Abstract
In a recent study, we identified and characterized the long-elusive replicative single-stranded DNA-binding protein of bacteriophage T5, which we showed is related to the eukaryotic transcription coactivator PC4. Here, we provide an extended discussion of these data, report several additional observations and consider implications for the recombination-dependent replication mechanism of the T5 genus, which is still poorly understood.
Keywords: T5, SSB, single-stranded DNA, PC4, replication, recombination, repair, 3R, RDR
Background
T5 is one of the seven classical Caudovirales or tailed bacteriophages that were originally designated T (for “Type”) phages by Delbrück and coworkers.1 Nowadays considered a separate genus within the Siphoviridae family, T5-like phages are characterized by a 250 nm non-contractile flexible tail, attached to a 90 nm icosahedral capsid.2 The capsid contains an approximately 120 kbp double-stranded (ds)DNA genome, the largest of the T-odd group (T1, T3, T5, and T7).3 Other distinguishing features of T5 are the single-stranded interruptions (nicks) that occur at well-defined positions in its genome3 and a unique two-step genome transfer mechanism that leads to transcription of pre-early genes before the remaining phage DNA has entered the host cell.4
Although the T5-like bacteriophages have been classified as a separate genus, recent electron microscopy work has revealed similarities to T4 as well as Lambda-like phages, suggesting unexpected evolutionary relationships.2 Interestingly, the resemblance to T4 extends beyond the mere morphological level: like T4, bacteriophage T5 encodes an ensemble of replication and recombination factors within a dedicated section of its genome, the so-called replication module.5 Even though only a subset of these factors show obvious homology to T4 counterparts, the co-localization of the two types of genes in the replication module strongly suggests that T5 uses a recombination-dependent replication (RDR) mechanism similar to that of T4.5 In apparent agreement with this idea, T5 contains large terminal redundancies in the form of direct repeats, while replication intermediates detected by electron microscopy show the highly complex, branched concatenates that are the hallmark of RDR.6
In contrast to T5, the DNA replication mechanism of T4 has been studied intensively, resulting in successful reconstitution of RDR reactions in vitro and detailed knowledge of the roles of the factors involved.5,7,8 In short, early rounds of T4 replication are initiated at several specific origins via transcription priming by the host RNA polymerase, followed by leading strand synthesis by the phage replisome. Concomitant lagging strand synthesis is primed by the viral primase, gp61, assisted by the helicase p41 and the helicase loader p59. Due to the linearity of the T4 genome, lagging strand synthesis in the initial rounds of replication is inevitably incomplete as priming cannot take place beyond the genome ends. This shortcoming is remedied in subsequent rounds of replication, also referred to as the “burst phase”, by means of a recombination-dependent mechanism. The latter relies on strand invasion of the unreplicated single-stranded extremity into the homologous, double-stranded redundancy at the opposite end of the genome, or into homologous regions of other genome copies already present in the cell. The free 3′-hydroxyl end of the invading strand then serves as a primer for the subsequent round of replication. Concurring strand invasion events in RDR frequently lead to topologically complex branched structures, which are eventually cleaved by gp49, a Holliday junction resolvase (endonuclease VII).
A virally encoded single-stranded DNA (ssDNA)-binding protein or SSB, gp32, is strictly required during the early transcription-dependent rounds of T4 replication as well as in the subsequent recombination-dependent cycles. In both phases, displaced DNA strands are transiently coated with gp32, until the SSB is removed by the replisome that performs lagging strand synthesis or during the events that lead up to the recombination process. In addition to protecting ssDNA from nucleolytic attack and preventing formation of secondary structure, gp32 acts as an interaction hub for downstream factors, particularly mediator proteins that direct the timely loading of specific enzymes onto ssDNA.9 At replication forks, for instance, gp32 recruits the helicase loader gp59, which is the essential first step in the process of lagging strand priming. On the single-stranded extremity of the lagging strand that remains at the end of the replication cycle, gp32 interacts with another mediator protein, UvsY. This factor then initiates formation of the so-called presynaptic filament by displacing the SSB from the ssDNA while recruiting UvsX, an ATP-dependent recombinase with homology to E. coli RecA and eukaryotic Rad51.
In sharp contrast to T4 and most other large dsDNA phages, no SSBs were detected until recently in any of the fully sequenced members of the T5 family. As pointed out by Weigel and Seitz,5 such a systematic lack of an SSB was rather surprising, considering the essential role that this type of protein plays in dsDNA phage replication in general and the RDR mechanism in particular.
