Abstract
Nuclear factor-κB (NF-κB) signaling contributes to human disease processes, notably inflammatory diseases and cancer. NF-κB has a role in tumorigenesis and tumor growth, as well as promotion of metastases. Mechanisms responsible for abnormal NF-κB activation are not fully elucidated; however, RelA phosphorylation, particularly at serine residues S536 and S276, is critical for RelA function. Kinases that phosphorylate RelA promote oncogenic behaviors, suggesting that phosphatases targeting RelA could have tumor-inhibiting activities; however, few RelA phosphatases have been identified. Here, we identified tumor inhibitory and RelA phosphatase activities of the protein phosphatase 2C (PP2C) phosphatase family member, PPM1A. We show that PPM1A directly dephosphorylated RelA at residues S536 and S276 and selectively inhibited NF-κB transcriptional activity, resulting in decreased expression of monocyte chemotactic protein-1/chemokine (C–C motif) ligand 2 and interleukin-6, cytokines implicated in cancer metastasis. PPM1A depletion enhanced NF-κB-dependent cell invasion, whereas PPM1A expression inhibited invasion. Analyses of human expression data revealed that metastatic prostate cancer deposits had lower PPM1A expression compared with primary tumors without distant metastases. A hematogenous metastasis mouse model revealed that PPM1A expression inhibited bony metastases of prostate cancer cells after vascular injection. In summary, our findings suggest that PPM1A is a RelA phosphatase that regulates NF-κB activity and that PPM1A has tumor suppressor-like activity. Our analyses also suggest that PPM1A inhibits prostate cancer metastases and as neither gene deletions nor inactivating mutations of PPM1A have been described, increasing PPM1A activity in tumors represents a potential therapeutic strategy to inhibit NF-κB signaling or bony metastases in human cancer.
Keywords: NF-κB, PPM1A, phosphatase, prostate cancer, tumor suppressor
INTRODUCTION
Nuclear factor-κB (NF-κB), comprised of a family of pluripotent transcription factors, has a fundamental role in inflammatory and immune responses, and aberrant NF-κB activity can directly contribute to tumorigenesis, neovascularization, tumor growth and metastases.1,2 NF-κB activation has been described in many tumors including HNSCC3,4 and breast cancer.5,6 Dysregulation of NF-κB in prostate cancer has been identified as a major driver of distant metastasis, which is the primary cause of death in this common male cancer.7–9 One transcriptional target of NF-κB, monocyte chemotactic protein-1 (MCP-1), also known as chemokine (C–C motif) ligand 2 (CCL2), has been implicated in prostate cancer migration, invasion and metastasis through effects on both tumor cells and the microenvironment.10 MCP-1 seems to be particularly implicated in bony metastases for prostate cancer and in other tumor types including renal cancer, bladder cancer and breast cancer.11 Although mechanisms governing MCP-1 expression are not fully described, the suspected importance of this cytokine in tumor progression is affirmed by the recent initiation of clinical trials using neutralizing antibody targeting MCP-1.
Because of the role of NF-κB in varied biological processes, NF-κB activity is intricately controlled at multiple levels. Inhibition of NF-κB through binding to IkBa with prevention of nuclear translocation has been well characterized; however, full activation of nuclear NF-κB transcriptional activity requires phosphorylation of RelA.12 Phosphor-acceptor sites of RelA have been characterized,12,13 particularly implicating serine residues S536 and S276, as critical for NF-κB activation.14,15 Although not as extensively studied, dephosphorylation of RelA is needed to prevent harmful effects of prolonged RelA signaling.16 To date, only two phosphatases, PP2A and Wip1/PPM1D, have been shown to directly dephosphorylate RelA, both having activity toward S536.17,18 Deficiency of PP2A contributes to the constitutive activation of RelA in melanoma cells,17 and mice lacking Wip1 have an inflammatory phenotype,18 suggesting that loss of RelA-targeting phosphatases can impact pathological processes including cancer.
PPM1A belongs to the protein phosphatase 2C (PP2C) family together with Wip1 and PPM1B. PPM1A has been implicated in the regulation of several signaling pathways including tumor growth factor-β/Smad,19 mitogen-activated protein kinase (c-Jun N-terminal kinase/p38), Cdk2 and Cdk620 and the nerve growth factor-activated Akt/ERK pathway.21 Regulation of these pathways has been attributed to PPM1A phosphatase activity toward important pathway components in some cases (for example, Smad2/3 and p38), but has not been fully characterized in others (for example, Akt/ERK, Cdk2 and Cdk6). In addition, PPM1A has been implicated in the regulation of proliferation,20 cell invasion and migration,22 but targets of PPM1A activity that regulate these activities have not been identified. Recently, PPM1A has been described as an indirect regulator of NF-κB through dephosphorylation and inactivation of IκB kinase β (IKKβ).23 Here we show that PPM1A directly dephosphorylated RelA at S536 and S276, with resultant inhibition of NF-κB transactivation and decreased expression of target genes, notably including MCP-1/CCL2. We report that PPM1A expression was downregulated in human metastatic prostate cancer, and that restoration of PPM1A decreased seeding and bony growth of prostate cancer cells in an animal vascular injection model. In addition, loss of PPM1A increased NF-κB-dependent invasion. These data suggest that PPM1A has tumor suppressor-like qualities that are, at least partially, dependent on the regulation of RelA.
