the Helsinki-II-declaration and approved by The Central Denmark Region Committees on Health Research (M-2007-0180, M-2009-0110) and by the Danish Data Protection Agency. All patients gave written informed consent.
Laboratory Investigations
Blood sampling
All samples were obtained from the antecubital vein with patients in supine position after 30 minutes of rest using vacuum tubes, a large bore needle (19 G), and a minimum of stasis.
Platelet characteristics and haematological parameters
Blood samples for haematological analyses were collected in 3.0 mL tubes containing EDTA (Terumo, Leuven, Belgium). In order to minimize and standardize time-dependent swelling of platelets, haematological analyses were performed within 60 minutes of blood sampling. Haematology parameters were measured using the Sysmex XE-2100 hematology analyser (Sysmex, Kobe, Japan) with upgraded software (XE IPF Master, Sysmex) allowing flow cytometric detection of immature platelets as previously described [2], [20]. In brief, platelet RNA was stained with flourescent dyes (polymethine and oxazine) before stained cells were passed through a semiconductor diode laser beam. The resulting flourescence intensity (RNA content) and forward light scatter (cell volume) were measured and a preset gate (XE IPF Master software, Sysmex) discriminated between mature and immature platelets. Absolute immature platelet count (IPC) was obtained, and immature platelet fraction (IPF) was calculated as the ratio of immature platelets to the total platelet count and reported in percent. Platelet volume parameters were derived from the platelet volume distribution. MPV was calculated by dividing the platelet crit by platelet impedance count. Platelet distribution width (PDW), a measure of platelet anisocytosis, was the width of the size distribution curve in femtoliters (fL) at the 20% level of the peak. The platelet large cell ratio (P-LCR) was defined as the number of cells falling above the 12-fL threshold divided by platelet count.
Thrombopoietin
Whole blood was allowed to clot at room temperature for 30 minutes before serum was separated by centrifugation at 1000 g for 15 minutes and stored at −80°C until analysis. Thrombopoietin concentrations were analysed in duplicate for all patients and the mean of the two results included in the statistical analyses. The coefficient of variance was 9%. Thrombopoietin analyses were performed by ELISA according to the manufacturer’s instructions (Human Tpo Immunoassay, R&D Systems Europe Ltd., Abingdon, UK)
Inflammatory markers
Blood for IL-6 analyses (cobas® 6000 analyser, E module, Roche, Mannheim, Germany) was collected in non-siliconized 5.0 mL tubes (Terumo, Leuven, Belgium) without anticoagulants. The blood was allowed to clot for one hour at 37°C before serum was separated by centrifugation at 2600 g for 10 minutes. Serum was stored at −80°C until analysis.
Blood for high-sensitive C-reactive protein (hs-CRP) analyses (KoneLab 30i, ILS Laboratories Scandinavia, Allerød, Denmark) was collected in 3.0 mL lithium-heparin tubes containing separating gel (Terumo, Leuven, Belgium). The measurement interval for hs-CRP was 0.2–10.0 mg/L. A total of 493 (85%) of the 581 patients were included in the hs-CRP analyses; 28 patients had hs-CRP levels >10.0 mg/L, and hs-CRP was not measured in 57 patients due to a change in laboratory procedures regarding measurements of hs-CRP during the study.
Statistics
If normally distributed, continuous data is presented as mean and standard deviation (SD), if not as median and interquartile range (IQR). Differences between two unpaired groups were tested with a two-sided t-test if data was normally distributed; otherwise the Mann-Whitney test was used. Proportions between two groups were tested using Fischer’s exact test and presented as absolute counts and percentages. Correlations were performed using Spearman’s rank coefficient. Multiple linear regression analyses were used to identify independent determinants of platelet turnover parameters. In the regression analyses, observations which were missing on the outcome variable or on any of the predictor variables were removed. A two-sided p-value <0.05 was considered statistically significant. Data was registered in Epidata® version 3.1 (EpiData Association, Odense Denmark). Statistical analyses were performed using Stata® version 11.0 (StataCorp LP, TX, USA) and graphs performed using GraphPad Prism® version 5.0 (GraphPad Software, CA, USA).
Results
Study Population
Clinical and biochemical characteristics of the study population are shown in Table 1. We studied a population of stable CAD patients with a relatively high-risk profile, as 92% of the patients had a history of prior myocardial infarction, 25% had type 2 diabetes and 17% had both.
