Abstract
In murine models of cancer, we have achieved efficient systemic activation of tumor-specific T cells by the local administration of a CTLA4-blocking antibody at low doses. Using a slow-release formulation, we could drastically lower the serum levels of the antibody, hence decreasing adverse effects and the risk of autoimmune reactions, without losing systemic efficacy.
Keywords: CTLA-4, abscopal effect, immunomodulating, local treatment, side-effects
Immunomodulation with monoclonal antibodies is a powerful new approach to anticancer immunotherapy. Recently, several antibody-based therapies have shown promising results in pre-clinical and clinical investigations, leading to the approval by the US FDA of a cytotoxic T lymphocyte-associated protein 4 (CTLA4)-blocking antibody, ipilimumab (Yervoy®), for the treatment of metastatic melanoma patients. CTLA4 is upregulated by CD4+ and CD8+ T cells upon activation, hence obstructing the positive co-stimulatory signals mediated by CD28 by binding to the same ligands: B7.1 (CD80) and B7.2 (CD86).1 CTLA4 has also been involved in the immunosuppressive functions of regulatory T cells, on which it is constitutively expressed.1 CTLA4 is one of the best-characterized molecules responsible for controlling T-cell responses against self tissues. Indeed, CTLA4 blockade improves antitumor T-cell responses, yet this is usually associated with severe autoimmune and inflammatory disorders, including dermatitis, colitis and hypophysitis.1
This risk of autoimmune and inflammatory complications upon the systemic administration of immunomodulatory antibodies has led to exploration of local intervention strategies. This concept coincides with growing evidence defining the suppressive effects of the tumor microenvironment and the unique position of tumor-draining lymph nodes (TDLNs). TDLNs can facilitate the priming of antitumor T cells but at the same time are directly influenced by the tumor microenvironment. In addition, TDLNs can serve as routes for malignant cells toward their metastatic dissemination to distant organs.2 Immunological processes of relevance for the tumor, be they immunostimulatory or immunosuppressive, mainly occur within neoplastic lesions and TDLNs.3
We have previously demonstrated that the delivery of a low dose of CD40 agonist antibodies in the slow-release formulation Montanide ISA-51 to the close proximity of malignant lesions efficiently activates antigen-specific CD8+ T-cell responses leading to tumor eradication. Importantly, the toxicity of this approach was strongly reduced as compared with the systemic administration. We demonstrated that this treatment was strictly local. Nevertheless, the resulting tumor-specific T-cell response was systemic and capable of eradicating distant tumors. We hypothesized that this protocol of administration could also be applicable to CTLA4-blocking antibody.4
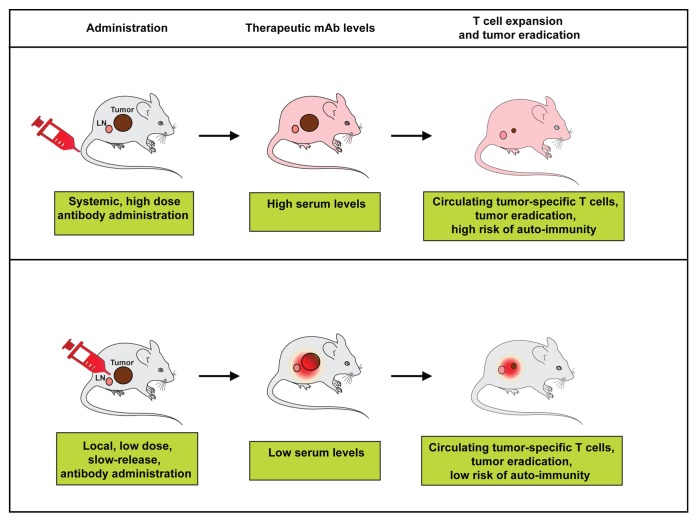
More recently, we demonstrated in several mouse models of cancer that the local injection of a CTLA4-blocking antibody in Montanide ISA-51 leads to effective anti-tumor CD8+ T-cell responses and tumor eradication while the serum levels of the antibody remain low. The treatment-elicited tumor-specific T-cell response consisted predominantly of CD8+ T cells, whereas CD4+ T cells did not play a major role in this setting. Similar to what was seen with the CD40 agonist antibody, the administration of CTLA4-targeting antibody was local but the effect on tumor-specific T-cell expansion was systemic (Fig. 1).5
Figure 1. Effects of systemic vs. local administration of immunomodulatory antibodies on their circulating levels and therapeutic activity. Importantly, in both cases distant neoplastic lesions are also rejected by CD8+ T-cell responses (not shown).
