Abstract
Through co-evolution insect herbivores have developed a myriad of strategies to manipulate host plant defense responses that include the synthesis of defensive compounds whose composition depends on the insect feeding mode. Among the plant-produced compounds are jasmonates (JAs), and Green Leafy Volatiles (GLVs), metabolites produced by the two parallel and competing branches of the oxylipin pathway. Here we provide evidence that chewing insects stimulate JA production but suppress the synthesis of GLVs through the transcriptional and post transcriptional reprogramming of critical genes in the corresponding pathway. We further establish that herbivore-derived elicitors known as Herbivore-Associated Molecular Patterns (HAMPs) are responsible for the reprogramming of these pathway genes. Through this strategy chewing herbivores coerce the plant signaling machinery that would otherwise leads to a reduction in the nutritional quality of the immediate and neighboring plants, and additionally shelters the herbivores from their natural enemies that are otherwise guided by the GLV cues to prey-infested plants.
Keywords: counter defense, green leafy volatiles (GLVs), herbivore insects, herbivore-associated molecular patterns (HAMPs), jasmonates, oxylipin pathway
Plant volatiles are communication signals that mediate intra-, inter-plant, and plant-insect interactions, and therefore they are key in maintaining ecological homeostasis. These signaling compounds are of diverse chemical nature generated from both primary and secondary metabolites, and their distinct signature, as defined by their quantitative and qualitative values, determines their effectiveness in maintaining the ecological balance.1
Insects make up the most diverse and abundant group of plant consumers and interaction between plants and insect herbivores is the most frequent known interspecies communication and a driving force of evolution.2,3 Among several classes of plant metabolites produced in response to insect attack are oxylipins—the oxygenated derivatives of fatty acids generated by parallel and competing branches of oxylipin pathway.4-7 The best studied oxylipin branches however are allene oxide synthase (AOS) and hydroperoxide lyase (HPL) responsible for production of jasmonates [jasmonic acid (JA), methyl jasmonate (MeJA) and their biosynthetic precursor, 12- oxophytodienoic acid (12-OPDA)], and the green leafy volatiles (GLVs), respectively8,9 (Fig. 1). Both these pathway metabolites are known to defend plants, directly or indirectly, against insects. Specifically, JA is an indispensable signaling component of the defense pathway that promotes resistance to a wide spectrum of insects,10-13 and GLVs are known to be involved in intra- and interplant defense signaling cascades and in directing tritrophic (plant-herbivore-natural enemy) interactions.7,9,14-17
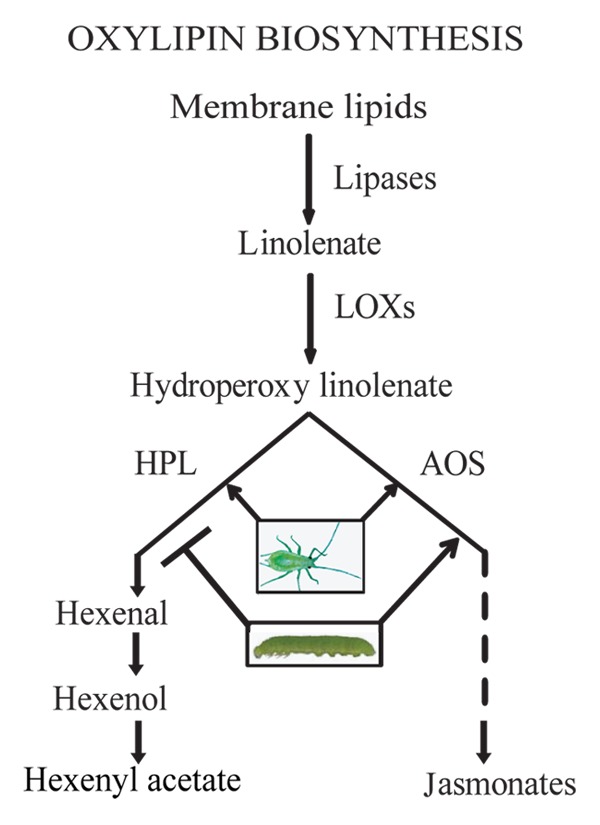
Figure 1. Simplified scheme of oxylipin biosynthesis pathway. Abbreviations: LOXs, lipoxygenases; AOS, allene oxide synthase; HPL, hydroperoxide lyase.