Table 1. Baseline characteristics of the study population, n = 581.
| Age, years | 64±9 |
| Body mass index, kg/m2 | 28±4 |
| Males | 460 (79) |
| Current smokers | 122 (21) |
| Blood pressure, systolic, mm Hg | 142±20 |
| Blood pressure, diastolic, mm Hg | 82±11 |
| Biochemistry | |
| B-Leukocytes, 109/L | 7.0±1.9 |
| B-Haemoglobin, mmol/L | 8.9±0.7 |
| B-Red blood cell count, 1012/L | 4.8±0.4 |
| B-Reticulocyte count, 109/L | 48±15 |
| B-Platelet count, 109/L | 231±59 |
| B-Immature platelet count, 109/L | 5.8 (4.4;7.9) |
| B-Immature platelet fraction, % | 2.5 (1.9;3.5) |
| B-Mean platelet volume, fL | 10.8±0.9 |
| B-Platelet distribution width, fL | 13.4±1.9 |
| B-Platelet large cell ratio, % | 32±7 |
| P-High-sensitive C-reactive protein, mg/L | 0.8 (0.4;1.6) |
| S-Interleukin-6, pg/mL | 2.2 (1.5;3.4) |
| S-Thrombopoietin, pg/mL | 45 (28;64) |
| P-Creatinine, µmol/L | 81 (71;94) |
| Cardiovascular morbidity | |
| Prior percutaneus coronary intervention | 562 (97) |
| Prior myocardial infarction | 533 (92) |
| Prior coronary artey bypass grafting | 49 (8) |
| Prior stroke | 25(4) |
| Type 2 Diabetes Mellitus | 148 (25) |
| Medication | |
| Aspirin | 581 (100) |
| Statins | 533 (92) |
| Beta-blockers | 440 (76) |
| ACE inhibitors | 265 (46) |
| Angiotensin receptor blockers | 80 (14) |
| Calcium antagonists | 111 (19) |
| Diuretics | 147 (25) |
| Proton pump inhibitors | 65 (11) |
| Insulina | 47 (32) |
| Oral antidiabetic medicationa | 129 (87) |
Data is presented as mean±SD, n(%) or median (25th;75th percentile).
a out of 148 patients with type 2 diabetes.
B: blood, S: serum, P: plasma.
Platelet Turnover Parameters
Correlation analyses between platelet parameters are listed in Table 2. As expected, all platelet turnover parameters were moderately or strongly associated. A total of 35 patients had high IPF values (≥6%, range 6–14%). These patients were older (68±1.2 vs. 64±0.4 years, p = 0.02) and more frequently had a history of prior myocardial infarction (83% vs. 17%, p = 0.05).
Table 2. Correlation analyses between platelet parameters, n = 581.
| IPF, % | IPC, 109/L | MPV, fL | PC, 109/L | PDW, fL | |
| IPF, % | / | / | / | / | / |
| IPC, 109/L | r = 0.84 | / | / | / | / |
| p<0.0001 | |||||
| MPV, fL | r = 0.78 | r = 0.64 | / | / | / |
| p<0.0001 | p<0.0001 | ||||
| PC, 109/L | r = −0.41 | r = 0.11 | r = 0.36 | / | / |
| p<0.0001 | p = 0.0070 | p<0.0001 | |||
| PDW, fL | r = 0.76 | r = 0.61 | r = 0.94 | r = −0.37 | / |
| p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 | ||
| P-LCR, % | r = 0.78 | r = 0.63 | r = 0.99 | r = −0.37 | r = 0.96 |
| p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 |
IPF: Immature platelet fraction, IPC: Immature platelet count, MPV: Mean platelet volume.
PC: Platelet count, PDW: Platelet distribution width, P-LCR: Platelet large cell ratio.
