Abstract
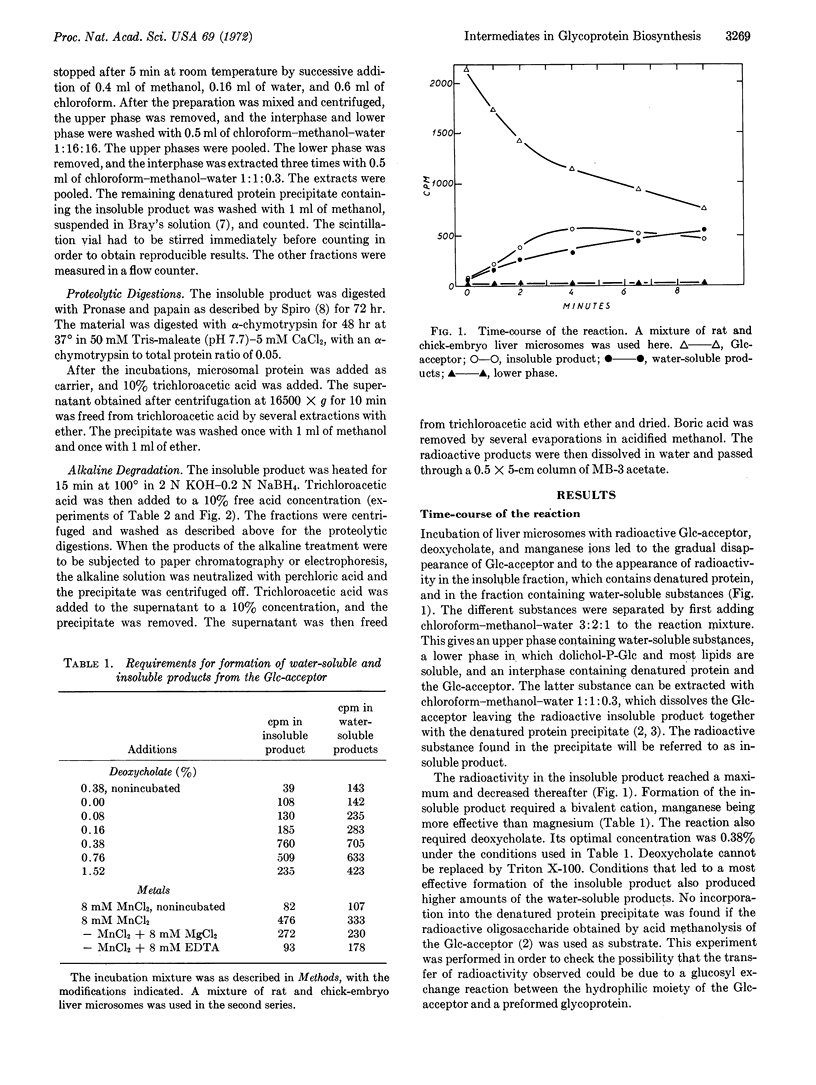
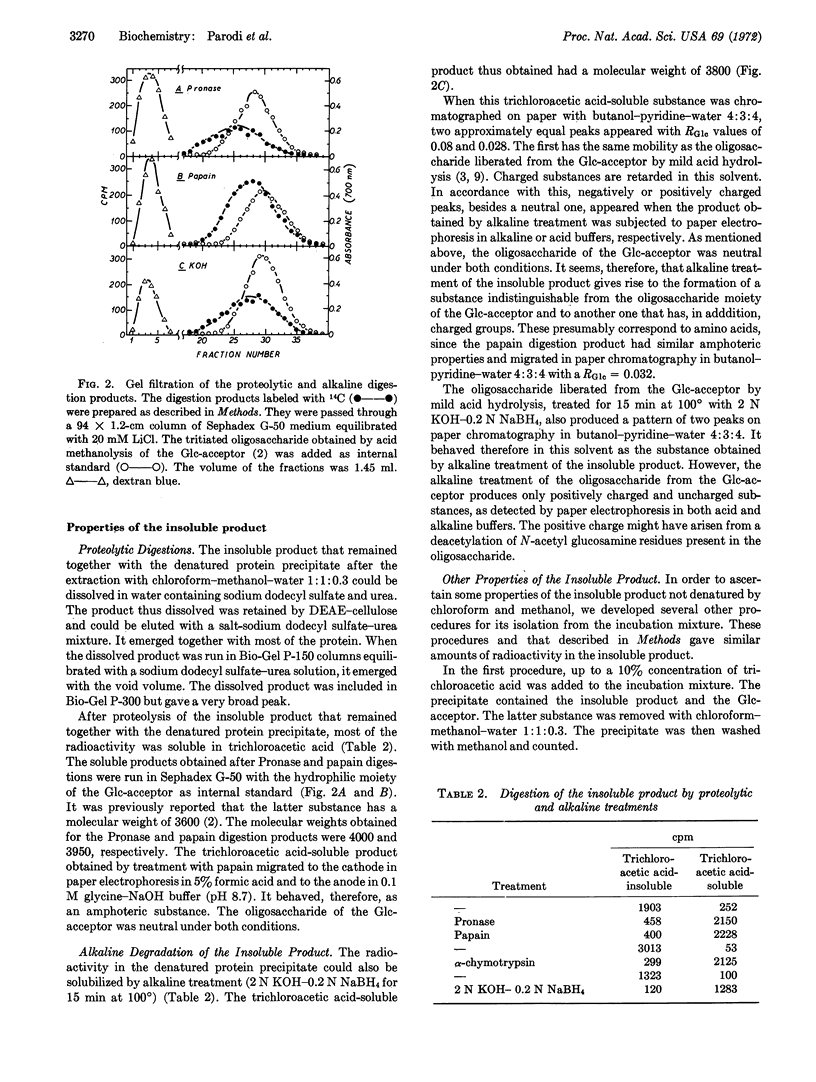
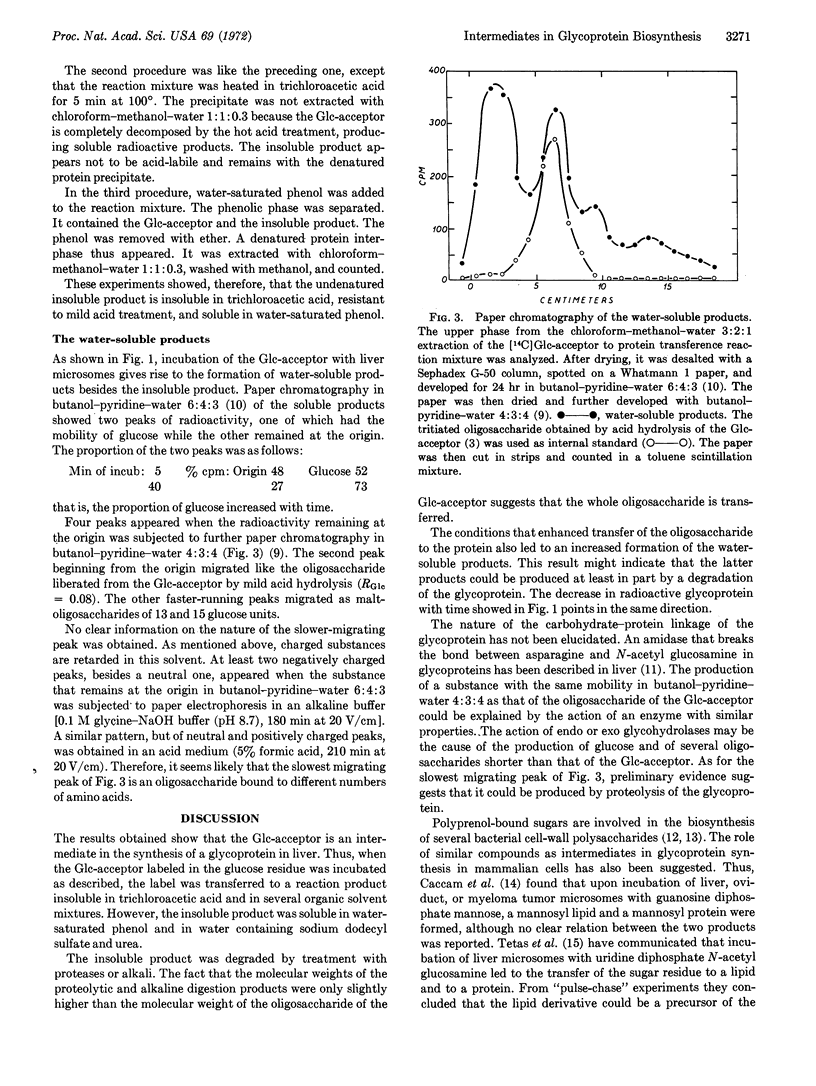
It has been reported that liver microsomes catalyze the transfer of glucose from uridine diphosphate glucose to dolichol monophosphate so as to produce dolichol monophosphate glucose. Dolichol is a polyprenol containing about 20 isoprene units. The glucosyl residue of dolichol monophosphate glucose is transferred to an endogenous acceptor on further incubation with liver microsomes. The glucosylated endogenous acceptor appears to be an oligosaccharide of about 20 monosaccharide units bound to dolichol through a phosphate or pyrophosphate bridge. In this paper it is reported that liver microsomes catalyze the transfer of the oligosaccharide from the glucosylated endogenous acceptor to an endogenous protein. This transfer reaction requires the presence of bivalent cations, manganese being more effective than magnesium. The presence of deoxycholate is also required. Besides the glycoprotein, several water-soluble products are also formed. Preliminary evidence indicates that they are glucose, iligosaccharides of different size, and possibly oligosaccharides bound to amino acids.