Identification of T5’s Replicative SSB
The missing T5 equivalent of gp32 was recently identified in a somewhat unexpected manner, when we found remote homologs of a eukaryotic transcription factor, positive cofactor 4 (PC4), encoded in all of the T5-like phage genomes that have been sequenced to date10 (see Table 1 for an overview). In eukaryotes, PC4 was originally identified as a potent coactivator of RNA polymerase II transcription,12 but unlike most other such cofactors the protein is not implicated in nucleosome remodelling or histone acetylation. Instead, PC4’s conserved core corresponds to a high-affinity ssDNA-binding domain,13 which in addition mediates homodimerization.14 Apart from its well-established activities in eukaryotic transcription regulation, which seem to depend to a large extent on protein-protein interactions,12 a number of recent reports have suggested that PC4 plays additional roles in replication, recombination and repair (3R) via the ssDNA-binding capacity of its core domain.15-19
Table 1. Fully sequenced T5-like bacteriophage genomes and the PC4 homologs they encode.
| Bacteriophage name | Genome accession number |
PC4-like SSB accession number |
% Identity to T5 SSB | % Identity to human PC4 (63–127) |
|---|---|---|---|---|
| Enterobacteria phage T5 | NC_005859.1 | YP_006943.1 | 100 | 23 |
| Enterobacteria phage SPC35 | NC_015269.1 | YP_004306588.1 | 100 | 23 |
| Enterobacteria phage H8 | AC171169.12 | AER23776.1 | 100 | 23 |
| Escherichia phage bV_EcoS_AKFV33 | NC_017969.1 | YP_006382417.1 | 100 | 23 |
| Enterobacteria phage T5 strain ATCC 11303-B5 | AY587007.1 | AAX12043.1 | 99 | 24 |
| Enterobacteria phage EPS7 | NC_010583.1 | YP_001837052.1 | 93 | 24 |
| Yersinia phage phiR201 | NC_019919.1 | YP_007237085.1b | 87 | 25 |
| Pectobacterium phage My1a | NC_018837.1 | YP_006906360.1 | 69 | 21 |
| Vibrio phage pVp-1 | NC_019529.1 | YP_007007848.1 | 52 | 15 |
Indicated are genome and protein accession numbers, homology level (percentage identical amino acids) with respect to the protein sequence of the SSB of T5 (YP_006943.1) and homology to the core domain (residues 63–127) of human PC4. Amino acid identity percentages were calculated following alignment by MAFFT.11 aA recently sequenced genome not yet included in the analysis by Steigemann et al.10 bThe protein sequence used for alignment was generated from the genomic DNA sequence, as the protein database entry listed appears to be N-terminally truncated.
Interestingly, the gene encoding the PC4 homolog in T5-like bacteriophages is highly conserved and invariably occupies the same position within the gene array of the T5 replication module.10 This is a strong indication that the PC4 homolog plays a critical role in phage DNA replication. We went on to determine the structure of the protein, which revealed that all of the critical ssDNA-binding features described for human PC420 are highly conserved in the T5 protein, including the detailed fold and the exact three-dimensional positioning of ssDNA-binding residues.10 Together, these data strongly suggest that the novel bacteriophage PC4 homolog corresponds to the elusive SSB of the T5 genus and the functional equivalent of T4’s gp32.
Comparison of gp32 and PC4
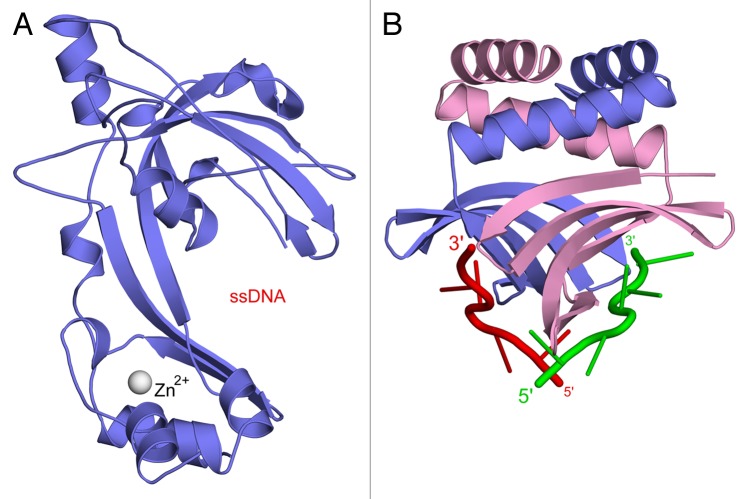
The structures of the gp32 ssDNA-binding core domain21 and the PC4-like SSB of T510 are compared in Figure 1. In contrast to the PC4-fold, which is only found in the eukaryotic transcription cofactor orthologs and the recently discovered homologs in bacteriophage T5 and a limited number of prokaryotes,10 gp32 belongs to the much broader OB (oligonucleotide- or oligosaccharide-binding)-fold family.22 OB-folds are present in the vast majority of currently known replicative SSBs, as well as in proteins that interact with a variety of unrelated ligands.23 In its simplest form, the OB-fold consists of a curved five-stranded β-barrel that has a Greek key topology, plus an α-helix that stabilizes the domain structure through interaction with one extremity of the barrel. The OB-fold of gp32 (Fig. 1A) deviates from this basic architecture through numerous helical insertions, including a small zinc-binding motif.