Platelet Turnover Parameters, Thrombopoietin and Low-grade Inflammation
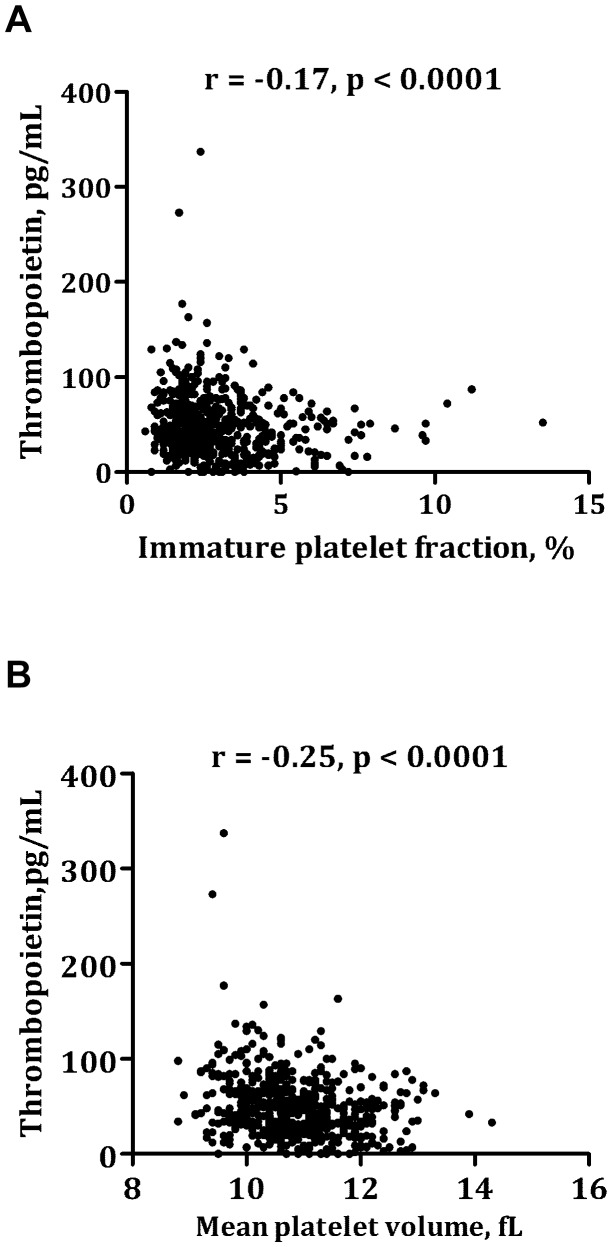
Thrombopoietin was inversely related to IPF (Figure 1A), MPV (Figure 1B), IPC, PDW and P-LCR (r = −0.17–−0.25, p<0.0001). There was a significant, yet weak, association between thrombopoietin and hs-CRP (r = 0.10, p = 0.03) and IL-6 (r = 0.10, p = 0.02). No association was found between thrombopoietin and platelet count (r = −0.01, p = 0.86). Although platelet counts were in the normal range (between 120 and 450∧109/L), five patients had very high thrombopoietin levels (>150 pg/mL) (Figure 1). Both hs-CRP and IL-6 were numerically elevated in these five patients as compared with patients having thrombopoietin levels <150 pg/mL (median hs-CRP mg/L [IQR]: 2.1 [0.7;2,5] vs. 0.9 [0.5;2.2], median IL-6 pg/mL [IQR]: 2.3 [1.7;6.6] vs. 2.2 [1.5;3.4]).
Figure 1. Correlation between thrombopoietin and immature platelet fraction (Figure 1A) and mean platelet volume (Figure 1B).
Hs-CRP was significantly, however weakly, correlated with IPC (r = 0.10, p = 0.03) and platelet count (r = 0.11, p = 0.01). IL-6 did not correlate with any of the platelet turnover parameters (data not shown).
Platelet Turnover Parameters and Clinical Characteristics
Platelet parameters and clinical characteristics of the study population are summarized in Table 3. Patients with diabetes had significantly increased PDW and slightly augmented IPC, MPV and P-LCR, however, this did not reach statistical significance. Smokers had significantly higher IPC and platelet counts than non-smokers. Females, patients aged ≥65 years and patients with an estimated glomerular filtration rate (eGFR) ≤60 mL/min had significantly increased platelet counts. Furthermore, females had higher IPF than males. Platelet turnover parameters did not differ between patients with or without prior myocardial infarction. Except for age and platelet count, all of the above-mentioned differences remained statistically significant after adjustment for diabetes, prior myocardial infarction, smoking, sex, age and eGFR in linear multivariate regression analyses (Table 3).
Table 3. Platelet parameters and clinical characteristics, n = 581.
| Immature | Immature | Mean platelet | ||||
| platelet fraction, % | platelet count, 10∧9/L | volume, fL | ||||
| No diabetes | 2.5 (1.9;3.5) | p = 0.15 | 5.7 (4.3;7.6) | p = 0.07§ | 10.7 (10.1;11.3) | p = 0.06§ |
| Type 2 diabetes | 2.6 (2.0;3.7) | 6.1 (4.5;8.4) | 10.9 (10.3;11.5) | |||
| No prior MI | 2.4 (1.8;3.7) | p = 0.99 | 5.4 (4.5;7.8) | p = 0.76 | 10.9 (10.2;11.5) | p = 0.71 |
| Prior MI | 2.5 (1.9;3.5) | 5.8 (4.4;7.8) | 10.7 (10.2;11.3) | |||
| No current smoking | 2.5 (1.9;3.4) | p = 0.13§ | 5.5 (4.2;8.7) | p = 0.001* | 10.8 (10.2;11.3) | p = 0.30 |
| Current smoking | 2.6 (1.9;3.8) | 6.7 (5.1;8.7) | 10.8 (10.2;11.5) | |||
| Female | 2.4 (1.7;3.4) | p = 0.04* | 5.7 (4.3;7.8) | p = 0.35 | 10.8 (10.1;11.3) | p = 0.51 |
| Male | 2.6 (1.9;3.6) | 6.0 (4.6;7.9) | 10.7 (10.2;11.4) | |||
| Age <65 years | 2.5 (1.9;3.4) | p = 0.67 | 5.9 (4.4;7.8) | p = 0.30 | 10.7 (10.2;11.4) | p = 0.82 |
| Age ≥65 years | 2.5 (1.9;3.6) | 5.6 (4.2;7.7) | 10.8 (10.2;11.3) | |||
| eGFR ≤60 mL/min | 2.5 (1.9;3.4) | p = 0.17 | 6.0 (4.5;8.0) | p = 0.59 | 10.7 (10.2;11.3) | p = 0.23 |