RESULTS
PPM1A directly dephosphorylates RelA
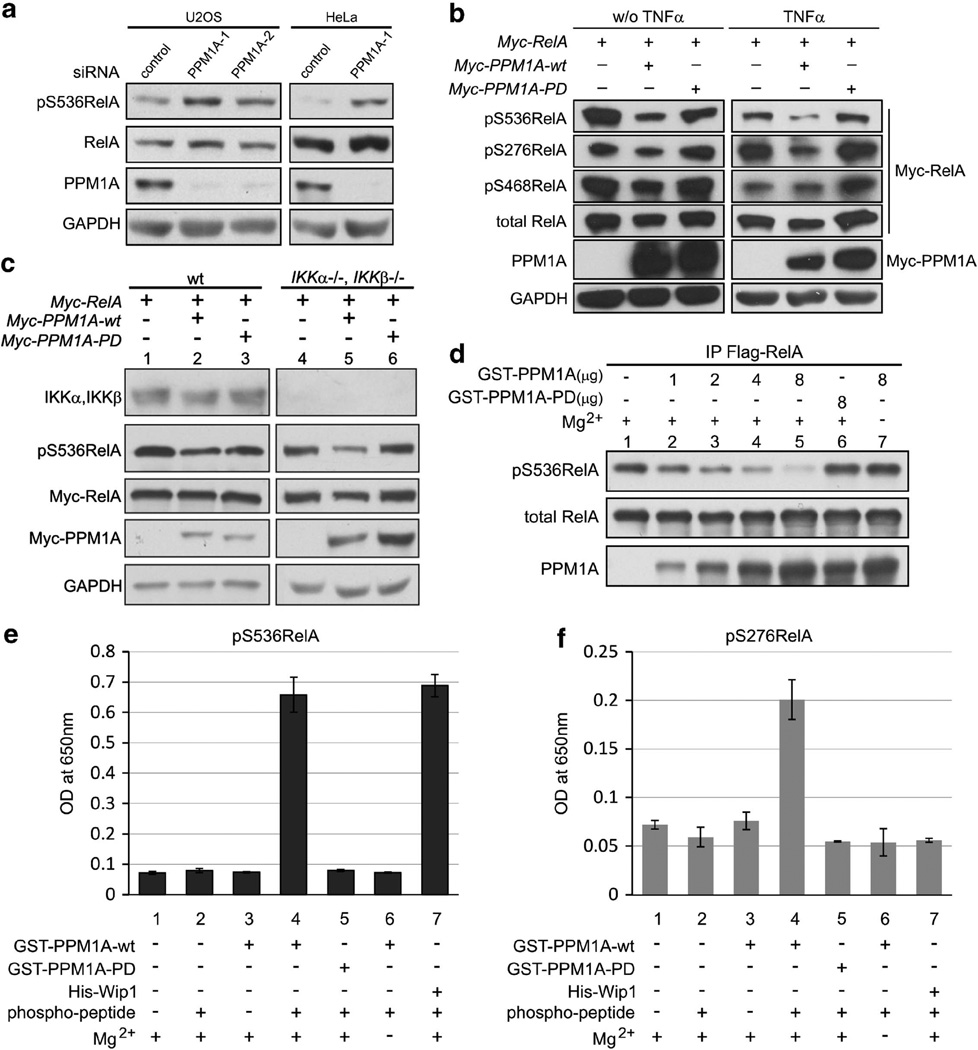
To better understand drivers of cancer progression and metas-tases, we sought to identify phosphatase inhibitors of NF-κB that work directly to dephosphorylate RelA. Because the PP2C family member Wip1 is one of the two described RelA phosphatases, we considered a PP2C family member, PPM1A, as a candidate RelA phosphatase. To begin exploring this possibility, the effect of endogenous PPM1A on RelA phosphorylation was determined in U2OS and HeLa cells. Following PPM1A depletion using two independent small interfering RNAs (siRNAs), RelA phosphorylation at S536 was increased in both cell lines, whereas total RelA levels were not altered (Figure 1a). Depletion of PPM1A further increased RelA phosphorylation following stimulation with tumor necrosis factor α (TNFα) (Supplementary Figure S1A). siRNA-mediated depletion of PPM1A did not alter total RelA levels (Supplementary Figure S1A) and did not affect PPM1B or Wip1 protein expression (Supplementary Figure S1B). Expression of wild-type PPM1A decreased S536 phosphorylation of ectopically expressed RelA both with and without TNFα stimulation, whereas phosphatase-dead (R174G) PPM1A had no effect (Figure 1b). Interestingly, RelA S276 phosphorylation was similarly decreased by the expression of PPM1A (Figure 1b) and siRNA-mediated depletion of PPM1A increased S276 phosphorylation after TNFα treatment (Supplementary Figure S1C). Phosphorylation of S468, a known transcriptional inhibition phosphorylation site of RelA,24,25 was unaffected by the expression of PPM1A (Figure 1b, 0.9 normalized to vector control as 1).
Figure 1.
PPM1A is a RelA phosphatase. (a) U2OS and HeLa cells were transfected with indicated siRNAs. PPM1A as well as endogenous phospho- and total RelA were visualized by immunoblotting. GAPDH served as loading control. (b) U2OS cells were transfected with indicated plasmids and proteins visualized by immunoblotting with GAPDH serving as loading control. (c) Wild-type (wt) mouse embryonic fibroblasts (MEFs) and IKKα−/−IKKβ −/− double-null MEFs were transfected with indicated plasmids. Endogenous IKKα and IKKβ and transfected phospho- and total RelA, as well as PPM1A, were visualized by immunoblotting. (d) Immunoprecipitated (IP) full-length Flag-RelA was used as substrate for in vitro phosphatase assay. Phospho- and total RelA were visualized by immunoblotting. Exclusion of PPM1A or magnesium in the phosphatase buffer served as negative controls. (e and f) S536- and S276-specific phosphorylated RelA peptides were incubated with equal amounts of glutathione S-transferase (GST)-PPM1A or His-Wip1 and free phosphate measured by malachite green assay. OD, optical density; PPM1A-PD, phosphatase dead PPM1A.
We confirmed reports that PPM1A decreases IKKα and IKKβ phosphorylation23 (Supplementary Figure S1D); therefore, decreased RelA phosphorylation observed following expression of PPM1A could be a result of direct activity to dephosphorylate RelA or be mediated by IKKs. To begin exploring these possibilities, PPM1A was expressed in IKKα −/−, IKKβ −/− double-null mouse embryonic fibroblasts. Notably, PPM1A expression resulted in decreased RelA S536 phosphorylation in the absence of IKKα or IKKβ (Figure 1c, lanes 4, 5) indicating that PPM1A regulates RelA independent of IKKs. In these cells, decreased RelA phosphorylation was also dependent on PPM1A phosphatase activity and did not alter in RelA protein levels (Figure 1c).
Although PPM1A activity toward RelA was independent of IKKs, PPM1A regulation of RelA phosphorylation could be mediated through unknown indirect activities or through direct dephos-phorylation of RelA. To begin distinguishing these possibilities, in vitro phosphatase assays using full-length RelA or RelA-specific phosphopeptides as substrates were performed. To determine PPM1A activity toward the full-length protein, expressed Flag-RelA was immunoprecipitated from 293T cells and then incubated with bacterially synthesized glutathione S-transferase-PPM1A proteins. Incubation with wild-type (Figure 1d, lanes 1–5) but not phosphatase-dead PPM1A (Figure 1d, lane 6), decreased RelA phosphorylation at S536 in a dose-dependent manner. As with all PP2C family members, PPM1A phosphatase activity was magnesium-dependent (Figure 1d, compare lanes 5 and 7). Because reliable phospho-specific antibodies are available only for S536 and to confirm findings of the full-length phosphatase assay, a peptide-based phosphatase assay was performed. Synthesized RelA phosphopeptides corresponding to phospho-S536 and S276 (pS536, pS276) (LifeTein, South Plainfield, NJ, USA) were used as substrates and dephosphorylation was quantified using malachite green assay.18 Wild-type, but not phosphatase-dead, PPM1A dephosphorylated the pS536 peptide with equivalent efficacy as the known RelA S536 phosphatase, Wip1 (Figure 1e, compare lanes 4 and 7). As opposed to Wip1, PPM1A also dephosphorylated the pS276 peptide (Figure 1f, compare lanes 4 and7). As expected, magnesium was required for the activity of both PPM1A and Wip1 (Figures 1e and f, lane 6) and the phosphatase-dead mutant of PPM1A had no activity toward either pS536 or pS276 peptides (Figure 1e and f, lane 5). Taken together, these data suggest that PPM1A is a direct RelA phosphatase and the first phosphatase with the potential activity toward pS276 of RelA.
