Abstract
By binding to the liver X receptor (LXR), oxysterols inhibit the expression of chemokine (C-C motif) receptor 7 (CCR7), hence impairing the migration of dendritic cells to secondary lymphoid organs and inhibiting antitumor immune responses. We have recently identified a new tumor-supporting activity of oxysterols, which recruit neutrophils within tumor microenvironment by a chemokine (C-X-C motif) receptor 2 (CXCR2)-dependent, LXR-independent mechanism.
Keywords: CXCR2, immune evasion, microenvironment, oxysterols, tumor-infiltrating neutrophils
Myeloid cells infiltrating the microenvironment of metastatic tumors are frequently endowed with a tumor-promoting, rather than -inhibiting, activity.1,2 Different tumor-derived factors have been shown to underlie such a tumor-supporting role of immune cells.3 Indeed, cytokines and growth factors such as interleukin (IL)-6, IL-10, transforming growth factor ? (TGF), granulocyte colony-stimulating factor (G-CSF), and vascular endothelial growth factor (VEGF), among others, have been identified as negative regulators of tumor-infiltrating immune cells as they inhibit the functions of antigen-presenting cells and T cells.3 Moreover, metabolic products generated in the course of tumor progression have also been involved in the establishment of immunosuppressive networks.4
Liver X receptor (LXR) ligands or the oxysterol family are cholesterol derivatives generated by the enzymatic and non-enzymatic oxidation of cholesterol. Most of the biological activity of oxysterols originates from the engagement of nuclear LXR? and LXR?.5 Oxysterols and their receptors are involved in the homeostasis of cholesterol and lipid metabolism.5 Recently, the LXR system has also been demonstrated to modulate innate and adaptive immune responses, playing a relevant role in inflammation and autoimmune diseases.6
We have previously reported that LXR ligands inhibit antitumor immune responses by reducing the expression of chemokine (C-C motif) receptor 7 (CCR7) on maturing dendritic cells (DCs), hence limiting their ability to migrate to tumor-draining lymph nodes, in an LXR-dependent manner.7 We also demonstrated that the pharmacologic or genetic inhibition of this pathway restores DC migration to tumor-draining lymph nodes, thereby supporting the elicitation of an immune response that ultimately delays disease progression or mediates tumor rejection in mice.
We have recently investigated whether oxysterols would exert additional tumor-supporting functions by affecting immune cells other than DCs within tumor microenvironment.8 To this aim, we analyzed the microenvironment of tumor grafts growing subcutaneously (RMA lymphomas and LLC lung carcinomas) or intraperitoneally (AB1 mesotheliomas) in mice. To avoid the secretion of bioactive oxysterols within tumor microenvironment, malignant cells were transduced with viral vectors encoding sulfotransferase family, cytosolic, 2B, member 1 (SULT2B1b), which is capable of inactivating oxysterols upon sulfation.7 The analysis of neoplastic lesions devoid of active oxysterols (i.e., SULT2B1b-expressing tumors) revealed a reduced size as well as a decrease in tumor-infiltrating neutrophils, as compared with control (i.e., mock-transfected) tumors. We reasoned that neutrophils could be implicated in tumor growth, and that the LXR system could be involved in the accumulation of neutrophils within the tumor microenvironment. By performing parabiosis experiments, we observed a continuous recruitment of neutrophils to neoplastic lesions, raising the intriguing hypothesis that oxysterols might behave as chemoattractants for neutrophils (Fig. 1). This hypothesis was confirmed by migration experiments in vitro. Noteworthy, the migration of neutrophils toward oxysterols turned out to require chemokine (C-X-C motif) receptor 2 (CXCR2) but not LXR, indicating that oxysterols released within tumor microenvironment may have a dual tumor-supporting role, i.e., the LXR-dependent inhibition of CCR7 expression on maturing DCs,7 and the LXR-independent activation of the G protein-coupled receptor CXCR2, resulting in neutrophil recruitment.8 Interestingly, we did not observe any variation in the amounts of chemokine (C-X-C motif) ligand (CXCL1) and CXCL5 (2 CXCR2-binding chemokines) released by SULT2B1B-expressing and control tumors.