| eGFR >60 mL/min | 2.5 (1.9;3.6) | 5.7 (4.3;7.8) | 10.8 (10.2;11.4) | |||
| Platelet count | Platelet distribution | Platelet large | ||||
| 10∧9/L | width, fL | cell ratio, % | ||||
| No diabetes | 227 (193;262) | p = 0.70 | 13.0 (12.0;14.0) | p = 0.02* | 31 (26;36) | p = 0.07§ |
| Type 2 diabetes | 219 (187;273) | 13.5 (12.0;15.0) | 32 (28;38) | |||
| No prior MI | 223 (193;272) | p = 0.60 | 13.9 (12.0;15.2) | p = 0.43 | 33 (27;38) | p = 0.69 |
| Prior MI | 226 (191;263) | 13.0 (12.0;14.0) | 31 (27;37) | |||
| No current smoking | 222 (190;257) | p = 0.003* | 13.0 (12.0;14.0) | p = 0.38 | 31 (27;36) | p = 0.40 |
| Current smoking | 236 (199;289) | 13.0 (12.0;15.0) | 32(26;38) | |||
| Female | 254 (221;298) | p<0.0001* | 13.0 (12.0;14.0) | p = 0.45 | 32 (27;36) | p = 0.50 |
| Male | 218 (186;255) | 13.0 (12.0;14.0) | 31 (27;37) | |||
| Age <65 years | 232 (198;273) | p = 0.001 | 13.0 (12.0;14.0) | p = 0.28 | 31 (26;37) | p = 0.92 |
| Age ≥65 years | 220 (185;256) | 13.0 (12.0;14.1) | 31 (27;36) | |||
| eGFR ≤60 mL/min | 236 (207;279) | p = 0.0003* | 13.0 (12.0;14.0) | p = 0.08 | 31 (26;36) | p = 0.27 |
| eGFR >60 mL/min | 221 (185;256) | 13.0 (12.0;14.4) | 32 (27;37) | |||
Data is presented as median (25th;75th percentile). Groups are compared using Mann-Whitney test.
p<0.05 in linear multivariate regression analyses adjusted for diabetes, prior MI, smoking, sex, age and eGFR.
p<0.10 in linear multivariate regression analyses adjusted for diabetes, prior MI, smoking, sex, age and eGFR.
MI: myocardial infarction, eGFR: estimated glomerular filtration rate.
Clinical Characteristics, Thrombopoietin and Low-grade Inflammation
Thrombopoietin levels were significantly increased in patients with diabetes (median pg/mL [IQR]: 50 [34;67] vs. 43 [26;64], p = 0.03), smokers (51 [34;72] vs. 43 [27;62], p = 0.003) and in patients without a history of myocardial infarction (53 [41;67] vs. 43 [27;64], p = 0.03). Thrombopoietin levels did not differ between females and males (43 [26;61] vs. 45 [29;65], p = 0.40).
Hs-CRP levels were significantly augmented in smokers (median mg/L [IQR]: 1.1 [0.6;2.4] vs. 0.7 [0.4;1.5], p = 0.001) and a trend was seen in patients with diabetes (1.1 [0.4;2.3] vs. 0.8 [0.4;1.4], p = 0.06). IL-6 was significantly increased in patients aged ≥65 years (pg/mL [IQR]: 2.4 [1.5;3.0] vs. 1.9 [1.5;3.0], p<0.0001) and in patients with eGFR >60 mL/min (2.3 [1.5;3.9] vs. 1.8 [1.5;2.8], p = 0.0001).
Discussion
To the best of our knowledge, this is the first study investigating platelet turnover parameters and the influence of low-grade inflammation and thrombopoietin in a large, homogeneous group of stable CAD patients. In our study, we found strong associations between the platelet turnover parameters IPF, IPC, MPV, PDW and P-LCR. Surprisingly, thrombopoietin was inversely related to IPF, IPC, MPV, PDW and P-LCR. Furthermore, thrombopoietin was significantly increased in patients with diabetes and in smokers. Regarding low-grade inflammation, hs-CRP correlated positively with IPC and platelet count and IL-6 correlated with thrombopoietin, but otherwise there were no associations between platelet turnover parameters and low-grade inflammatory markers.