PPM1A inhibits RelA transcription activity, decreases NF-κB-dependent cell invasion, sensitizes cell to TNFα, but does not alter RelA nuclear localization or DNA binding
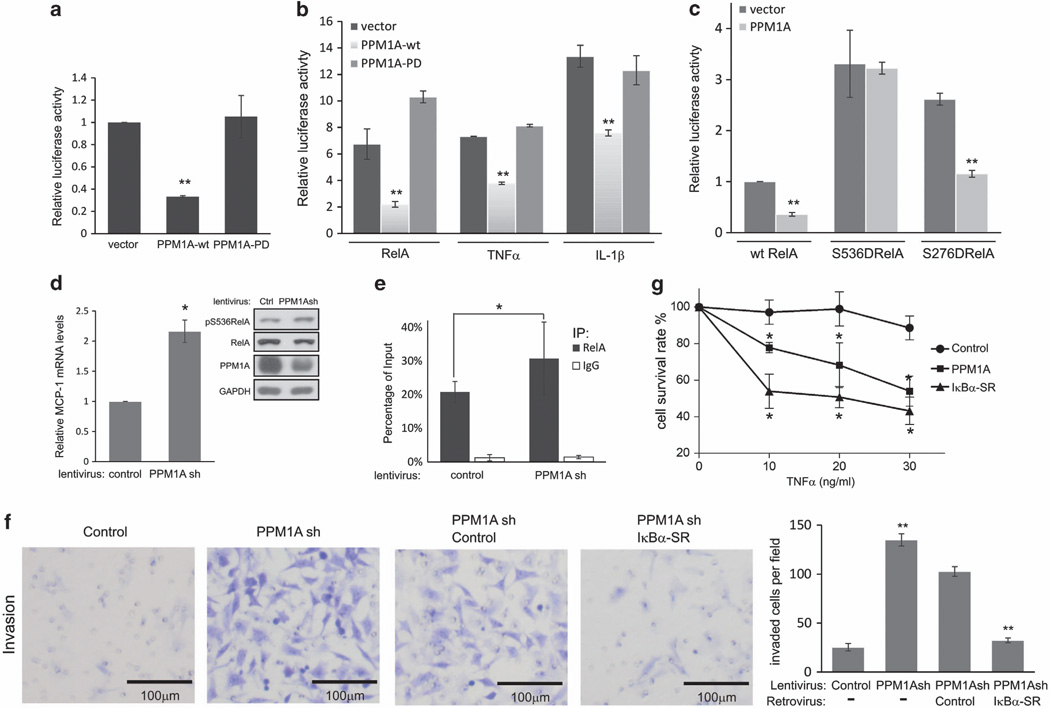
Phosphorylation at serine residues S536 and S276 is critical for RelA transactivation activity.26 To determine if dephosphorylation by PPM1A inhibits RelA transcriptional activity, a dual-luciferase reporter assay was performed.3 In U2OS cells, endogenous NF-κB transcriptional activity was decreased following ectopic coexpression of wild-type PPM1A- and NF-κB-responsive reporter (Figure 2a). Likewise, PPM1A also inhibited endogenous NF-κB transcriptional activity after stimulation by TNFα or IL-1β and reversed luciferase transcription increased by ectopic expression of RelA (Figure 2b), whereas PPM1A did not inhibit transcription of control reporter without NF-κB-responsive element (Supplementary Figure S2A). Consistent with our observations that phosphatase activity was required for PPM1A to alter RelA phosphorylation, phosphatase-dead PPM1A had no effect on RelA transcriptional activity (Figure 2a and b). S536 and S276 contribute to RelA transcriptional activity and both residues were dephosphorylated by PPM1A. To explore the relative contribution of these two residues to PPM1A-mediated RelA inhibition, RelA mutants mimicking phosphorylation at these sites (RelAS536D and RelAS276D) were expressed with or without PPM1A. S536D and S276D mutants displayed increased transcriptional activity compared with wild-type RelA (Figure 2c). Ectopically expressed PPM1A inhibited transcription by RelA S276D (P<0.001), but not by RelA S536D (P=0.86). Of note, PPM1A inhibited slightly less efficiently the transcription by the S276D mutant than wild-type RelA (inhibition rate 56 vs 64%, P<0.01). Taken together, these data suggest that dephosphorylation of S276 by PPM1A may contribute to inhibit RelA transcriptional activity, but the majority of PPM1A activity to inhibit RelA transcription relies on dephos-phorylation of S536 of RelA.
Figure 2.
PPM1A inhibits NF-κB transcription activity, NF-κB-dependent cell invasion and sensitizes cells to TNFα. (a) U2OS cells were co-transfected with indicated plasmids and Renilla luciferase and the NF-κB-responsive reporter 3κB-ConA-LUC firefly luciferase. In this and subsequent luciferase assays, firefly luciferase activity was normalized to Renilla luciferase and normalized firefly luciferase activity from cells transfected with control plasmids was assigned a value of 1. Error bars, s.d. derived from three analyses. **P<0.01. (b) NF-κB-dependent luciferase activity was determined as described in a in cells expressing wild-type (wt) or phosphatase dead PPM1A and either ectopic expression of RelA or 4 h treatment with TNFα or IL-1β; **P<0.01. (c) Luciferase activity was determined following transfection of U2OS with plasmids encoding wt or mutant RelA (S536D or S276D) with or without PPM1A as indicated; **P<0.01. (d) Quantitative real-time PCR (q-RT-PCR) analyses of MCP-1 expression in HeLa cells selected after infection of PPM1A short hairpin RNA (shRNA) encoding lentivirus. Error bars = s.d.; *P<0.05. PPM1A, pS536RelA and RelA levels were confirmed by immunoblotting. (e) ChIP assays of the binding of RelA to MCP-1 proximal promoter. Samples from HeLa cells as described in d were prepared and analyzed using antibodies specific for RelA or immunoglobulin G (IgG) as control. Immunoprecipitated (IP) DNA fragments and input DNA were analyzed by real-time PCR. Values were normalized to input DNA in each group. Error bars = s.d.; *P<0.05. (f) HeLa cells infected with indicated viruses were plated on transwells with matrigel and invasion measured by direct counting of trespassed cells. Representative photomicrographs are shown (scale bars, 100 µm). Quantification of cell invasion in results represents cell counts from 10 randomly selected low-powered fields (× 200); **P<0.01. PPM1A and kBα-super-repressor (IκBα-SR) expression levels were confirmed by immunoblotting (Supplementary Figure S2D). (g) HT1080 cells were infected as indicated and treated with indicated doses of TNFα for 24 h. Cells were stained with Trypan blue and live cells were counted. Error bars = s.d.; *P<0.05.