Figure 1. Comparison of the core domain of gp32, the SSB of bacteriophage T4 (A, PDB entry 1GPC),21 to the PC4-like SSB encoded by T5 (B, PDB entry 4BG7).10 Protein monomers are shown in blue and pink. For the T5 SSB, a tentative model for bound ssDNA strands (in red and green) has been added, based on superposition of the crystal structure of human PC4 bound to an oligonucleotide (PDB code 2C62).20 The ssDNA backbones are shown as coils, the bases as sticks. For gp32, no comparably detailed ssDNA model is currently available; instead, the location of the ssDNA-binding channel has been indicated. Images were prepared using PyMOL 0.99rc6 (DeLano Scientific).
Although the OB- and PC4-fold both bind ssDNA by means of a concave β-surface, major differences between the binding sites are apparent. First, structural studies indicate that the much deeper binding channel of gp32 accommodates approximately three nucleotides,21 whereas the rather shallow but more extended channel of human PC4 was shown to bind five residues.20 Analysis of gp32-ssDNA cocrystals by Shamoo et al. did not reveal sufficiently detailed electron density to warrant deposition of atomic coordinates for the ssDNA, but a tentative model places the nucleic acid in an orientation where its bases are bound on the inside of the hydrophobic channel.21 Indeed, this arrangement is fully consistent with the orientation observed in other OB-fold-ssDNA complexes whose structures have been determined more recently.24 The PC4-fold on the other hand has a binding mode that primarily involves phosphate backbone contacts, whereas DNA bases remain to a large extent exposed to the solvent.20 In addition, the ssDNA-binding surface of the homodimeric PC4-fold is of an inherently composite nature and due to its 2-fold symmetry facilitates simultaneous binding of the two channels to opposing ssDNA strands that run in opposite directions.13,20 The core domain of gp32 is not known to form symmetrical dimers under physiological conditions, but by means of its additional N-terminal domain (the so-called basic or B-domain) the full-length protein can self-interact and bind ssDNA cooperatively.25 Whether or not the homodimeric T5 SSB is able to bind ssDNA in a similarly cooperative manner remains to be determined, but little or no cooperativity was observed for the interaction of human PC4 or its core domain with ssDNA.13
In addition to the central ssDNA-binding domain and the N-terminal B-domain, gp32 contains an acidic C-terminal region, the so-called A-domain, which interacts with downstream factors such as UvsY and the helicase loader gp59. Moreover, this A-domain has been shown to reduce the ssDNA-binding affinity of the gp32 core, presumably by interfering with the ssDNA-binding channel in the absence of intermolecular protein-protein interactions.25 The SSB of T5 does not contain a region homologous to the A-domain of gp32, but our X-ray structure has revealed an N-terminal tail within the protein that may have a similar autoinhibitory function, as it was found to physically block one of the two ssDNA-binding channels of the homodimer in the asymmetric unit of the crystal.10
Consequences for the Replication Mechanism of T5
Although T4 and T5 both use a form of RDR, the comparison of gp32 and the PC4-like SSB in the previous section shows that intriguing differences are likely to exist between the detailed replication mechanisms of the two phages and the roles of their cognate SSBs. Of note, extensive homology searches that we performed in all of the T5 genomes listed in Table 1 failed to reveal homologs of UvsX and UvsY, the two factors that act in concert with gp32 to mediate strand exchange in T4. Although it cannot be ruled out that some of the as yet uncharacterised proteins encoded by the T5 replication module provide UvsX- and UvsY-like functionalities, it is also conceivable that the PC4-like SSB itself has recombination-stimulatory properties, which could obviate the requirement for a RecA-like recombinase in T5. The structure of the human PC4-ssDNA complex is highly suggestive in this respect, as it reveals juxtaposed strands of ssDNA that are kept in an inside-out arrangement, i.e., with the phosphate backbones pointing inward and bases turned outward.20 The unwound DNA in the complex thus seems poised for sequence-specific recognition of its bases, the purpose of which may well be to facilitate processes such as DNA repair and recombination. Although activity of eukaryotic PC4 in homologous recombination remains to be investigated, recent reports show that the protein is able to stimulate double-stranded break repair by non-homologous end-joining (NHEJ),18,19 presumably through a mechanism that depends on interaction with DNA. Thus, it seems tempting to speculate that the RDR mechanism of T5 is evolutionarily related to PC4-mediated DNA repair in eukaryotes, just like RDR in T4 is highly similar to eukaryotic repair mechanisms that involve Rad51. Indeed, bacteriophage T5 could turn out to be a valuable model system for currently emerging PC4-dependent repair pathways.