Platelet Turnover in Coronary Artery Disease
We found strong associations between IPF, IPC, MPV, PDW and P-LCR, which is consistent with previous studies investigating platelet turnover parameters in atherosclerotic patients [14]–[17], [20], [26], [27]. Compared with a group of healthy controls included in a previous study by our group [15], the stable CAD patients included in the present study had higher IPF, MPV and PDW, indicating increased platelet turnover in stable CAD patients. Furthermore, the presence of high IPF values (>6%) was associated with increased age, which is consistent with results from the study by Cesari et al. [14].
Platelet Turnover and Thrombopoietin
We found an unexpected, significant, inverse relation between thrombopoietin and IPF, IPC, MPV, PDW and P-LCR. A similar inverse relation has been reported in several other studies, although in different clinical settings than ours [28]–[30]. The regulation of thrombopoietin is complex and is not fully understood. Under normal physiologic conditions, decreased platelet production and turnover rate result in increased levels of unbound thrombopoietin, thereby enabling a compensatory response of megakaryocytes to the increased demand for peripheral blood platelets [23]. One may hypothesize that the inverse relation between thrombopoietin and platelet turnover parameters found in our study could be explained by an inclination of immature platelets to take up circulating thrombopoietin, thus decreasing free thrombopoietin levels in the setting of increased platelet turnover. Others have suggested megakaryocyte mass as the major determinant of thrombopoietin levels as opposed to circulating platelet count and size [23], [29], [30]. However, we did not measure megakaryocyte mass in our study.
Limited data exits on the relation between thrombopoietin and the platelet turnover parameters IPF and IPC in stable CAD patients. In a previous study by our group, we found that thrombopoietin was an independent predictor of IPF and IPC in patients with stable CAD [20]. In a study by Seneran et al., increased levels of MPV and thrombopoietin were reported in CAD patients as compared with healthy individuals [31]. MPV and thrombopoietin were positively correlated, which is contrasting our results [31]. However, patients included in the study by Seneran et al. were in the acute phase of myocardial infarction or unstable angina as opposed to our patients with stable phase of CAD. This may offer an explanation for the contrasting results, since the interaction between thrombopoietin and thrombopoiesis may be altered during acute coronary syndrome. In accordance with this hypothesis, Lupia et al. demonstrated higher levels of thrombopoietin in patients with unstable angina than in patients having stable angina [32].
Thrombopoietin and Clinical Characteristics
In the present study, thrombopoietin levels were significantly increased in patients with diabetes, which is in accordance with previous results from our group [20]. Furthermore, smokers had significantly augmented thrombopoietin levels. Consistent with our results, Lupia et al. demonstrated significantly increased thrombopoietin levels in smokers as compared with non-smokers [33]. However, their study was fairly small (n = 40) and the study population consisted of healthy individuals. In long-term smokers, platelet aggregability is enhanced leading to subsequent alterations in the clotting-cascade in favor of a pro-thrombotic state [34]. Platelets are capable of releasing thrombopoietin when stimulated [35]. Thus, a potential explanation of increased thrombopoietin levels in smokers could be that platelets themselves are contributors to thrombopoietin release. To the best of our knowledge, no other studies have investigated the distribution of thrombopoietin in smokers with stable CAD.
Platelet Turnover and Low-grade Inflammation
Hs-CRP reflects chronic low-grade inflammation and is a marker of cardiovascular risk [36]. In our study, hs-CRP correlated significantly, yet weakly, with IPC, platelet count and thrombopoietin. Recently, Sahin et al. reported that hs-CRP was independently related to MPV in stable CAD patients and suggested that high MPV values may be part of the chronic low-grade inflammatory state in stable CAD patients [10]. Lupia et al. found significantly higher levels of thrombopoietin and CRP in patients with unstable angina as compared with patients having stable angina and suggested that the acute-phase response related to acute coronary syndrome may play a role in increasing thrombopoietin levels [32].
Even though thrombopoietin is considered to be the key hormone in the regulation of platelet production, it is thought to function in conjunction with numerous cytokines [23]. In vitro, IL-6 seems to act as a megakaryocyte maturation factor and infusion of IL-6 has been shown to induce modest thrombocytosis [37], [38]. Recently, in vivo experimental studies confirmed the regulatory role of IL-6 in thrombopoiesis [39], [40]. However, in the present study, including stable, high-risk CAD patients, we found no associations between IL-6 and any platelet turnover parameters, although, IL-6 and thrombopoietin were weakly associated.
Platelet Turnover and Clinical Characteristics
In agreement with a previous study [41], we found that CAD patients with diabetes had significantly higher PDW than patients without diabetes and a trend for augmented IPC, MPV and P-LCR. A number of studies have reported significant associations between type 2 diabetes and increased platelet volume indices in CAD patients [10], [15], [42], [43]. Potential mechanisms explaining this relation include increased platelet turnover [44] and osmotic swelling of platelets on the basis of raised blood glucose and increased glucose metabolites levels [45].
