To the Editor:
Chronic rhinosinusitis (CRS) is an inflammatory disease of the nasal and paranasal sinuses that persists for at least 12 weeks and affects up to 30 million Americans.1 CRS is often divided into 2 clinically and phenotypically distinct classifications: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).2 Despite the high prevalence of this disease, little is known regarding the mechanisms that underlie its pathogenesis, thus limiting effective treatment options. While CRSwNP and CRSsNP in Caucasian patients have classically been described as TH2- and TH1-associated diseases, respectively, it is becoming increasingly clear that the inflammatory responses within the affected sinus tissues of these patients are complex. For example, among other mediators, we have reported elevated levels of IL-63 and B-cell–activating factor of the TNF family4 as well as increased numbers of B-lineage cells5 in CRSwNP tissue than in controls.
Many inflammatory cytokines mediate their effects via the JAK-STAT signaling pathways. Under normal conditions, these pathways have built-in negative feedback mechanisms to prevent chronic activation of inflammatory responses. One important group of regulatory molecules is the suppressor of cytokine signaling (SOCS) proteins, which negatively regulate JAK-STAT pathways by binding to JAK proteins and inhibiting the phosphorylation of STAT proteins.6 Of the 7 SOCS proteins, SOCS3 is one of the best characterized and it is induced by STAT3 activation.6 SOCS3 directly binds to and inhibits JAK1, JAK2, and TYK2, but not JAK3.6 Previous reports have suggested that SOCS3 expression is elevated in TH2 cells and that SOCS3 may play a role in TH2 skewing in asthma and atopic diseases.7 A recent study by Park et al8 from South Korea found that levels of SOCS3 and SOCS1 were elevated in CRS ethmoid tissue compared with control tissue. Although SOCS proteins are known to negatively regulate inflammatory signaling pathways, Park et al suggested that elevated levels of SOCS1 and SOCS3 might contribute to disease pathogenesis in CRS.8 Their report has prompted us to communicate contrasting results on SOCS3 expression in CRS from our studies in Chicago.
We used Western blots and densitometry to analyze the levels of total STAT3, activated STAT3 (pSTAT3), and total SOCS3 in uncinate tissue (UT) from 5 control patients, 18 patients with CRSsNP, and 24 patients with CRSwNP as well as 31 nasal polyp (NP) samples from patients with CRSwNP (see Table E1 in this article's Online Repository at www.jacionline.org). All patients were recruited from the Allergy-Immunology and Otolaryngology Clinics of the Northwestern Medical Faculty Foundation and the Northwestern Sinus Center at the Northwestern Medical Faculty Foundation. UT and NP tissues were obtained during routine functional endoscopic sinus surgery from patients with CRS. CRS was defined by the American Academy of Otolaryngology–Head and Neck Surgery Chronic Rhinosinusitis Task Force. Patients with an aspirin-exacerbated respiratory disease, established immunodeficiency, pregnancy, coagulation disorder, classic allergic fungal sinusitis, or cystic fibrosis were excluded from the study. Control tissues were obtained from subjects without a history of sinonasal inflammation during endoscopic skull-base tumor excisions, intranasal procedures for obstructive sleep apnea, and facial fracture repairs. Asthma was physician diagnosed, and atopy was defined by positive skin-prick testing results. Two of the 31 patients with CRSwNP were on nasal steroids. All subjects provided informed consent, and the study was approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine. Western blots were performed as previously described5 by using the following primary antibodies: mouse antihuman STAT3 (1:1000; Cell Signaling Technology, Danvers, Mass), rabbit antihuman pSTAT3 (1:1000; Cell Signaling Technology), mouse antihuman SOCS3 (1:250; BioLegend, San Diego, Calif), and mouse antihuman β-actin (1:10,000; Sigma-Aldrich, St Louis, Mo). The relative density of STAT3, pSTAT3, and SOCS3 bands was normalized to β-actin. STAT3 genomic sequencing was performed as previously described.9 Comparisons between the groups were done by using 1-way ANOVA with Tukey's adjustment for multiple comparisons, and correlations were calculated by using Spearman r value with GraphPad Prism v5.0b (GraphPad Software, La Jolla, Calif); P value of less than .05 was considered significant.