Given that PPM1A robustly inhibited RelA transcription (Figures 2a – c), we explored NF-κB target genes regulated by endogenous PPM1A by comparing expression between HeLa cells with and without lentivirus-driven PPM1A knockdown. Of tested genes (RT2 Profiler PCR Array; SABiosciences, Valencia, CA, USA), expression of selective RelA-responsive genes (Table S1), including MCP-1 and IL-6, were increased at least 1.5-fold following PPM1A depletion. These data are consistent with earlier reports23,27 that PPM1A regulates IL-6 expression, as we confirmed in PC3 (human prostate cancer) cells (Supplementary Figure S2B). Expression of MCP-1, a chemokine that is linked to tumor progression and metastases, was confirmed by real-time polymerase chain reaction (PCR) (Figure 2d). To determine if PPM1A directly alters the ability of NF-κB to bind the promoter region of the MCP-1 gene28,29 RelA chromatin immunoprecipitation (ChIP) was performed following PPM1A depletion in HeLa cells. Following PPM1A depletion, endogenous RelA association with the MCP-1 promoter was observed (Supplementary Figure S2C). Compared to control cells, cells with PPM1A knockdown showed a moderate but significant increase (48% increase, 20.8 vs 30.8%, P<0.05) of endogenous RelA binding to the MCP-1 proximal promoter region (Figure 2e). These data suggest that PPM1A selectively regulates NF-κB transcription activity.
We previously showed that the tumor suppressor LZAP inhibits NF-κB activity and diminishes HeLa cell invasion.3 To determine if PPM1A inhibition of RelA similarly decreased HeLa invasion, matrigel invasion was measured after PPM1A depletion. Knockdown of PPM1A increased HeLa cell invasion by approximately fivefold (Figure 2f). IκBα degradation leads to release and nuclear localization of NF-κB dimmers with the RelA/p50 heterodimer representing the most abundant and the primary target of IκBα.30,31 To determine if increased invasion associated with PPM1A depletion was dependent on NF-κB and especially RelA, a non-degradable IκBα, termed IkBα-super-repressor, was expressed in PPM1A-depleted cells. Inhibition of NF-κB abrogated the increased invasion observed following PPM1A knockdown (Figure 2e). These data suggest that depletion of PPM1A enhances NF-κB-dependent cell invasion.
TNFα activates apoptotic pathways but rarely results in massive cell death due to simultaneous induction of NF-κB transcription.32 To determine if PPM1A-mediated inhibition of NF-κB would sensitize cells to TNFα, PPM1A was expressed in HT1080 cells before treatment with increasing doses of TNFα. Remarkably, PPM1A sensitized HT1080 cells to TNFα-induced cell death, albeit to a lesser extent than IkBα-super-repressor (Figure 2f). In the absence of expressed PPM1A or IκBα-super-repressor, HT1080 cell survival was minimally impacted by TNFα concentrations tested.
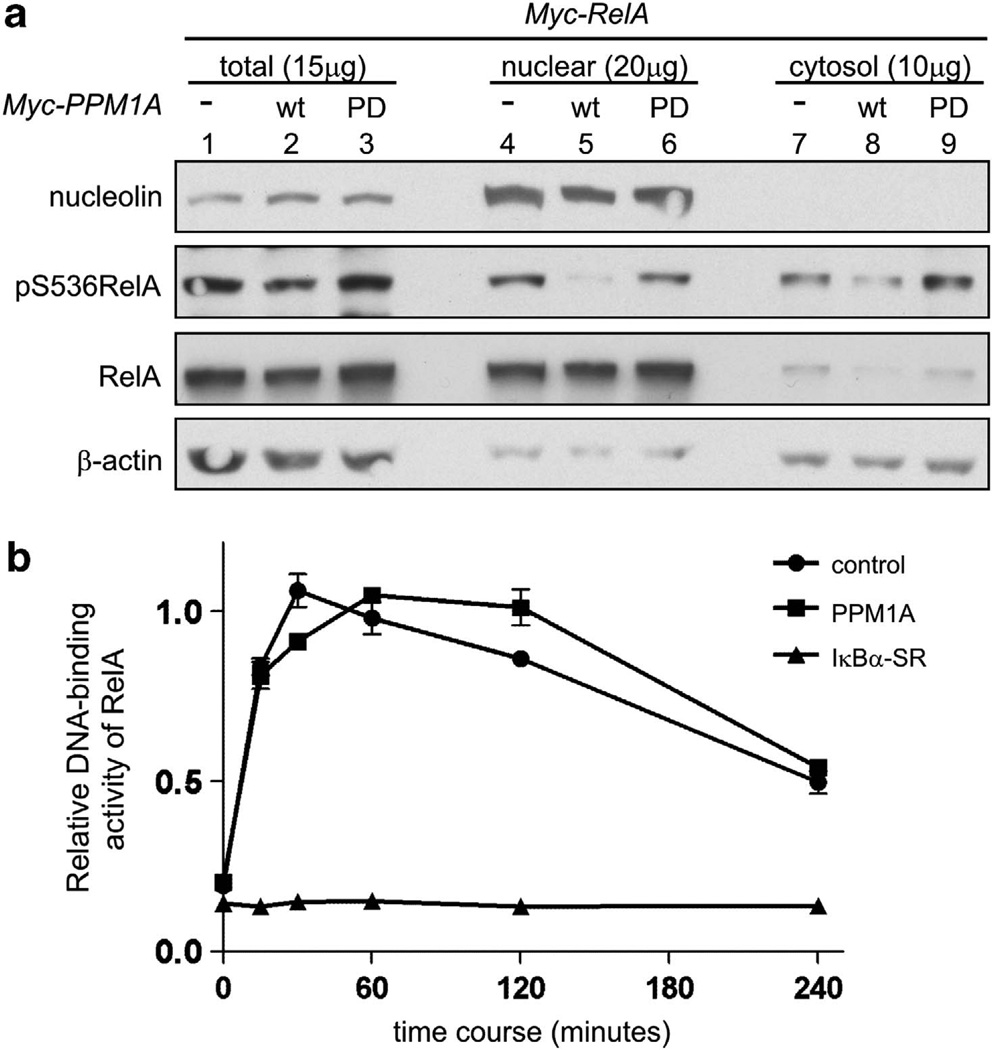