Smoking is a major risk factor for thrombosis and is associated with adverse cardiovascular events, in part due to enhanced platelet aggregation [34]. We found that smokers had significantly increased IPC and platelet counts compared with non-smokers. This is consistent with previous studies reporting increased IPF in smokers compared with non-smokers [15], [46].
In the present study, patients with eGFR ≤60 mL/min had significantly higher platelet counts compared with patients having normal renal function. This is contrasting recent results by Ucar et al. who reported significantly lower platelet counts in stable CAD patients with eGFR <60 mL/min compared with eGFR ≥60 mL/min [42].
Several previous studies have reported increased MPV [7], [47] and IPF [15] in the acute phase of ST-elevation myocardial infarction. In our study investigating stable CAD patients, platelet turnover parameters did not differ between patients with or without prior myocardial infarction, suggesting the increase in platelet turnover in myocardial infarction is related to the acute event.
Strenghts and Limitations
The overall strength of our study is the inclusion of a large, homogeneous study population with stable CAD. Our study population is very similar to the average CAD patient and thus, the present study has broad applicability. We deliberately excluded patients with low platelet counts or thrombocytosis in order to study platelet turnover in a stable population with platelet counts within the normal range. However, being an observational study, we were not able to establish causality between platelet turnover parameters, thrombopoietin and markers of low-grade inflammation. Accordingly, the conclusions from this study should be seen as being hypothesis-generating.
Conclusion
In stable, high-risk CAD patients, platelet turnover parameters were strongly associated with each other. Thrombopoietin levels were inversely associated with platelet turnover parameters and were significantly increased in patients with diabetes and in smokers. Low-grade inflammation did not seem to have a substantial impact on platelet turnover parameters.
Acknowledgments
We gratefully acknowledge the help of technician Vivi Bo Mogensen, who provided laboratory assistance and performed ELISA analyses.
Funding Statement
The study was financially supported be the Danish Agency for Science Technology and Innovation (grant no. 2101-05-0052), The Danish Heart Foundation, A. P. Møller Foundation, Department of Clinical Medicine, Aarhus University, Denmark, The Eva and Henry Fraenkel Foundation, The Aase and Ejnar Danielsen Foundation, The Korning Foundation, The Physician’s assurance association anno 1891 and The Sophus Jacobsen and Spouse Astrid Jacobsen Foundation. Carl and Ellen Hertz's legate to Danish medical and natural science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ault KA, Rinder HM, Mitchell J, Carmody MB, Vary CP, et al. (1992) The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am J Clin Pathol 98: 637–646. [DOI] [PubMed] [Google Scholar]
- 2. Briggs C, Kunka S, Hart D, Oguni S, Machin SJ (2004) Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol 126: 93–99. [DOI] [PubMed] [Google Scholar]
- 3. Italiano JE Jr, Shivdasani RA (2003) Megakaryocytes and beyond: the birth of platelets. J Thromb Haemost 1: 1174–1182. [DOI] [PubMed] [Google Scholar]
- 4. Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR (1982) Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol 50: 509–519. [DOI] [PubMed] [Google Scholar]
- 5. Martin JF, Trowbridge EA, Salmon G, Plumb J (1983) The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res 32: 443–460. [DOI] [PubMed] [Google Scholar]
- 6. Brown AS, Martin JF (1994) The megakaryocyte platelet system and vascular disease. Eur J Clin Invest 24 Suppl 19–15. [DOI] [PubMed] [Google Scholar]
- 7. Martin JF, Kristensen SD, Mathur A, Grove EL, Choudry FA (2012) The causal role of megakaryocyte-platelet hyperactivity in acute coronary syndromes. Nat Rev Cardiol 9: 658–670. [DOI] [PubMed] [Google Scholar]
- 8. Klovaite J, Benn M, Yazdanyar S, Nordestgaard BG (2011) High platelet volume and increased risk of myocardial infarction: 39,531 participants from the general population. J Thromb Haemost 9: 49–56. [DOI] [PubMed] [Google Scholar]
- 9. Martin JF, Bath PM, Burr ML (1991) Influence of platelet size on outcome after myocardial infarction. Lancet 338: 1409–1411. [DOI] [PubMed] [Google Scholar]