Recently we have examined the influence of chewing insect feeding and mechanical wounding on the HPL- and AOS- derived metabolic signatures, by using three Arabidopsis genotypes, namely Col-0 accession, a natural loss-of-function mutation in hpl18 as the control, engineered Col-0 lines that overexpress a rice HPL under the control of a 35S promoter (OsHPL3 OE)19 and wild-type Wassilewskija (Ws) ecotype that contain a functional HPL driven by the native promoter.20 These genotypes were damaged either mechanically or by insects of different feeding guilds (piercing aphids, generalist chewing caterpillars “Spodoptera exicua” and specialist chewing caterpillars “Pieris rapae”). Subsequent metabolic analyses established that emission of GLVs is stimulated by wounding incurred mechanically or by aphids, but the release of these volatiles is constitutively impaired by both generalist and specialist chewing insects (Fig. 2). Simultaneously however, these chewing herbivores stimulated JA production, demonstrating targeted suppression of the HPL branch of the oxylipin pathway. Employment of lines engineered to express HPL constitutively, in conjunction with qRT-PCR-based expression analyses, established a combination of transcriptional and posttranscriptional reprogramming of the HPL-pathway genes as the mechanistic basis of insect-mediated suppression of the corresponding metabolites. Additional studies established that insect-inflicted suppression of GLV emission is caused by herbivore oral secretion (OS), as a strategy to counteract the plant’s ability to produce defensive metabolites and to reduce nutritional quality central to herbivore fitness. Subsequent no-choice feeding trials on plants that had or had not been exposed to GLVs from a neighboring damaged plant determined that caterpillars fed more on plants that had not been primed by GLVs emitted by wounded plants, suggesting that plant-plant communication triggered by these volatiles altered the insect feeding behavior. This insect-mediated suppression of GLV production is also instrumental for the removal of this broad ecological signal that could otherwise attract and direct herbivore’s natural enemies.
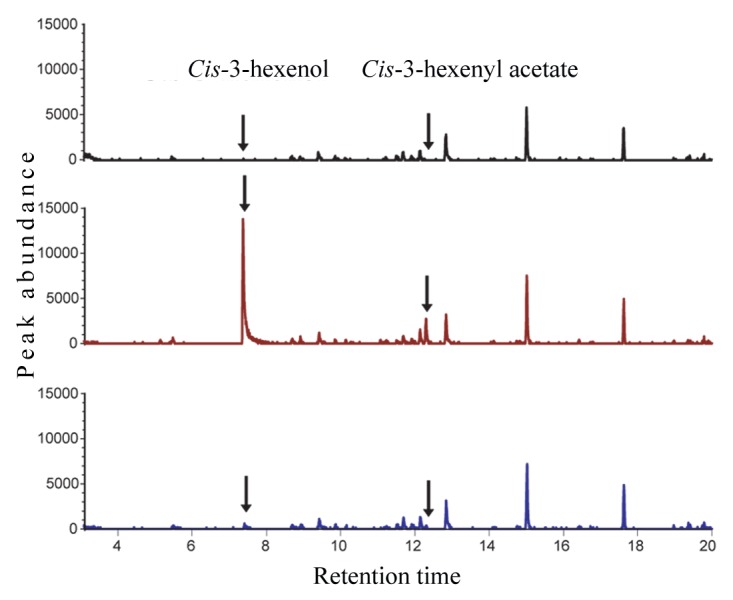
Figure 2. Emission of GLVs is stimulated by mechanical wounding, but is suppressed by chewing insects. Gas chromatography-mass spectrometry (GC-MS) ion chromatograms (m/z 67) showing cis-3-hexenol and cis-3-hexenyl acetate in volatile samples collected from control (black line), wounded (red line), and caterpillar damaged (blue line) Arabidopsis plants. Volatiles were captured for 3 h on mini-columns filled with Porapak Q® 100/120 (Alltech Associates, Inc.) using an open-flow system, bound compounds were eluted with 300 µl of dichloromethane and analyzed by GC-MS.