Inhibition of RelA nuclear translocation is a well-described mechanism for the regulation of NF-κB. Our data suggest that PPM1A is a direct RelA phosphatase, and although phosphoryla-tion of RelA is not required for nuclear translocation, it is possible that PPM1A could inhibit both RelA phosphorylation and nuclear localization. To explore this possibility, subcellular distribution of expressed RelA and phospho-RelA were determined following coexpression of RelA with wild-type or phosphatase-dead PPM1A. Immunoblotting of cell fractions revealed that neither wild-type nor phosphatase-dead PPM1A inhibited nuclear translocation of RelA (Figure 3a, compare lane 4 with lanes 5 and 6). Consistent with our finding that PPM1A decreased RelA phosphorylation (Figures 1a – c), pS536 RelA was markedly decreased in the nuclear fraction following expression of wild-type, but not phosphatase-dead, PPM1A. PPM1A was primarily localized to the nucleus (data not shown and Lin et al19), suggesting that PPM1A may act to dephosphorylate RelA after translocation to the nucleus. Next, we determined if overall DNA-binding activity of RelA was altered by PPM1A. RelA DNA binding was stimulated by TNFα and DNA-bound RelA identified and quantitated (Active Motif, Carlsbad, CA, USA). PPM1A expression did not alter TNFα-stimulated DNA-binding activity of RelA compared to control cells at any time point tested (Figure 3b). As a positive control, ectopic expression of IκBα-super-repressor efficiently inhibited DNA binding by RelA at all the time points. Collectively, these data suggest that PPM1A inhibition of RelA does not depend on inhibition of RelA nuclear translocation and that PPM1A regulation of RelA transcription is gene- or promoter-specific.
Figure 3.
PPM1A does not interfere with nuclear translocation or DNA binding of RelA. (a) U2OS cells transfected with indicated plasmids were lysed and total or p-RelA visualized by immuno-blotting from total lysate or subcellular fractions. Nucleolin and β-actin served as nuclear and cytosol controls, respectively. Amount of protein loaded is indicated. (b) Following TNFα stimulation, nuclear extracts were prepared from HeLa cells stably expressing PPM1A, IκBα-super-repressor (IκBα-SR) or control. RelA DNA-binding activity was measured using TransAM NF-κB p65 transcription factor kit. Error bars = s.d. Wt, wild type.
PPM1A inhibits NF-κB activation, MCP-1 expression and cell invasion in prostate cancer cell lines
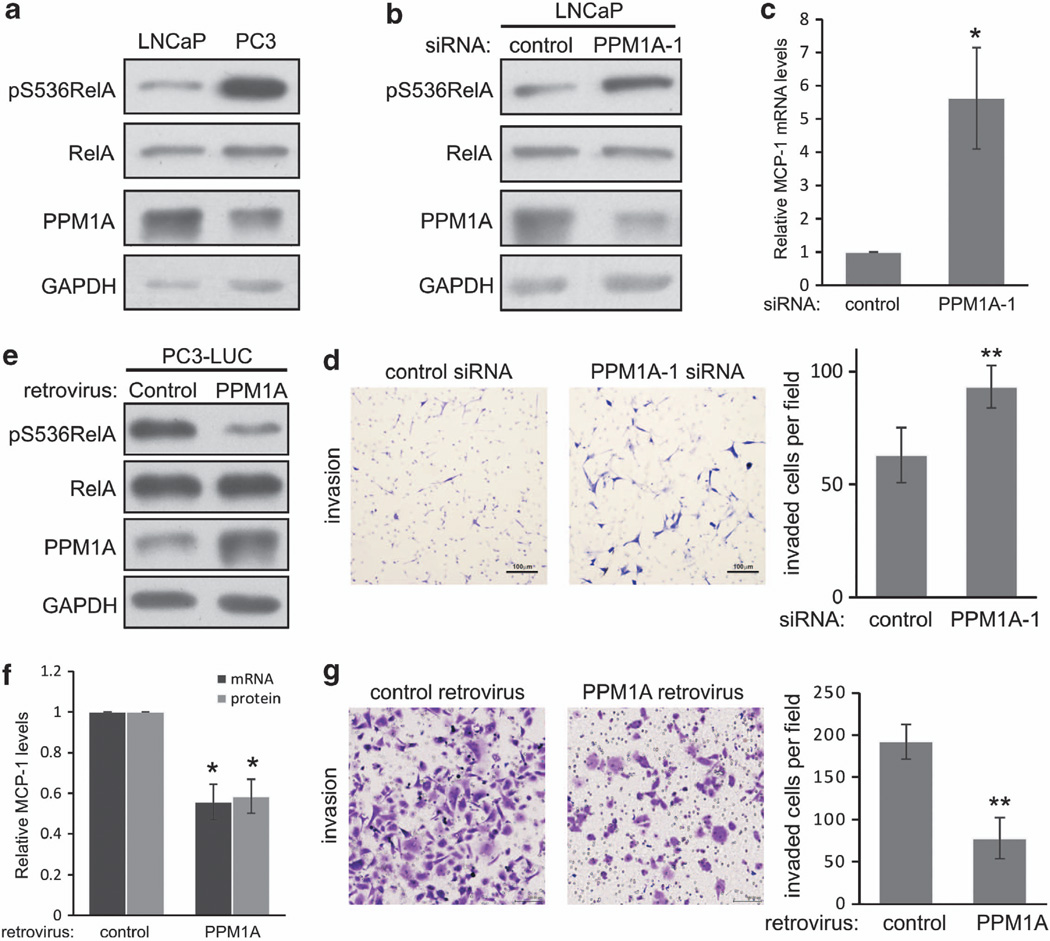
We were intrigued that PPM1A regulated the expression of MCP-1 (Figure 2d), a chemokine whose expression has been strongly linked to bony metastasis in prostate and advanced breast cancers.33,34 To begin exploring a potential role of PPM1A in regulating bony metastases in prostate cancer, we explored PPM1A expression and the effect of PPM1A manipulation in a pair of prostate cancer cell lines with well-described metastatic and invasive potential, PC3 and LNCaP. LNCaP cells are androgen-dependent with low metastatic potential, whereas PC3 cells are androgen-independent and highly metastatic. Perhaps related to their aggressive metastatic potential, PC3 cells express higher levels of MCP-1.35 We observed that PPM1A protein (Figure 4a) and mRNA (data not shown) expressions were approximately twofold lower in PC3 cells compared with LNCaP. Although total RelA expression was similar in the two cell lines, RelA S536 phosphorylation was markedly higher in PC3 cells compared with LNCaP (Figure 4a).
