Abstract
Plant organ size and thus plant size is determined by both cell proliferation and cell expansion. The bHLH transcription factor SPATULA (SPT) was originally identified as a regulator of carpel patterning. It has subsequently been found to control growth of the organs of the shoot. It does this at least in part by controlling the size of meristematic regions of organs in parallel to gibberellic acid (GA). It also acts downstream of several environmental signals, influencing growth in response to light and temperature. We have recently demonstrated that SPT functions to repress the size of the root meristem and thus root growth and size. It appears to do this using a similar mechanism to its control of leaf size. Based on the recent work on SPT, we propose that it is a growth repressor that acts to limit the size of meristems in response to environmental signals, perhaps by regulating auxin transport.
Keywords: SPT, organ size, root growth, gibberellic acid, auxin, environmental signals
The size of plant organs is controlled by both cell division and cell expansion and regulation of these processes is an area of active investigation. Many genes have been identified that influence final organ size either through control of cell division, expansion or both and the relationship between the two processes is complex (reviewed in refs. 1–3). Recently the bHLH transcription factor SPATULA (SPT) has emerged as an important regulator of organ size in Arabidopsis thaliana. Although first identified for its effects on pistil morphology,4 SPT has emerged as a more general repressor of organ growth. Loss of function mutants in SPT have larger cotyledons, longer hypocotyls and larger leaves while overexpression leads to smaller organs.5-7 Depending on the organ, the difference in size is the result of changes in cell number and/or cell size, suggesting that SPT can regulate both processes.
SPT functions in both cotyledons and leaves. In cotyledons it acts to repress expansion in parallel to the gibberellin (GA)-dependent DELLAs.5 SPT and GA share some common target genes in this organ and SPT is negatively regulated by DELLAs, suggesting a complex relationship between GA and SPT. In contrast to the cotyledon, SPT restricts cell division in leaves. In this organ a proliferative zone is found between the developing blade and petiole.8 This zone is established early in leaf development and produces cells that populate both blade and petiole. Expression of a SPT enhancer trap line is found in the marginal region of this proliferative zone.6,8 Consistent with this expression, in spt leaves the meristematic region of the leaf primordia was found to have more cells than in wild type.6 This data suggests that SPT is important for regulating the size of the meristematic region of leaves and that the larger leaf size seen in spt plants is a result of expanded meristematic identity.
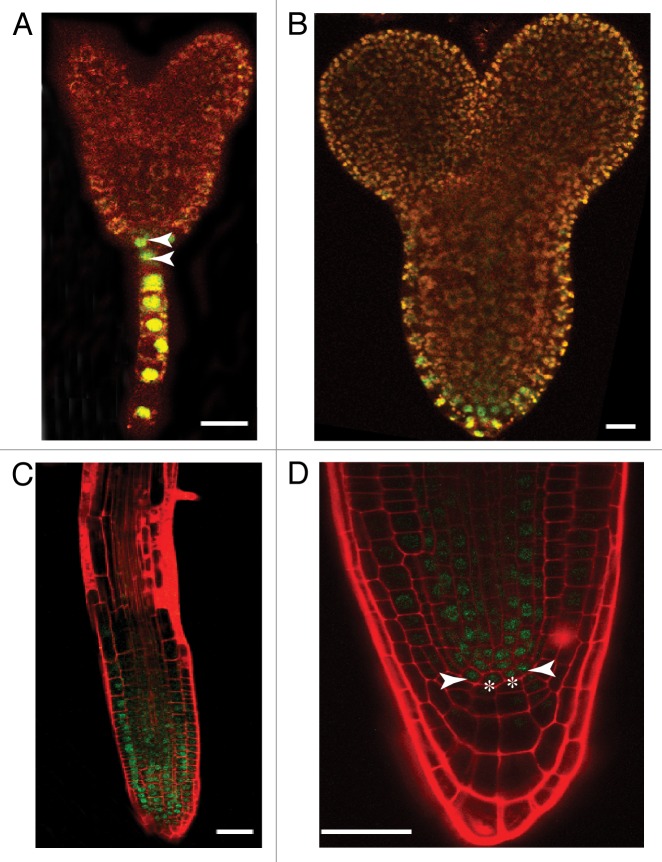
Although it had previously been reported that SPT is expressed in the roots,9 its function in this area of the plant had not been examined. SPT expression in Arabidopsis is first detected in the embryonic hypophysis, then the forming root apical meristem (RAM). It continues to be expressed in the RAM and stele after germination (Fig. 1 and ref. 9). Our studies revealed that spt mutants have longer roots and an increase in the number of cortical cells around the diameter of the root,10 suggesting that SPT represses growth in all axes of