In line with our findings, other researchers have reported on the successful use of local CTLA4-blocking antibodies. Combined with either Toll-like receptor (TLR) ligands such as CpG oligodeoxynucleotides plus antibodies specific for the tumor necrosis factor α receptor family member OX40 (CD134), or with a granulocyte macrophage colony-stimulating factor (GM-CSF)-secreting cellular vaccine, the local administration of CTLA4-targeting antibodies was very successful in activating tumor-specific T cell responses and eradicating established lesions.6,7 In one of these studies, the intratumoral administration of both CTLA4- and OX40-targeting antibodies was shown to deplete regulatory T cells more efficiently than the systemic treatment, presumably through antibody-dependent cell-mediated cytotoxicity. Both these studies showed that local delivery results in strongly decreased circulating levels of therapeutic antibodies.
Local administration seems especially fitted for combinations of immunomodulatory antibodies, including antibodies against additional inhibitory receptors such as PD-1 (CD279) or against the TNFR family members CD27 and 4–1BB (CD137), because the risk of adverse effects including autoimmune reactions is drastically lowered. Moreover, therapies that are deemed too powerful (e.g., potentially causing a cytokine storm) are perhaps feasible upon local administration. Finally, combining this approach with other immunostimulatory agents such as TLR ligands, cytokines, selected chemotherapeutics or synthetic long peptide vaccines is a possibility worth exploring.8
Local approaches to cancer therapy are not novel. Surgical resection is local for obvious reasons, and new radiotherapy techniques, including photon-based radiation therapy, have significantly increased the spatial accuracy of this strategy. Even the possibility of administering chemotherapy locally is being tested, for instance by isolated perfusions or targeted delivery particles.9 Especially upon radiotherapy, systemic effects of a local treatment, termed “abscopal effects,” have been observed, possibly due to the activation of systemic immune responses. This emphasizes the efficacy of local treatments and the therapeutic potential of combinatorial regimens involving this approach.10
The tumor microenvironment and even more so TDLNs are key sites for the elicitation of optimal antitumor responses, and are therefore the quintessential targets of immunomodulatory interventions in subjects affected by solid tumors. Both the priming of tumor-specific T-cell responses and immunosuppression occur in this area. Local therapies designed to tip this equilibrium toward an effective antitumor response while not causing adverse effects are to be preferred over systemic regimens. The local immunomodulation of TDLNs and the tumor microenvironment (as opposed to the systemic administration of immunotherapeutic agents), as well as the possibility to combine this approach with other local or systemic therapies should be a focus of future efforts toward the development of novel antineoplastic regimens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Arens R, Melief CJ, Fransen M. Local immunomodulation for cancer therapy: Providing treatment where needed. OncoImmunology 2013; 2:e26493; 10.4161/onci.26493
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26493
References
- 1.Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143–55. doi: 10.1111/j.1600-065X.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 2.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6:659–70. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 3.Bindea G, Mlecnik B, Fridman WH, Pagès F, Galon J. Natural immunity to cancer in humans. Curr Opin Immunol. 2010;22:215–22. doi: 10.1016/j.coi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17:2270–80. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 5.Fransen MF, van der Sluis TC, Ossendorp F, Arens R, Melief CJ. Controlled Local Delivery of CTLA-4 Blocking Antibody Induces CD8+ T-Cell-Dependent Tumor Eradication and Decreases Risk of Toxic Side Effects. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-0781. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 6.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–63. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons AD, Moskalenko M, Creson J, Fang J, Yi S, VanRoey MJ, Allison JP, Jooss K. Local secretion of anti-CTLA-4 enhances the therapeutic efficacy of a cancer immunotherapy with reduced evidence of systemic autoimmunity. Cancer Immunol Immunother. 2008;57:1263–70. doi: 10.1007/s00262-008-0451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arens R, van Hall T, van der Burg SH, Ossendorp F, Melief CJ. Prospects of combinatorial synthetic peptide vaccine-based immunotherapy against cancer. Semin Immunol. 2013;25:182–90. doi: 10.1016/j.smim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363:2434–43. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Zitvogel L. Abscopal but desirable: The contribution of immune responses to the efficacy of radiotherapy. Oncoimmunology. 2012;1:407–8. doi: 10.4161/onci.20074. [DOI] [PMC free article] [PubMed] [Google Scholar]