These findings reveal a new facet in the co-evolutionary processes that shape insect-plant interactions at a molecular level, whereby herbivore-derived elicitors denoted as Herbivore-Associated Molecular Patterns (HAMPs)21,22 mediate the reprogramming of HPL-pathway genes. Among several intriguing questions arising from these observations are: the chemical nature of insect OS component responsible for suppression of HPL pathway, and mechanisms by which signals are sensed and relayed into downstream signaling cascades involved in constitutive deactivation of HPL-defense responsive pathways.
Acknowledgments
This project is supported by the National Science Foundation grant IOS-1036491 (K.D).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24136
References
- 1.Hare JD. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol. 2011;56:161–80. doi: 10.1146/annurev-ento-120709-144753. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal AA, Hastings AP, Johnson MT, Maron JL, Salminen JP. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science. 2012;338:113–6. doi: 10.1126/science.1225977. [DOI] [PubMed] [Google Scholar]
- 3.Züst T, Heichinger C, Grossniklaus U, Harrington R, Kliebenstein DJ, Turnbull LA. Natural enemies drive geographic variation in plant defenses. Science. 2012;338:116–9. doi: 10.1126/science.1226397. [DOI] [PubMed] [Google Scholar]
- 4.Creelman RA, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA. 1992;89:4938–41. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase-inhibitors. Plant Cell. 1992;4:129–34. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blechert S, Brodschelm W, Hölder S, Kammerer L, Kutchan TM, Mueller MJ, et al. The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA. 1995;92:4099–105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bate NJ, Rothstein SJ. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998;16:561–9. doi: 10.1046/j.1365-313x.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 8.Matsui K, Kurishita S, Hisamitsu A, Kajiwara T. A lipid-hydrolysing activity involved in hexenal formation. Biochem Soc Trans. 2000;28:857–60. doi: 10.1042/BST0280857. [DOI] [PubMed] [Google Scholar]
- 9.Chehab EW, Kaspi R, Savchenko T, Rowe H, Negre-Zakharov F, Kliebenstein D, et al. Distinct roles of jasmonates and aldehydes in plant-defense responses. PLoS ONE. 2008;3:e1904. doi: 10.1371/journal.pone.0001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 11.Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8:2067–77. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA. 1997;94:5473–7. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldwin IT. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA. 1998;95:8113–8. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arimura G, Ozawa R, Horiuchi J, Nishioka T, Takabayashi J. Plant-plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem Syst Ecol. 2001;29:1049–61. doi: 10.1016/S0305-1978(01)00049-7. [DOI] [Google Scholar]
- 15.Paré PW, Alborn HT, Tumlinson JH. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc Natl Acad Sci USA. 1998;95:13971–5. doi: 10.1073/pnas.95.23.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stotz HU, Kroymann J, Mitchell-Olds T. Plant-insect interactions. Curr Opin Plant Biol. 1999;2:268–72. doi: 10.1016/S1369-5266(99)80048-X. [DOI] [PubMed] [Google Scholar]
- 17.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA. 2004;101:1781–5. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan H, Huang MY, Palacio K, Schuler MA. Variations in CYP74B2 (hydroperoxide lyase) gene expression differentially affect hexenal signaling in the Columbia and Landsberg erecta ecotypes of Arabidopsis. Plant Physiol. 2005;139:1529–44. doi: 10.1104/pp.105.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chehab EW, Raman G, Walley JW, Perea JV, Banu G, Theg S, et al. Rice HYDROPEROXIDE LYASES with unique expression patterns generate distinct aldehyde signatures in Arabidopsis. Plant Physiol. 2006;141:121–34. doi: 10.1104/pp.106.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savchenko T, Pearse IS, Ignata L, Karban R, Dehesh K. Insect herbivores selectively supress the HPL branch of the oxylipin pathway in host plants. Plant J. 2012;73:653–62. doi: 10.1111/tpj.12064. [DOI] [PubMed] [Google Scholar]
- 21.Felton GW, Tumlinson JH. Plant-insect dialogs: complex interactions at the plant-insect interface. Curr Opin Plant Biol. 2008;11:457–63. doi: 10.1016/j.pbi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Mithöfer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008;146:825–31. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]