the root. However, overall patterning of the root is not disrupted. The size increase is due to an expanded region of cell proliferation in the RAM. Closer examination of spt roots revealed that the number of cells in the quiescent center (QC) is increased as examined both morphologically and with molecular markers. The changes seen in spt roots is similar to those seen in spt leaves, i.e., an increase in the size of the meristematic region leading to increased cell proliferation and increased organ size and expands the role of SPT as a growth repressor throughout the plant.
Figure 1.SPT is expressed in the root starting in embryogenesis. Confocal micrographs of SPTp::gSPT-GFP embryos (A and B) and seedlings (C and D) stained with propidium iodide as in Reference 10. (A) Transition stage embryo. Arrowheads indicate expression in the upper and lower hypophyseal cells. (B) Torpedo stage embryo with expression in the presumptive RAM. (C) 7 d after germination (DAG) root. SPT is expressed throughout the division zone. (D) Root tip of a 7DAG seedling. Asterisks indicate QC cells while arrowheads indicate initial cells. Scale bars indicate 50 µm in (A and B) and 100 µm in (C and D).
In the root SPT acts in parallel to GA.10 This is consistent with results in the shoot,5 supporting the idea that the molecular mechanism by which SPT represses growth may be similar in these regions. SPT regulates at least one DELLA target gene in the root suggesting that co-regulation by SPT and the GA pathway maybe a feature throughout the plant. spt mutant roots have broader auxin maxima at their tips and altered expression of the auxin efflux carrier PIN4.10 Although spt roots respond normally to exogenous auxin, they are hypersensitive to auxin transport inhibitors. This suggests that SPT regulates auxin transport. This is consistent with its regulation of genes related to this transport in the flower11 and recovery of the spt carpel phenotype by application of auxin transport inhibitors.12
The role of SPT in carpel and fruit development has been extensively analyzed,4,11,13-15 In this context SPT does not seem to act merely as a growth repressor but to regulate patterning of the septum, style and stigma in the carpel and subsequently dehiscence zone development in the silique.4,15 Interestingly, GA positively regulates SPT in this organ independently of DELLA proteins.16 In carpels and fruits SPT is partially redundant with its paralog ALCATRAZ (ALC).15 However, ALC-like genes are confined to a subset of angiosperms consisting of at least the Brassicaceae.13,15 The function of ALC outside of the flower has not been examined; however, ALC is expressed in hypocotyls, the lateral margins of leaves and in leaf vasculature.9 Some of this expression overlaps that of SPT, especially in the leaf margins, suggesting that the functional redundancy between ALC and SPT could extend to control of leaf growth. ALC is expressed in emerging lateral roots and the root-lateral root junction, and in the stele but not the root tip.9 Earlier expression in the embryo has not been reported. Since ALC is not expressed in the root tip, it seems unlikely that SPT is functionally redundant with this gene in controlling the size of the RAM. However, a function in the stele that is masked in spt mutants by the presence of a wild type ALC locus is possible. Examination of non-floral phenotypes of spt; alc plants should be undertaken to determine to what extent these two genes are redundant.