Keywords: dolichol monophosphate glucose, lipid intermediates, liver microsomes
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGOS J., HEMMING F. W., PENNOCK J. F., MORTON R. A. DOLICHOL: A NATURALLY-OCCURRING C100 ISOPRENOID ALCOHOL. Biochem J. 1963 Sep;88:470–482. [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Leloir L. F. Dolichol monophosphate glucose: an intermediate in glucose transfer in liver. Proc Natl Acad Sci U S A. 1970 May;66(1):153–159. doi: 10.1073/pnas.66.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Parodi A. J., Leloir L. F. Glucose transfer from dolichol monophosphate glucose: the product formed with endogenous microsomal acceptor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2857–2860. doi: 10.1073/pnas.68.11.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccam J. F., Jackson J. J., Eylar E. H. The biosynthesis of mannose-containing glycoproteins: a possible lipid intermediate. Biochem Biophys Res Commun. 1969 May 22;35(4):505–511. doi: 10.1016/0006-291x(69)90375-1. [DOI] [PubMed] [Google Scholar]
- Helting T., Peterson P. A. Galactosyltransfer in mouse mastocytoma: synthesis of a galactose-containing polar metabolite of retinol. Biochem Biophys Res Commun. 1972 Jan 31;46(2):429–436. doi: 10.1016/s0006-291x(72)80156-6. [DOI] [PubMed] [Google Scholar]
- Helting T., Rodén L. Biosynthesis of chondroitin sulfate. I. Galactosyl transfer in the formation of the carbohydrate-protein linkage region. J Biol Chem. 1969 May 25;244(10):2790–2798. [PubMed] [Google Scholar]
- Helting T., Rodén L. Biosynthesis of chondroitin sulfate. II. Glucuronosyl transfer in the formation of the carbohydrate-protein linkage region. J Biol Chem. 1969 May 25;244(10):2799–2805. [PubMed] [Google Scholar]
- Johnston I. R., McGuire E. J., Jourdian G. W., Roseman S. Incorporation of N-acetyl-D-glucosamine into glycoproteins. J Biol Chem. 1966 Dec 10;241(23):5735–5737. [PubMed] [Google Scholar]
- Mahadevan S., Tappel A. L. Beta-aspartylglucosylamine amido hydrolase of rat liver and kidney. J Biol Chem. 1967 Oct 25;242(20):4568–4576. [PubMed] [Google Scholar]
- McGuire E. J., Jourdian G. W., Carlson D. M., Roseman S. Incorporation of D-galactose into glycoproteins. J Biol Chem. 1965 Oct;240(10):4112–4115. [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Mordoh J., Krisman C. R., Leloir L. F. In vitro synthesis of particulate glycogen from uridine diphosphate glucose. Arch Biochem Biophys. 1969 Jun;132(1):111–117. doi: 10.1016/0003-9861(69)90342-7. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Telser A., Dorfman A. Studies on biosynthesis of the linkage region of chondroitin sulfate-protein complex. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1859–1866. doi: 10.1073/pnas.56.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetas M., Chao H., Molnar J. Incorporation of carbohydrates into endogenous acceptors of liver microsomal fractions. Arch Biochem Biophys. 1970 May;138(1):135–146. doi: 10.1016/0003-9861(70)90292-4. [DOI] [PubMed] [Google Scholar]