- 10.Sahin DY, Gur M, Elbasan Z, Yildirim A, Akilli RE, et al.. (2012) Mean Platelet Volume Associated With Aortic Distensibility, Chronic Inflammation, and Diabetes in Patients With Stable Coronary Artery Disease. Clin Appl Thromb Hemost. [DOI] [PubMed]
- 11. Kario K, Matsuo T, Nakao K (1992) Cigarette smoking increases the mean platelet volume in elderly patients with risk factors for atherosclerosis. Clin Lab Haematol 14: 281–287. [DOI] [PubMed] [Google Scholar]
- 12. Coban E, Ozdogan M, Yazicioglu G, Akcit F (2005) The mean platelet volume in patients with obesity. Int J Clin Pract 59: 981–982. [DOI] [PubMed] [Google Scholar]
- 13. Ritchie JL, Harker LA (1977) Platelet and fibrinogen survival in coronary atherosclerosis. Response to medical and surgical therapy. Am J Cardiol 39: 595–598. [DOI] [PubMed] [Google Scholar]
- 14. Cesari F, Marcucci R, Caporale R, Paniccia R, Romano E, et al. (2008) Relationship between high platelet turnover and platelet function in high-risk patients with coronary artery disease on dual antiplatelet therapy. Thromb Haemost 99: 930–935. [DOI] [PubMed] [Google Scholar]
- 15. Grove EL, Hvas AM, Kristensen SD (2009) Immature platelets in patients with acute coronary syndromes. Thromb Haemost 101: 151–156. [PubMed] [Google Scholar]
- 16. Khode V, Sindhur J, Kanbur D, Ruikar K, Nallulwar S (2012) Mean platelet volume and other platelet volume indices in patients with stable coronary artery disease and acute myocardial infarction: A case control study. J Cardiovasc Dis Res 3: 272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ranjith MP, Divya R, Mehta VK, Krishnan MG, KamalRaj R, et al. (2009) Significance of platelet volume indices and platelet count in ischaemic heart disease. J Clin Pathol 62: 830–833. [DOI] [PubMed] [Google Scholar]
- 18. Freynhofer MK, Bruno V, Brozovic I, Grove EL, Kristensen SD, et al. (2011) Is increased platelet turnover responsible for low responsiveness to different thienopyridienes? A case report of recurrent stent thromboses. Thromb Haemost 106: 182–184. [DOI] [PubMed] [Google Scholar]
- 19. Wurtz M, Grove EL, Wulff LN, Kaltoft AK, Tilsted HH, et al. (2010) Patients with previous definite stent thrombosis have a reduced antiplatelet effect of aspirin and a larger fraction of immature platelets. JACC Cardiovasc Interv 3: 828–835. [DOI] [PubMed] [Google Scholar]
- 20. Grove EL, Hvas AM, Mortensen SB, Larsen SB, Kristensen SD (2011) Effect of platelet turnover on whole blood platelet aggregation in patients with coronary artery disease. J Thromb Haemost 9: 185–191. [DOI] [PubMed] [Google Scholar]
- 21. Guthikonda S, Alviar CL, Vaduganathan M, Arikan M, Tellez A, et al. (2008) Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol 52: 743–749. [DOI] [PubMed] [Google Scholar]
- 22. Cesari F, Marcucci R, Gori AM, Caporale R, Fanelli A, et al. (2013) Reticulated platelets predict cardiovascular death in acute coronary syndrome patients. Insights from the AMI-Florence 2 Study. Thromb Haemost 109: 846–853. [DOI] [PubMed] [Google Scholar]
- 23. Deutsch VR, Tomer A (2013) Advances in megakaryocytopoiesis and thrombopoiesis: from bench to bedside. Br J Haematol 161: 778–793. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt M, Maeng M, Jakobsen CJ, Madsen M, Thuesen L, et al. (2010) Existing data sources for clinical epidemiology: The Western Denmark Heart Registry. Clin Epidemiol 2: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen SB, Neergaard-Petersen S, Grove EL, Kristensen SD, Hvas AM (2012) Increased platelet aggregation and serum thromboxane levels inaspirin-treatedpatients with prior myocardial infarction. Thromb Haemost 108. [DOI] [PubMed]
- 26. Gonzalez-Porras JR, Martin-Herrero F, Gonzalez-Lopez TJ, Olazabal J, Diez-Campelo M, et al. (2010) The role of immature platelet fraction in acute coronary syndrome. Thromb Haemost 103: 247–249. [DOI] [PubMed] [Google Scholar]
- 27. Lordkipanidze M (2012) Platelet turnover in atherothrombotic disease. Curr Pharm Des 18: 5328–5343. [DOI] [PubMed] [Google Scholar]
- 28. Panasiuk A, Prokopowicz D, Zak J, Panasiuk B (2004) Reticulated platelets as a marker of megakaryopoiesis in liver cirrhosis; relation to thrombopoietin and hepatocyte growth factor serum concentration. Hepatogastroenterology 51: 1124–1128. [PubMed] [Google Scholar]