Concluding Remarks
Clearly, gaining a better understanding of the replication mechanism of T5 will require a more detailed analysis of the properties and activities of the PC4-like SSB and the other protein factors involved, as well as, ultimately, reconstitution of T5 replication reactions in vitro. Such efforts may be motivated by the observation that T5 replication seems to deviate in several ways from the well-established RDR paradigm of bacteriophage T4 and could constitute a model system for as yet poorly characterized eukaryotic 3R mechanisms that rely on PC4. In addition, the purely lytic life cycle, high burst rate, as well as complete absence of pathogenicity islands, antibiotic resistance genes, and integrases suggest that T5-like phages are promising candidates for the development of medical applications.26,27 Together, these features may lead to a renewed interest in this classical but still relatively poorly characterized bacteriophage.
Disclosure of Potential Conflicts of Interest
There are no potential conflicts of interest.
Citation: Werten S. Identification of the ssDNA-binding protein of bacteriophage T5: Implications for T5 replication. Bacteriophage 2013; 3:e27304; 10.4161/bact.27304
Footnotes
Previously published online: www.landesbioscience.com/journals/bacteriophage/article/27304
References
- 1.Demerec M, Fano U. Bacteriophage-resistant mutants in Escherichia coli. Genetics. 1945;30:119–36. doi: 10.1093/genetics/30.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effantin G, Boulanger P, Neumann E, Letellier L, Conway JF. Bacteriophage T5 structure reveals similarities with HK97 and T4 suggesting evolutionary relationships. J Mol Biol. 2006;361:993–1002. doi: 10.1016/j.jmb.2006.06.081. [DOI] [PubMed] [Google Scholar]
- 3.McCorquodale DJ, Warner HR. Bacteriophage T5 and related phages. In: The bacteriophages (Calender R, editor), Plenum Press, New York (1988). [Google Scholar]
- 4.Letellier L, Boulanger P, Plançon L, Jacquot P, Santamaria M. Main features on tailed phage, host recognition and DNA uptake. Front Biosci. 2004;9:1228–339. doi: 10.2741/1333. [DOI] [PubMed] [Google Scholar]
- 5.Weigel C, Seitz H. Bacteriophage replication modules. FEMS Microbiol Rev. 2006;30:321–81. doi: 10.1111/j.1574-6976.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- 6.Bourguignon GJ, Sweeney TK, Delius H. Multiple origins and circular structures in replicating T5 bacteriophage DNA. J Virol. 1976;18:245–59. doi: 10.1128/jvi.18.1.245-259.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Morrical SW. Assembly and dynamics of the bacteriophage T4 homologous recombination machinery. Virol J. 2010;7:357. doi: 10.1186/1743-422X-7-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreuzer KN, Brister JR. Initiation of bacteriophage T4 DNA replication and replication fork dynamics: a review in the Virology Journal series on bacteriophage T4 and its relatives. Virol J. 2010;7:358. doi: 10.1186/1743-422X-7-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleuit JS, Xu H, Ma Y, Wang T, Liu J, Morrical SW. Mediator proteins orchestrate enzyme-ssDNA assembly during T4 recombination-dependent DNA replication and repair. Proc Natl Acad Sci U S A. 2001;98:8298–305. doi: 10.1073/pnas.131007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steigemann B, Schulz A, Werten S. Bacteriophage T5 encodes a homolog of the eukaryotic transcription coactivator PC4 implicated in recombination-dependent DNA replication. J Mol Biol. 2013;425:4125–33. doi: 10.1016/j.jmb.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser K, Stelzer G, Meisterernst M. The coactivator p15 (PC4) initiates transcriptional activation during TFIIA-TFIID-promoter complex formation. EMBO J. 1995;14:3520–7. doi: 10.1002/j.1460-2075.1995.tb07358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werten S, Langen FW, van Schaik R, Timmers HT, Meisterernst M, van der Vliet PC. High-affinity DNA binding by the C-terminal domain of the transcriptional coactivator PC4 requires simultaneous interaction with two opposing unpaired strands and results in helix destabilization. J Mol Biol. 1998;276:367–77. doi: 10.1006/jmbi.1997.1534. [DOI] [PubMed] [Google Scholar]