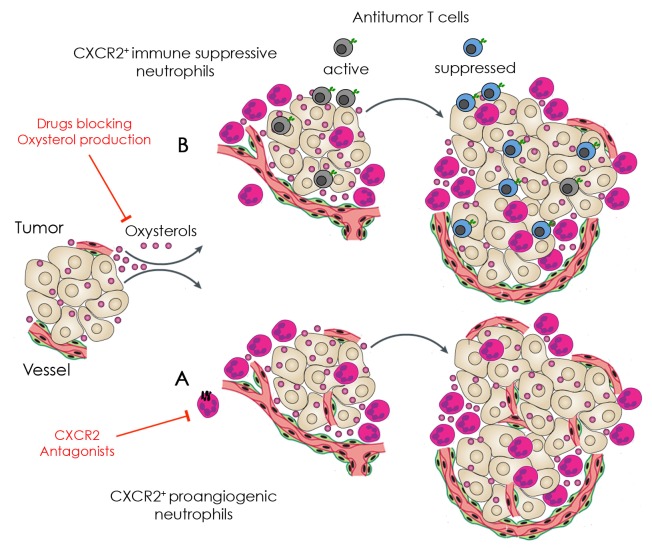
Figure 1. Involvement of oxysterols in the recruitment of neutrophils to neoplastic lesions and possible points of therapeutic intervention. (A and B) Cancer cells release oxysterols in the extracellular milieu, resulting in the recruitment of tumor-supporting neutrophils. Such neutrophils mediate pro-angiogenic effects by releasing matrix metalloproteinase 9 (MMP9) and prokineticin 2 (PROK2, also known as BV8) (A), as well as immunosuppressive functions, as they inhibit antigen-specific T cells (B). Agents that antagonize chemokine (C-X-C motif) receptor 2 (CXCR2) (A) or block oxysterols (B) may inhibit the recruitment of tumor-supporting neutrophils, hence restoring antitumor immune responses that may delay disease progression or even eradicate established lesions.
Tumor-infiltrating neutrophils may exert a tumor-supporting role by stimulating neoangiogenesis, a process that involves the release of the pro-angiogenic factors matrix metallopeptidase 9 (MMP9) and prokineticin 2 (PROK2, also known as BV8), or by suppressing the activity of antigen-specific T cells within the tumor microenvironment.9 We thus assessed whether oxysterol-recruited neutrophils would be able to induce neoangiogenesis, immunosuppression or both in the three tumor models. In RMA and AB1 tumors, neutrophils mainly effected neoangiogenic functions (Fig. 1A). Instead, neutrophils isolated from LLC tumors were endowed with a potent immunosuppressive activity (Fig. 1B), yet they failed to exert proangiogenic effects. These variations may depend on the cellular and molecular context of each tumor, and deserve further investigation in spontaneous tumor models. Noteworthy, the dual (i.e., LXR-dependent and -independent) role of tumor-derived oxysterols was confirmed in Nr1h3-/- bone marrow (BM) chimeras. In particular, we observed that the growth of oxysterol-releasing tumors was slower in Nr1h3-/- BM chimeras than in ?wild type? BM chimeras. However, the growth rate of oxysterol-releasing tumors was invariably higher than that of their SULT2B1b-expressing counterparts, in which oxysterols are inactivated (unpublished results). These findings indicate that oxysterols dampen antitumor immune responses by exploiting both LXR-dependent and -independent mechanisms. Whether other immunosuppressive mechanisms are activated by tumor-derived oxysterols remains to be clarified. Furthermore, oxysterols released by stromal cells or inflammatory cells (i.e., macrophages),10 for instance upon exposure to pro-inflammatory stimuli, may also play a relevant tumor-promoting role, a possibility that should be carefully investigated.
We also demonstrated that the treatment of tumor-bearing mice with a synthetic CXCR2 antagonist delays tumor growth, reduces the degree of neo-vascularization and limit tumor infiltration by neutrophils. This suggests that CXCR2 antagonists should be considered as anticancer agents in combination with conventional or experimental therapies (Fig. 1).
In summary, we have characterized a new mechanism whereby oxysterols dampen antitumor immune responses.8 In particular, tumor-derived oxysterols turned out to recruit tumor-supporting neutrophils within neoplastic lesions in a CXCR2-dependent manner. Based on this mechanism and on previous findings from our laboratory,7 we propose that agents blocking oxysterol synthesis as well as CXCR2 antagonist constitute promising candidates for the development of novel anticancer therapies (Fig. 1).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- DC
dendritic cell
- LXR
liver X receptor
- SULT2B1b
sulfotransferase family, cytosolic, 2B, member 1
Citation: Raccosta L, Fontana R, Traversari C, Russo V. Oxysterols recruit tumor-supporting neutrophils within the tumor microenvironment: The many facets of tumor-derived oxysterols. . OncoImmunology 2013; 2:e26469; 10.4161/onci.26469
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26469
References
- 1.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 4.Villalba M, Rathore MG, Lopez-Royuela N, Krzywinska E, Garaude J, Allende-Vega N. From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol. 2013;45:106–13. doi: 10.1016/j.biocel.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–81. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 6.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–7. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 7.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Sanvito F, Ponzoni M, Valentinis B, Bregni M, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 8.Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, Chiricozzi E, Trincavelli ML, Daniele S, et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711–28. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 10.Spann NJ, Glass CK. Sterols and oxysterols in immune cell function. Nat Immunol. 2013;14:893–900. doi: 10.1038/ni.2681. [DOI] [PubMed] [Google Scholar]