Abstract
Effector T cells become rapidly inactivated after antigen exposure due to extracellular as well as intrinsic signals. We have recently demonstrated that the deletion of diacylglycerol kinases, intrinsic inhibitors of T-cell signaling, enhances the activity of adoptively transferred T cells expressing a chimeric antigen receptor (CAR) specific for a tumor-associated antigen.
Keywords: Chimeric Antigen Receptor, T cell hypofunction, diacylglycerol kinase, tumor immunosuppression, tumor microenvironment
Chimeric antigen receptor (CAR)-expressing T cells are patient-derived blood lymphocytes genetically engineered to express a fusion protein that consists of an antigen-specific single chain antibody as the extracellular domain linked to intracellular signaling domains that mediate T-cell activation. Owing to this unique construction, CARs combine the effector functions of T lymphocytes with the ability of antibodies to recognize predefined surface antigens with high specificity and avidity, independent of MHC restriction.1,2 Recently, dramatic tumor regressions in patients with blood-borne malignancies have been achieved with CAR-expressing T cells targeting the B-cell antigen CD19.3,4 This has resulted in growing enthusiasm to apply the same approach to treat solid tumors.
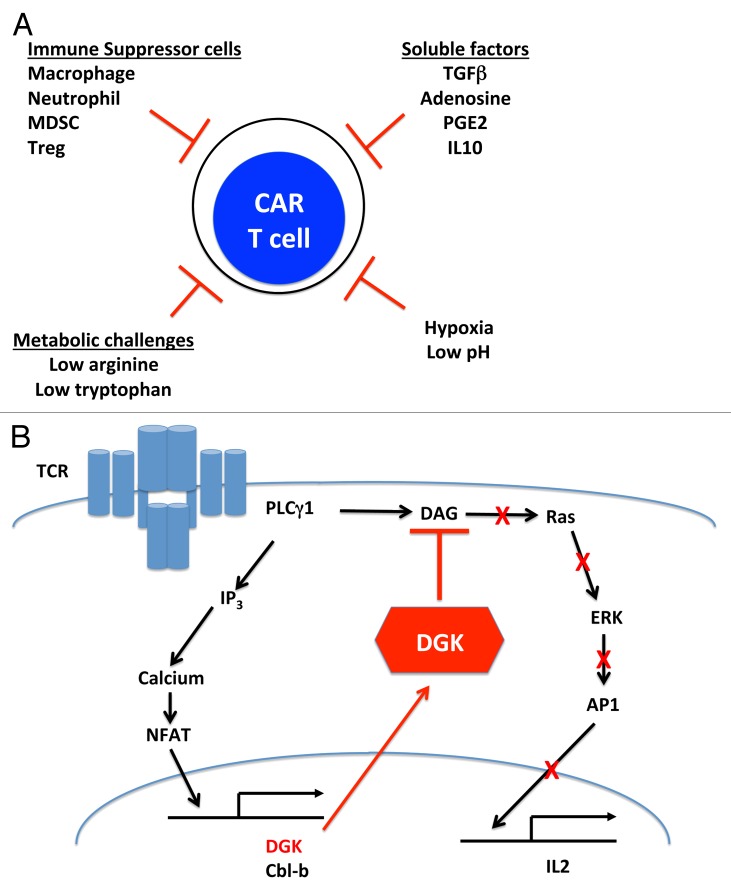
However, CAR-expressing T cells, similar to endogenous effector T cells or tumor-infiltrating lymphocytes (TILs) expanded ex vivo, will face a number of challenges within the microenvironment of solid tumors that are likely to limit their efficacy (Fig. 1A). These obstacles include: (1) the immunosuppressive effects of multiple tumor-infiltrating cells, including macrophages, neutrophils, myeloid-derived suppressor cells, and regulatory T cells; (2) the inhibitory effects of tumor-derived soluble factors, such as transforming growth factor ? (TGF?), prostaglandin E2 (PGE2), and adenosine; (3) metabolic challenges, such as restrictive amounts of arginine or tryptophan; and (4) a microenvironment characterized by hypoxia and low pH. Moreover, previous studies with mouse and human TILs suggest that 2 additional inhibitory mechanisms will limit the antineoplastic activity of CAR-expressing T cells. First, as these cells encounter their cognate antigen within the tumor microenvironment, they are likely to upregulate inhibitory receptors causing the inhibition of their tumoricidal activity. A growing number of these receptors (and the corresponding ligands) are being characterized, including CTLA4/B7?1, PD-1/PD-L1, LAG3/MHC class II molecules, 2B4/CD48, and TIM3/Galectin-9. Second, T cells are endowed with a robust intracellular inhibitory system that can be induced upon T-cell activation, hence limiting T-cell receptor (TCR)-dependent signaling pathways and functions (presumably as a means to prevent autoimmune reactions). Components of this system that have been shown to operate in TILs include: (1) phosphatases that oppose immunostimulatory kinases, such as SHP-1, which dephosphorylates some of the TCR-associated kinases, such as LCK and ZAP70; (2) deubiquitinases (i.e., cbl-b); and (3) inhibitory kinases, such as diacylglycerol kinases (DGKs), which physically translocate to subcellular compartments important for TCR signaling.5
Figure 1. Impact of diacylglycerol kinases on the antineoplastic activity of CAR-expressing T cells. (A) Obstacles for CAR-expressing T cells in tumor microenvironment. The tumor microenvironment is inhibitory for T cells and CAR-expressing T cells. At least in part, this results from: (1) the immunosuppressive effects of various tumor-infiltrating cells, (2) the inhibitory effects of tumor-derived soluble factors, (3) metabolic challenges, and (4) a microenvironment characterized by hypoxia and low pH. (B) DGKs are central inhibitors of TCR-mediated RAS/ERK signaling in functionally impaired T cells. Prolonged TCR signaling upregulates the expression of cell-intrinsic immunosuppressive factors such as diacylglycerol kinases (DGKs) and cbl-b. In functionally impaired T cells, high levels of DGKs mediate the phosphorylation of the second messenger diacylglycerol (DAG), blunting the activation of the RAS/ERK/AP-1 signaling pathway.