Figure 4.
PPM1A inhibits NF-κB and prostate cancer cell invasion. (a) Endogenous total and p-RelA (S536) and PPM1A were visualized in LNCaP and PC3 cells by immunoblotting. GAPDH serves as loading control. (b) LNCaP cells were transfected with indicated siRNA and indicated phospho- or total proteins visualized by immunoblotting. (c) MCP-1 expression was measured by quantitative real-time PCR (q-RT–PCR) in LNCaP cells transfected as indicated. Error bars = s.d.; *P<0.05; (d) Transwell invasion of LNCaP cells following transfection with indicated constructs were quantified as described in Figure 2f; **P<0.001. (e) PC3-LUC cells were infected with indicated retrovirus, selected using puromycin and expression of indicated phospho- or total proteins visualized by immunoblotting. (f) PC3-LUC cells were infected with indicated retroviruses and expression of MCP-1 mRNA or protein, determined by q-RT–PCR or enzyme-linked immunosorbent assay, Error bars = s.d.; *P<0.05. (g) PC3-LUC cells were infected with indicated retroviruses and transwell invasion determined as in d; **P<0.001.
As LNCaP cells expressed higher levels of PPM1A, effects of PPM1A on RelA phosphorylation, MCP-1 expression and cellular invasion were determined by depletion of PPM1A. Consistent with results from other cell lines, PPM1A depletion in LNCaP cells increased RelA S536 phosphorylation (Figure 4b), MCP-1 mRNA expression (Figure 4c) and cell invasion (Figure 4d). To determine the effect of PPM1A in PC3-LUC cells with lower endogenous levels of PPM1A, PPM1A was expressed. Ectopic PPM1A expression did not alter total RelA expression, but markedly decreased RelA S536 phosphorylation (Figure 4e) and resulted in a 50% reduction of MCP-1 mRNA expression and a similar reduction of secreted MCP-1 detected in the conditioned media (Figure 4f). Remarkably, expression of PPM1A reduced invasion of otherwise aggressive PC3 cells by >50% (Figure 4g).
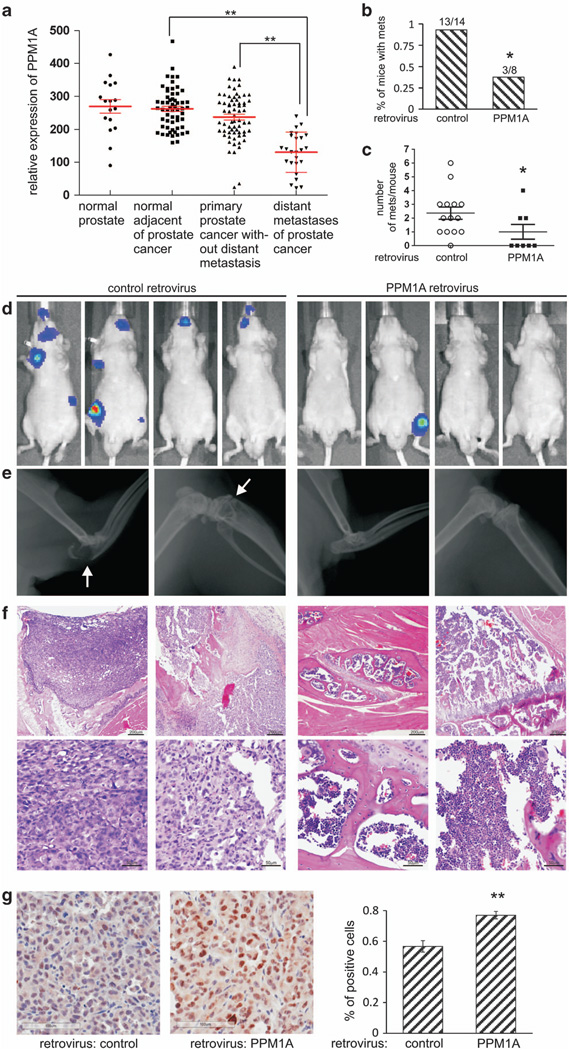
PPM1A expression is lower in metastatic human prostate cancer and PPM1A expression inhibits metastases in a mouse model
As PPM1A expression inversely correlated with MCP-1 expression and invasiveness of two prostate cancer cell lines (Figure 4), we examined PPM1A expression in publically available expression data derived from human prostate cancers to determine if PPM1A expression differed in metastatic lesions compared with primary tumors. Indeed, PPM1A expression was twofold lower in distant prostate cancer metastases compared with primary prostate tumors in patients without distant metastases (Figure 5a, NCBI Gene Expression Omnibus, GDS254636,37). To begin exploring a potential role of PPM1A in metastasis, an intracardiac injection model was used to study critical behaviors for prostate cancer cell metastases: extravasation and growth in distant sites. Aggressive PC3 cells were chosen since LNCaP cells injected intracardially fail to develop metastasis.38 PC3 cells expressing luciferase (PC3-LUC) with or without stable PPM1A expression (Figure 4e) were injected intracardially into nude mice and bony metastases quantified. Bioluminescent imaging was performed on mice 1h after injection and weekly for 4 weeks (example shown in Supplementary Figure S3). Expression of PPM1A in PC3-LUC cells significantly reduced the incidence of metastases in injected mice (38 vs 93%; Figure 5b) and the number of metastases per mouse (Figure 5c). Metastases were confirmed by X-ray imaging (Figure 5d, white arrows indicate osteolytic lesions) and hematoxylin/eosin staining showing metastases of control PC3-LUC cells to the bone marrow cavity (Figure 5e, left) and normal bone tissues from mice injected with PPM1A-expressing PC3-LUC cells for comparison (Figure 5e, right). Immunostaining of cleaved-caspase-3 reveals that apoptosis (cleaved-caspase-3 positive/total cells) in metastases of PPM1A expression cells increased about half of that in control tumors (Figure 5f; 56.7 vs 77%, P<0.01).