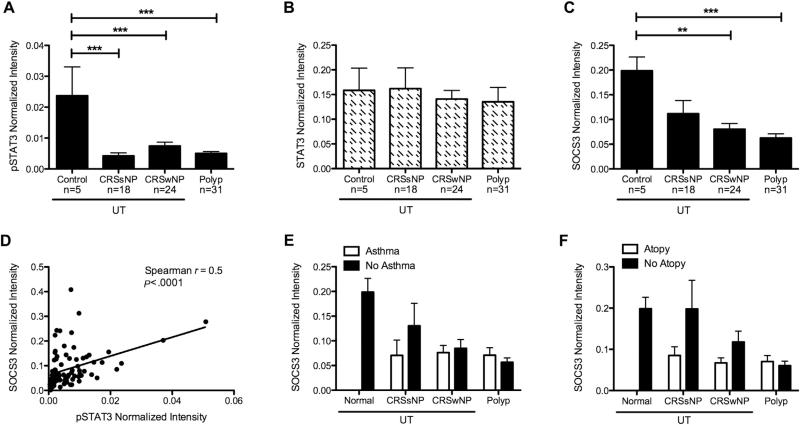
We have previously reported that despite elevated levels of the STAT3-activating cytokine IL-6 and the soluble IL-6 receptor in NPs, levels of pSTAT3 were diminished in these same tissues compared to control tissues.3 In the current study, we first confirmed and extended these results and found that pSTAT3 levels were also diminished in UT, as well as NPs, from both CRS groups compared to control UTs (P <.001, Fig 1, A), while levels of total STAT3 were not different among the groups (Fig 1, B). To exclude the possibility that germline or somatic mutations in STAT3, like those associated with decreased STAT3 signaling in hyper-IgE syndrome,9 were not the cause of the decreased pSTAT3 in these patients, the STAT3 gene was sequenced in a subset of 18 patients with CRSwNP. No STAT3-coding mutations were identified (data not shown). Because SOCS3 is known to inhibit STAT3 phosphorylation, we investigated whether levels of SOCS3 was elevated in CRS tissues. Surprisingly, we found that levels of SOCS3 were significantly decreased in CRSwNP UTand NP tissues compared to controls (P <.01 and .001, respectively, Fig 1, C). In fact, levels of SOCS3 were significantly positively correlated with levels of pSTAT3 in our patient samples (Spearman r = 0.5, P < .001; Fig 1, D). Moreover, we found no association with asthmatic or atopic status on the levels of pSTAT3 (data not shown) or SOCS3 (Fig 1, E and F) in these tissues. Because many factors may play a role in diminishing pSTAT3 levels in CRS tissues, we also investigated whether soluble gp130 or eosinophilic cationic protein might play a role, but found no correlation between these factors and pSTAT3 levels (data not shown).
FIG 1.
pSTAT3 and SOCS3 are diminished in CRSwNP. Protein from UT or NP tissue was analyzed by using Western blotting and normalized to β-actin for pSTAT3 (A), total STAT3 (B), and SOCS3 (C). D, Correlation of pSTAT3 and SOCS3. Effect of asthmatic status (E) or atopic status (F) on SOCS3 levels. Data in Fig 1, A, B, C, E, and F represent mean ± SEM; **P < .01 and ***P < .001 by 1-way ANOVA with Tukey's adjustment.