- 29. Koike Y, Yoneyama A, Shirai J, Ishida T, Shoda E, et al. (1998) Evaluation of thrombopoiesis in thrombocytopenic disorders by simultaneous measurement of reticulated platelets of whole blood and serum thrombopoietin concentrations. Thromb Haemost 79: 1106–1110. [PubMed] [Google Scholar]
- 30. Emmons RV, Reid DM, Cohen RL, Meng G, Young NS, et al. (1996) Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood 87: 4068–4071. [PubMed] [Google Scholar]
- 31. Senaran H, Ileri M, Altinbas A, Kosar A, Yetkin E, et al. (2001) Thrombopoietin and mean platelet volume in coronary artery disease. Clin Cardiol 24: 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lupia E, Bosco O, Bergerone S, Dondi AE, Goffi A, et al. (2006) Thrombopoietin contributes to enhanced platelet activation in patients with unstable angina. J Am Coll Cardiol 48: 2195–2203. [DOI] [PubMed] [Google Scholar]
- 33. Lupia E, Bosco O, Goffi A, Poletto C, Locatelli S, et al. (2010) Thrombopoietin contributes to enhanced platelet activation in cigarette smokers. Atherosclerosis 210: 314–319. [DOI] [PubMed] [Google Scholar]
- 34. Takajo Y, Ikeda H, Haramaki N, Murohara T, Imaizumi T (2001) Augmented oxidative stress of platelets in chronic smokers. Mechanisms of impaired platelet-derived nitric oxide bioactivity and augmented platelet aggregability. J Am Coll Cardiol 38: 1320–1327. [DOI] [PubMed] [Google Scholar]
- 35. Folman CC, Linthorst GE, van MJ, van WG, de JE, et al. (2000) Platelets release thrombopoietin (Tpo) upon activation: another regulatory loop in thrombocytopoiesis? Thromb Haemost 83: 923–930. [PubMed] [Google Scholar]
- 36. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, et al. (2004) C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 350: 1387–1397. [DOI] [PubMed] [Google Scholar]
- 37. Laterveer L, van DJ, Willemze R, Fibbe WE (1993) Continuous infusion of interleukin-6 in sublethally irradiated mice accelerates platelet reconstitution and the recovery of myeloid but not of megakaryocytic progenitor cells in bone marrow. Exp Hematol 21: 1621–1627. [PubMed] [Google Scholar]
- 38. Sosman JA, Aronson FR, Sznol M, Atkins MB, Dutcher JP, et al. (1997) Concurrent phase I trials of intravenous interleukin 6 in solid tumor patients: reversible dose-limiting neurological toxicity. Clin Cancer Res 3: 39–46. [PubMed] [Google Scholar]
- 39. Senchenkova EY, Komoto S, Russell J, Almeida-Paula LD, Yan LS, et al. (2013) Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am J Pathol 183: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang L, Lukowski R, Gaertner F, Lorenz M, Legate KR, et al. (2013) Thrombocytosis as a response to high interleukin-6 levels in cGMP-dependent protein kinase I mutant mice. Arterioscler Thromb Vasc Biol 33: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 41. De Luca G, Venegoni L, Iorio S, Secco GG, Cassetti E, et al. (2010) Platelet distribution width and the extent of coronary artery disease: results from a large prospective study. Platelets 21: 508–514. [DOI] [PubMed] [Google Scholar]
- 42.Ucar H, Gur M, Koyunsever NY, Seker T, Turkoglu C, et al.. (2013) Mean platelet volume is independently associated with renal dysfunction in stable coronary artery disease. Platelets. (doi:10.3109/09537104.2013.805406). In press. [DOI] [PubMed]
- 43. Tavil Y, Sen N, Yazici H, Turfan M, Hizal F, et al. (2010) Coronary heart disease is associated with mean platelet volume in type 2 diabetic patients. Platelets 21: 368–372. [DOI] [PubMed] [Google Scholar]
- 44. Watala C, Boncler M, Pietrucha T, Trojanowski Z (1999) Possible mechanisms of the altered platelet volume distribution in type 2 diabetes: does increased platelet activation contribute to platelet size heterogeneity? Platelets 10: 52–60. [DOI] [PubMed] [Google Scholar]
- 45. Keating FK, Sobel BE, Schneider DJ (2003) Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am J Cardiol 92: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 46. Butkiewicz AM, Kemona-Chetnik I, Dymicka-Piekarska V, Matowicka-Karna J, Kemona H, et al. (2006) Does smoking affect thrombocytopoiesis and platelet activation in women and men? Adv Med Sci 51: 123–126. [PubMed] [Google Scholar]
- 47. Martin JF, Plumb J, Kilbey RS, Kishk YT (1983) Changes in volume and density of platelets in myocardial infarction. Br Med J (Clin Res Ed) 287: 456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]