SPT is related to a group of bHLH proteins, the PHYTOCHROME INTERACTING FACTORS (PIFs), but differs in having lost the active phytochrome-binding domain (APB).13 SPT is involved in regulating growth in response to both light and temperature in seeds, leaves and carpels.7,13,14,17 Recently it has been suggested that a light-regulated module functioning in shade avoidance was recruited to carpel development after the loss of the APB domain from SPT-like genes.13 Roots, like carpels, develop in dark and shaded conditions, supporting the view that loss of the APB domain allowed expansion of expression and function of SPT-like genes into non-light regulated pathways. Phytochromes have been implicated in regulation of root architecture, controlling the emergence of lateral roots partly by regulating auxin distribution,18 similar to the regulation of root growth by SPT via control of auxin transport.10 It is possible that light, temperature or other environmental signals are integrated into root growth by SPT. This hasn’t been examined as all work in our paper was done under standard long day conditions and normal temperature regimes.10
In conclusion, SPT is a central hub in the regulation of organ size in Arabidopsis (Fig. 2). It integrates environmental signals, most prominently light, with GA and controls the size of meristematic regions by restricting cell proliferation as well as controlling cell expansion in some organs. SPT regulates auxin transport, directly or indirectly, and shares a subset of GA targets. Further experimentation is necessary to determine the exact mechanisms by which SPT functions and the origins of its organ specific functions.
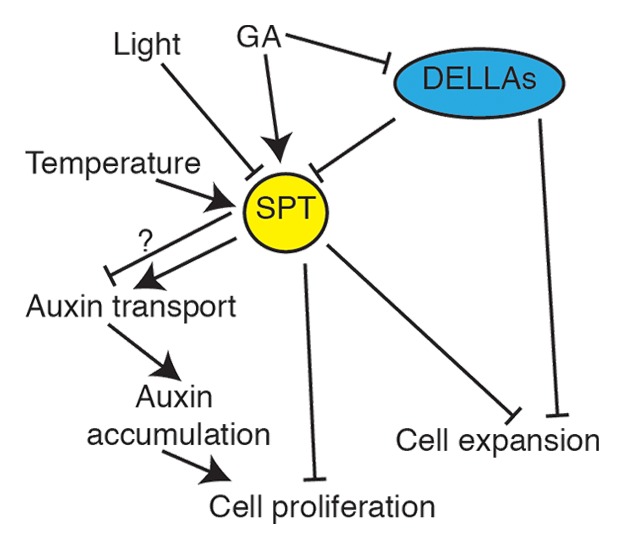
Figure 2. A model for SPT action. SPT acts as a central hub in the control of organ size. Hormonal and environmental inputs regulate the expression and stability of SPT which in turn regulates auxin transport, either directly or indirectly, as well as other genes to negatively regulate cell division and expansion in parallel to the GA-regulated DELLAs.
Acknowledgments
We thank Dr J.C. Jang, Dr Iris Meier and Dr Patrice Hamel (The Ohio State University) for discussions about SPT function. This work was supported in part by a grant from the National Science Foundation (MCD-0418891) to R.S.L. and funds from The Ohio State University.