Many studies (in both mouse and human systems) have shown that, owing to these mechanisms, TILs become hypofunctional (i.e., unable to kill malignant cells and release immunostimulatory cytokines) upon recruitment to neoplastic lesions. We believe the same phenomenon would affect CAR-expressing T cells. This idea is supported by recent preclinical studies from our laboratory showing that human CAR-expressing T cells injected into immunodeficient mice bearing human tumors developed profound functional defects. Thus, for CAR-based therapies to succeed in the clinic, it will be important to design strategies that will render T cells more resistant to tumor-induced functional impairment. To date, this goal has been approached by engineering T cells with genes other than the CAR-coding one, including genes that encode cytokines (i.e., IL-12), stimulatory proteins (i.e., constitutively active AKT1), or antagonists of inhibitory proteins (i.e., a dominant-negative TGF? receptor).6-8 It should also be possible to block the expression or activities of inhibitory factors such as PD-1, SHP-1, or CBL-B using short-hairpin RNAs or intracellular antibodies.
We recently evaluated the role of the isoforms of one intrinsic inhibitor of TCR signaling, namely DGKs, on effector T-cell functions.9 DGKs are key enzymes that inactivate diacylglycerol (DAG), the essential second messenger of signal transduction cascades that are essential for T-cell activation, most notably the RAS/ERK pathway. DAG activity is terminated through its conversion into phosphatidic acid by 1 of 2 isoforms of DGK present within T cells, DGK? or DGK?. The deletion of DGKs has been shown to counteract anergy in CD4+ cells,10 and DGKs are known to be upregulated in functionally impaired TILs (Fig. 1B).
In our recent study,9 we investigated T cells from mice deficient in 1 or both isoforms of DGK. We found that the deletion of DGK-coding genes has profound effects on effector T cells, both downstream of the TCR in ovalbumin-specific T cells and downstream of the CAR in T cells expressing a CAR specific for the tumor-associated antigen mesothelin. The ablation of DGK? augmented ERK activation in retrovirally-transduced CAR-expressing T cells upon antigen ligation, resulting in enhanced cytokine production and target cell killing relative to their wild-type counterparts. The ablation of both DGK? and DGK? resulted in an even greater enhancement of the effector functions of CAR-transduced cells and prolonged the persistence of adoptively transferred T cells, ultimately resulting in superior antineoplastic effects in mice. In addition, the deletion of DGKs was observed to protect T cells from inactivation by inhibitory stimuli such as PGE2, adenosine, and TGF?. Importantly, using an in vitro model of functional impairment of human CAR-expressing T cells, pharmacologic inhibition of DGKs was also shown to prevent the loss of cytotoxic activity and cytokine release.
Our study demonstrates that targeting specific enzymes that inhibit T-cell signaling (DGKs) has the potential to blunt tumor-induced functional defects in T cells. Given the ease by which additional genes can be inserted into CAR-expressing T cells, we believe that knocking-down or inhibiting DGKs could be a useful approach to improve adoptive T cell transfer-based strategies for the treatment of human neoplasms. We are currently attempting to genetically suppress DGK activity in human CAR-expressing T cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Wang LC, Riese MJ, Moon EK, Albelda SM. Overcoming intrinsic inhibitory pathways to augment the antineoplastic activity of adoptively transferred T cells: Re-tuning your car before hitting a rocky road. OncoImmunology 2013; 2:e26492; 10.4161/onci.26492
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26492
References
- 1.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–12. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter D, Levine B, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–56. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 6.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Dotti G, Huye LE, Foster AE, Savoldo B, Gramatges MM, Spencer DM, Rooney CM. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther. 2010;18:2006–17. doi: 10.1038/mt.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer S, Wang ZG, Akhtari M, Zhao W, Seth P. Targeting TGFbeta signaling for cancer therapy. Cancer Biol Ther. 2005;4:261–6. doi: 10.4161/cbt.4.3.1566. [DOI] [PubMed] [Google Scholar]
- 9.Riese MJ, Wang L-CS, Moon EK, Joshi RP, Ranganathan A, June CH, Koretzky GA, Albelda SM. Enhanced effector responses in activated CD8+ T cells deficient in diacylglycerol kinases. Cancer Res. 2013;73:3566–77. doi: 10.1158/0008-5472.CAN-12-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–81. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]