- 14.Brandsen J, Werten S, van der Vliet PC, Meisterernst M, Kroon J, Gros P. C-terminal domain of transcription cofactor PC4 reveals dimeric ssDNA binding site. Nat Struct Biol. 1997;4:900–3. doi: 10.1038/nsb1197-900. [DOI] [PubMed] [Google Scholar]
- 15.Pan Z-Q, Ge H, Amin AA, Hurwitz J. Transcription-positive cofactor 4 forms complexes with HSSB (RPA) on single-stranded DNA and influences HSSB-dependent enzymatic synthesis of simian virus 40 DNA. J Biol Chem. 1996;271:22111–6. doi: 10.1074/jbc.271.36.22111. [DOI] [PubMed] [Google Scholar]
- 16.Wang J-Y, Sarker AH, Cooper PK, Volkert MR. The single-strand DNA binding activity of human PC4 prevents mutagenesis and killing by oxidative DNA damage. Mol Cell Biol. 2004;24:6084–93. doi: 10.1128/MCB.24.13.6084-6093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortusewicz O, Roth W, Li N, Cardoso MC, Meisterernst M, Leonhardt H. Recruitment of RNA polymerase II cofactor PC4 to DNA damage sites. J Cell Biol. 2008;183:769–76. doi: 10.1083/jcb.200808097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batta K, Yokokawa M, Takeyasu K, Kundu TK. Human transcriptional coactivator PC4 stimulates DNA end joining and activates DSB repair activity. J Mol Biol. 2009;385:788–99. doi: 10.1016/j.jmb.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Volkert MR. Differential requirement for SUB1 in chromosomal and plasmid double-strand DNA break repair. PLoS One. 2013;8:e58015. doi: 10.1371/journal.pone.0058015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werten S, Moras D. A global transcription cofactor bound to juxtaposed strands of unwound DNA. Nat Struct Mol Biol. 2006;13:181–2. doi: 10.1038/nsmb1044. [DOI] [PubMed] [Google Scholar]
- 21.Shamoo Y, Friedman AM, Parsons MR, Konigsberg WH, Steitz TA. Crystal structure of a replication fork single-stranded DNA binding protein (T4 gp32) complexed to DNA. Nature. 1995;376:362–6. doi: 10.1038/376362a0. [DOI] [PubMed] [Google Scholar]
- 22.Murzin AG. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 1993;12:861–7. doi: 10.1002/j.1460-2075.1993.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arcus V. OB-fold domains: a snapshot of the evolution of sequence, structure and function. Curr Opin Struct Biol. 2002;12:794–801. doi: 10.1016/S0959-440X(02)00392-5. [DOI] [PubMed] [Google Scholar]
- 24.Theobald DL, Mitton-Fry RM, Wuttke DS. Nucleic acid recognition by OB-fold proteins. Annu Rev Biophys Biomol Struct. 2003;32:115–33. doi: 10.1146/annurev.biophys.32.110601.142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giedroc DP, Khan R, Barnhart K. Overexpression, purification, and characterization of recombinant T4 gene 32 protein22-301 (g32P-B) J Biol Chem. 1990;265:11444–55. [PubMed] [Google Scholar]
- 26.Raya RR, Oot RA, Moore-Maley B, Wieland S, Callaway TR, Kutter EM, Brabban AD. Naturally resident and exogenously applied T4-like and T5-like bacteriophages can reduce Escherichia coli O157:H7 levels in sheep guts. Bacteriophage. 2011;1:15–24. doi: 10.4161/bact.1.1.14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu YD, Stanford K, Kropinski AM, Ackermann HW, Johnson RP, She YM, Ahmed R, Villegas A, McAllister TA. Genomic, proteomic and physiological characterization of a T5-like bacteriophage for control of Shiga toxin-producing Escherichia coli O157:H7. PLoS One. 2012;7:e34585. doi: 10.1371/journal.pone.0034585. [DOI] [PMC free article] [PubMed] [Google Scholar]