Glossary
Abbreviations:
- SPT
SPATULA
- bHLH
basic helix-loop-helix
- GA
gibberellic acid
- RAM
root apical meristem
- QC
quiescent center
- ALC
ALCATRAZ
- PIF
PHYTOCHROME INTERACTING FACTOR
- APB
active phytochrome binding domain
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24140
References
- 1.Gonzalez N, Vanhaeren H, Inzé D. Leaf size control: complex coordination of cell division and expansion. Trends Plant Sci. 2012;17:332–40. doi: 10.1016/j.tplants.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Powell AE, Lenhard M. Control of organ size in plants. Curr Biol. 2012;22:R360–7. doi: 10.1016/j.cub.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Krizek BA, Anderson JT. Control of flower size. J Exp Bot. 2013 doi: 10.1093/jxb/ert025. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez J, Smyth DR. CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development. 1999;126:2377–86. doi: 10.1242/dev.126.11.2377. [DOI] [PubMed] [Google Scholar]
- 5.Josse EM, Gan Y, Bou-Torrent J, Stewart KL, Gilday AD, Jeffree CE, et al. A DELLA in disguise: SPATULA restrains the growth of the developing Arabidopsis seedling. Plant Cell. 2011;23:1337–51. doi: 10.1105/tpc.110.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichihashi Y, Horiguchi G, Gleissberg S, Tsukaya H. The bHLH transcription factor SPATULA controls final leaf size in Arabidopsis thaliana. Plant Cell Physiol. 2010;51:252–61. doi: 10.1093/pcp/pcp184. [DOI] [PubMed] [Google Scholar]
- 7.Sidaway-Lee K, Josse EM, Brown A, Gan Y, Halliday KJ, Graham IA, et al. SPATULA links daytime temperature and plant growth rate. Curr Biol. 2010;20:1493–7. doi: 10.1016/j.cub.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Ichihashi Y, Kawade K, Usami T, Horiguchi G, Takahashi T, Tsukaya H. Key proliferative activity in the junction between the leaf blade and leaf petiole of Arabidopsis. Plant Physiol. 2011;157:1151–62. doi: 10.1104/pp.111.185066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groszmann M, Bylstra Y, Lampugnani ER, Smyth DR. Regulation of tissue-specific expression of SPATULA, a bHLH gene involved in carpel development, seedling germination, and lateral organ growth in Arabidopsis. J Exp Bot. 2010;61:1495–508. doi: 10.1093/jxb/erq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makkena S, Lamb RS. The bHLH transcription factor SPATULA regulates root growth by controlling the size of the root meristem. BMC Plant Biol. 2013;13:1. doi: 10.1186/1471-2229-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girin T, Paicu T, Stephenson P, Fuentes S, Körner E, O’Brien M, et al. INDEHISCENT and SPATULA interact to specify carpel and valve margin tissue and thus promote seed dispersal in Arabidopsis. Plant Cell. 2011;23:3641–53. doi: 10.1105/tpc.111.090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemhause JL, Feldman LJ, Zambryski PC. Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development. 2000;127:38770–88. doi: 10.1242/dev.127.18.3877. [DOI] [PubMed] [Google Scholar]
- 13.Reymond MC, Brunoud G, Chauvet A, Martínez-Garcia JF, Martin-Magniette ML, Monéger F, et al. A light-regulated genetic module was recruited to carpel development in Arabidopsis following a structural change to SPATULA. Plant Cell. 2012;24:2812–25. doi: 10.1105/tpc.112.097915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foreman J, White J, Graham I, Halliday K, Josse EM. Shedding light on flower development: phytochrome B regulates gynoecium formation in association with the transcription factor SPATULA. Plant Signal Behav. 2011;6:471–6. doi: 10.4161/psb.6.4.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groszmann M, Paicu T, Alvarez JP, Swain SM, Smyth DR. SPATULA and ALCATRAZ, are partially redundant, functionally diverging bHLH genes required for Arabidopsis gynoecium and fruit development. Plant J. 2011;68:816–29. doi: 10.1111/j.1365-313X.2011.04732.x. [DOI] [PubMed] [Google Scholar]
- 16.Fuentes S, Ljung K, Sorefan K, Alvey E, Harberd NP, Østergaarda L. Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell. 2012;24:3982–96. doi: 10.1105/tpc.112.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penfield S, Josse EM, Kannangara R, Gilday AD, Halliday KJ, Graham IA. Cold and light control seed germination through the bHLH transcription factor SPATULA. Curr Biol. 2005;15:1998–2006. doi: 10.1016/j.cub.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Salisbury FJ, Hall A, Grierson CS, Halliday KJ. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007;50:429–38. doi: 10.1111/j.1365-313X.2007.03059.x. [DOI] [PubMed] [Google Scholar]