Figure 5.
PPM1A expression is lower in metastatic human prostate cancer and inhibits a model of prostate cancer cells metastasis. (a) A Gene Expression Omnibus search indicated that PPM1A expression is lower in metastatic deposits of prostate cancer compared to: primary prostate cancers without distant metastases (P= 2.43E — 9, fold change 1.82); normal prostate tissue adjacent to cancer (P= 9.77E — −14, fold change 2.00); or normal prostate (P=2.17E –7, fold change 2.06). (b) Percentage of mice developed metastases. On top is the number of animals that develop metastases/the number of animals successfully injected; *P<0.05. (c) The number of metastases in each mouse; *P<0.05. (d) Xenogen bioluminescent images of representative mice 4 weeks after intracardiac injection of PC3-LUC cells infected with control retrovirus or retrovirus driving expression of PPM1A. (e) X-ray images of representative mice in b. Lytic bone lesions are indicated by white arrows. (f) Representative hematoxylin/eosin staining of bone and adjacent tissue samples obtained from mice in b are shown. Magnification: top, × 40; bottom, × 200. (g) Sections of bony metastases described in b were immunostained with cleaved-caspase-3. Percentage of positive staining cells in each group was measured from at least three representative tumors and in each tumor at least five randomly chose fields (× 200). Error bars, s.d.; **P<0.001.
DISCUSSION
RelA phosphorylation is necessary for transcriptional competence of nuclear NF-κB,39 and dephosphorylation of RelA is an important mechanism for homeostatic downregulation of NF-κB activity.16 In many human tumors, NF-κB activity enhances tumorigenic behavior with upstream kinases largely implicated in inappropriate NF-κB transcription.40,41 Data we present here identify a new RelA phosphatase and suggest that loss of RelA phosphatases may be equally important or an alternative mechanism of NF-κB activation in human cancer.
We found that PPM1A, a PP2C family member, is a direct RelA phosphatase at S536 as indicated by activity of bacterially synthesized PPM1A toward both RelA phosphopeptides and immunoprecipitated full-length RelA. Data presented here also support PPM1A as a phosphatase for the S276 site of RelA. S/TQ and TXY are the only consensus target sequences for PP2C phosphatases.42,43 As S536 and S276 of RelA are not consensus targets, additional studies with more endogenous PP2C targets may reveal additional consensus sequences. Previous reports show that PPM1A targets NF-κB through dephosphorylation of IKKα and IKKβ.23 Our data reveal that PPM1A inhibited RelA phosphorylation independent of IKKs (Figure 1c). Inability of PPM1A to inhibit RelA nuclear translocation further suggests that IKKs are not the dominant mechanism of RelA inhibition by PPM1A. Taken together, PPM1A inhibits NF-κB through at least two mechanisms: (1) inhibition of upstream IKKs, and (2) direct dephosphorylation of RelA. We observed PPM1A-mediated inhibition of RelA with and without stimulation, whereas PPM1A activity to inhibit IKKs was observed only at later time points and after TNFα stimulation,23 suggesting that whether PPM1A regulation of RelA directly or through IKKs may depend on timing and cellular and signaling context.
NF-κB regulation to alter selectively transcription has been widely reported and also varies from cell type to cell type.3,44,45 For example, the known RelA phosphatase, Wip1, inhibits a subset of NF-κB targets.18 We found that among others, PPM1A decreased expression of NF-κB targets IL-6, as reported previously,23,27 and MCP-1. ChIP assay showed depletion of PPM1A increased RelA associated with the MCP-1 gene distal promoter (Figure 2e), but the phosphorylation status of promoter-associated RelA could not be determined because of the absence of suitable antibodies. Increased mRNA levels of MCP-1 under similar circumstances suggest that promoter-associated NF-κB was transcriptionally active and therefore phosphorylated. These data suggest that PPM1A regulates RelA phosphorylation, resulting in altered expression of select NF-κB targets.
IL-6 and MCP-1 are implicated in metastases, particularly in cancers of the prostate,46 colon47,48 and breast.33,49 Distant metastases in breast and prostate cancer are tightly associated with poor patient outcomes. Targeting MCP-1 is an effective therapeutic approach to prevent metastases in animal models of prostate and breast cancer,50 and a phase II clinical trial using a neutralizing monoclonal antibody against MCP-1 is being tested in metastatic prostate cancer (NCT00992186). IL-6 and IL-6 receptor are also therapeutic targets for prevention of inflammation, tumor progression and metastasis, and their inhibition has shown promise in preclinical models.51 A phase II trial was completed to determine efficacy of anti-IL-6 chimeric monoclonal antibody in patients with metastatic hormone-resistant prostate cancer (NCT00433446). Given that PPM1A inhibited expression of IL-6 and MCP-1, we explored the role of PPM1A in metastases. Expression data from human prostate cancers revealed that metastatic prostate deposits had significantly lower PPM1A expression when compared with primary tumors in patients without metastases. This exciting finding combined with increased invasion associated with PPM1A depletion (Figure 2e) led us to examine the effect of PPM1A expression in an androgen-independent highly metastatic prostate cancer cell line, PC3. Interestingly, PC3 cells express lower levels of PPM1A when compared with androgen-dependent and less aggressive LNCaP cells. PPM1A expression significantly inhibited PC3 cell invasion (Figure 4g) and abrogated PC3 bony metastases in an intra-vascular metastases model (Figure 5b – d). Notably, mean PPM1A expression is also decreased in breast cancer and colorectal cancers compared with normal tissue (Oncomine, PPM1A gene, TCGA breast, TCGA colorectal and colorectal 2 data sets), and a xenograft model of breast cancer MCF7 cells revealed that PPM1A depletion increases tumorigenic potential and tumor growth.52 These data suggest that loss or decreased PPM1A activity may increase aggressive behavior in different tumor types. Although survival data was not included in the prostate cancer data set to correlate PPM1A expression with prognosis, it will be informative to explore when larger public data sets become available.
Our data suggest that PPM1A is a direct RelA phosphatase with tumor suppressor-like activity that, at least partially, depends on PPM1A ability to inhibit NF-κB. In addition to NF-κB, PPM1A targets tumor centric proteins including Smad2/3, p38 and cdk2 implicating PPM1A in cell cycle regulation, as well as tumor growth factor-β and MAPK signaling pathways.19,20 Although data presented here suggest that invasion in HeLa cells was completely dependent on NF-κB, regulation of tumor invasion, progression and metastasis by PPM1A likely involves additional PPM1A activities. Decreased expression of PPM1A in distant metastases of human prostate cancer coupled with in vivo data suggest that increased PPM1A expression inhibits bony metastases. Neither deletions nor inactivating mutations of PPM1A have been described, suggesting that strategies to increase PPM1A expression or activity in cancer cells could be explored as a therapeutic strategy in human cancers.