Previous work has suggested that elevated expression of SOCS3 is associated with TH2-mediated diseases.7 Given that CRSwNP is characterized by TH2-associated inflammation and eosinophilia, we expected to find that SOCS3 levels were elevated in CRSwNP. Our results indicate that rather than being elevated, SOCS3 levels were diminished, along with pSTAT3, in CRSwNP sinonasal tissues regardless of asthmatic or atopic status. Together with the positive correlation between pSTAT3 and SOCS3 levels in our patient samples, these data suggest that factors other than SOCS3 are involved in the reduction of pSTAT3 levels in CRS tissues. Overall, low levels of SOCS3 in diseased tissue may suggest that there is an absence of an important anti-inflammatory regulatory mechanism that may promote chronic inflammatory signaling in CRS. In addition, our data indicate that there is a baseline level of pSTAT3 in control tissue that may be important for the maintenance of tissue homeostasis. Interestingly, however, our results are in direct contrast with the results of the study by Park et al.8 It is worth noting that the study by Park et al did not include analysis of NP tissue, nor did it include any patients with asthma or atopy.8 Similarly, while we have previously reported elevated expression of IL-6 in NP tissue,3 Park et al8 found no difference in IL-6 expression in their samples. One possible explanation for this discrepancy may be the use of ethmoid tissue by Park et al versus UT and NP in our studies. We have found that gene expression patterns vary widely within the sinus mucosa,10 and it is possible that UT and ethmoid differ. Alternatively, although the study by Park et al found that the level of IL-13 was elevated in CRSwNP and the level of IFN-γ was elevated in CRSsNP, in line with phenotypes of Caucasian populations, it is possible that the discrepancy between our results could be due to differences in CRS phenotypes that have been previously noted between CRS in Asian and Caucasian populations.11 Taken together, however, these data suggest that tissue sampling and patient demographics may be important factors to consider when comparing results of similar studies.
Our current work may provide a valuable insight into the mechanisms that drive chronic inflammation in CRSwNP, and ongoing studies are focused on further elucidating these mechanisms. A better understanding of CRS pathogenesis will hopefully uncover strategies for the development of improved therapeutic strategies for patients suffering from CRS.
Supplementary Material
Acknowledgments
This study was funded by National Institutes of Health grants R37 HL068546, R01 HL078860, R01 AI072570, and T32 AI083216 and the Ernest S. Bazley Trust.
Footnotes
Disclosure of potential conflict of interest: R. P. Schleimer has consultant arrangements with Interesect ENT, GlaxoSmithKline, Allakos, and Aurasense. L. C. Grammer has received grants and travel support for meetings or other purposes from the National Institutes of Health (NIH); has received grants from the Bazley Foundation; has consultant arrangements with Atellas Pharmaceuticals; is employed by Northwestern University and the Northwestern Medical Faculty Foundation; has grants/grants pending with the NIH, the Food Allergy Network, and S&C Electric; has received payment for lectures, including service on speakers’ bureaus, from the American Academy of Allergy, Asthma & Immunology and Mount Sinai; and receives royalties from Lippincott, UpToDate, BMJ, and Elsevier. B. K. Tan has received grants from the NIH and the Triological Society/American College of Surgeons; has consultant arrangements with Acclarent, Inc, and has received travel/accommodations/meeting expenses from the Foundation for Innovation, Education, and Research in Otorhinolaryngology. T. R. Torgerson has consultant arrangements with Baxter Biosciences and BD Biosciences; has grants/grants pending with Baxter Biosciences and CSL Behring; has received payment for lectures, including service on speakers’ bureaus, from Baxter Biosciences; receives royalties from New England Biolabs; and receives payment for the development of educational presentations from Baxter Biosciences. K. E. Harris has received grants from the NIH/National Heart Lung and Blood Institute (grant no. R37L068546 and grant no. R01HL078860), the NIH/National Institute for Allergy and Infectious Disease (grant no. T32A1083216), and the Ernest S. Bazley Trust. A. T. Peters has received payment for lectures, including service on speakers’ bureaus, from Baxter. A. Kato has received a grant from the NIH (grant no. R21HL113913). The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–6. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125:397–403. e10. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. 1392, e1–2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–83. 1083, e1–7. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babon JJ, Kershaw NJ, Murphy JM, Varghese LN, Laktyushin A, Young SN, et al. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity. 2012;36:239–50. doi: 10.1016/j.immuni.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–54. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 8.Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, et al. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol. 2013;131:772–80. doi: 10.1016/j.jaci.2012.12.671. [DOI] [PubMed] [Google Scholar]
- 9.Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–7. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seshadri S, Rosati M, Lin DC, Carter RG, Norton JE, Choi AW, et al. Regional differences in the expression of innate host defense molecules in sinonasal mucosa. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2013.05.042. [Epub 2013 Jul 26] http://dx.doi.org/10.1016/j.jaci.2013.05.042. [DOI] [PMC free article] [PubMed]
- 11.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.