MATERIALS AND METHODS
Expression plasmid and small hairpin RNA expression constructs
Myc-PPM1A, Myc-PPM1A (R174G) mutant, glutathione S-transferase-PPM1A and glutathione S-transferase-PPM1A (R174G) were gifts from Dr Sun (Baylor College of Medicine, Houston, TX, USA).23 His-Wip1 was a gift from Dr Donehower (Baylor College of Medicine).53 PPM1A was PCR amplified and subcloned into the pHIT-dell2-puro retroviral expression vector. Other constructs were described previously.3 A pSuper-retro vector (provided by Dr Reuven Agami, the Netherlands Cancer Institute, Amsterdam, the Netherlands) was used to generate short hairpin RNA plasmids for PPM1A with the following target sequence 5′-AAGTACCTGGAATGCAGAGTA-3′.23 Lentiviral vector pGIPZ and plasmid coding for PPM1A-targeting short hairpin RNA (Clone ID V2LHS_35113) were from Open Biosystems (Waltham, MA, USA). PPM1A-1 (catalog no. SI02659258, Hs_PPM1A_5) and PPM1A-2 (catalog no. SI02659265, Hs_PPM1A_6) siRNA were purchased from Qiagen (Germantown, MD, USA). Control siRNA (non-targeting no. 1) was from Dharmacon (Waltham, MA, USA).
Cell lines, cell culture, transfection and virus infection
IKKα −/−, IKKβ −/− (IKK1 −/−, IKK2 −/−) double-null mouse embryonic fibroblasts (MEFs) were a gift from Dr Verma (the Salk Institute for Biological Studies, Laboratory of Genetics, San Diego, CA, USA). PC3 and LNCaP cell lines were provided by Dr Renjie Jin (Vanderbilt University, Nashville, TN, USA). Cell lines were maintained according to ATCC protocol. TransIT-2020 (Mirus, Madison, WI, USA) was used for MEFs’ transfection. SiRNA was transfected at 20 nM using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA). All transfection reagents were used according to the manufacturer’s instructions. Cells expressing PPM1A or short hairpin RNA targeting PPM1A were infected with indicated virus-containing medium with 4 mg/ml polybrene (Sigma-Aldrich, St Louis, MO, USA). For transient expression, cells were used after 24 h of infection. Stable cell lines were selected for 10 days in puromycin (1 µg/ml).
Antibodies and reagents
Rabbit anti-RelA (C20), anti-p-S276 RelA (sc101749), anti-glyceraldehyde 3-phosphate dehydrogenase, anti-IκBα (C21), anti-IKKα/β (sc7607) and horse radish peroxidase-conjugated secondary donkey anti-mouse and anti-rabbit antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse and rabbit anti-PPM1A were from Abcam (Cambridge, MA, USA) (p6c7) and GeneTex (Irvine, CA, USA) (GTX109744), respectively. Rabbit anti-p-S468 RelA (Ab31473) was from Abcam. Mouse anti-Flag M2, anti-Flag M2 affinity gel and 3 × Flag peptide were from Sigma-Aldrich. Anti-p-S536 RelA (Cell Signaling, Boston, MA, USA). TNFα and IL-1β were from PeproTech (Rocky Hill, NJ, USA).
Immunoprecipitation and immunoblotting
Cells were lysed in 0.5% (v/v) Nonidet P-40 lysis buffer supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN, USA) and phosphatase inhibitor III (Sigma-Aldrich). Total cell lysate was incubated with anti-Flag M2 affinity gel (Sigma-Aldrich) and immunoprecipitation was performed according to the manufacturer’s instruction. Immunoblotting was performed as described previously.54
In vitro phosphatase reaction and malachite green phosphatase assay
The experiment was performed as described,23 with modified phosphatase 2C buffer (50 mM Tris-HCl (pH 7.5), 0.1 mM ethylene glycol tetraacetic acid, 0.02% 2-mercaptoethanol ± 25 mM MgCl2). Flag-RelA protein was used as substrate after elution with 3 × Flag peptide (Sigma-Aldrich).Phosphopeptide analysis was performed as described,18 with modified PP2C buffer and malachite green assay kit (no. POMG-25H; BioAssay Systems, Hayward, CA, USA). Phosphopeptides synthesized by LifeTein: RelAS536 (GDEDFSpSIADMD) and RelAS276 (QLRRPpSDRELS).18 His-Wip1 was purified using Ni beads as described.53
Luciferase reporter gene assay
Dual-Luciferase Reporter Assay (Promega, Madison, WI, USA) was performed as described previously.3
cDNA synthesis and real-time PCR
mRNA was extracted using RNeasy mini (Qiagen). cDNA synthesis was performed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Real-time PCR was performed using iQ SYBR green supermix (Bio-Rad). RT-PCR Array (PAHS-025A, SABiosciences) was used according to the manufacturer’s instructions. MCP-1 primer sequences were as follows: forward, 5′-CTGCTCATAGCAGCCACCTT-3′; reverse, 5′-GCACTGAGATCTTCC-TATTGGTG-3′. GAPDH was used as control.
ChIP assays
ChIP assays were performed using Magna ChIP A/G (no. 17–10085; EMD Millipore, Billerica, MA, USA) following the manufacturer’s instructions. The 3 × 106 HeLa cells were used for each assay. Anti-RelA antibody (SC-109 X) and normal rabbit IgG (Santa Cruz Biotechnology) were used for immunoprecipitation. Purified DNA was analyzed by real-time PCR. Primers ((NM_002982.3 (–) 03 kb) for the κB binding site on MCP-1 promoter were from SABioscience/Qiagen.
In vitro cell invasion assay
Transwell invasion assay was performed as described.3 The 2 × 104 HeLa cells, 5 × 104 PC3-LUC cells or 1 × 105 LNCaP cells were seeded into the upper chamber and analyzed after 21, 42 and 46 h, respectively. Membranes mounted to slides were scanned at × 1.25, × 10 and × 20 using the Ariol SL-50 platform at Vanderbilt Epithelial Biology Center or under microscope with × 200 magnifications.
Cell fractionation
Cell fractionation was performed as described,55 with modified hypotonic buffer (10mM HEPES (pH 7.1), 50mM NaCl, 0.3m sucrose, 0.1 mM ethylenediaminetetraacetic acid, 0.5% Triton X-100, 1 mM dithiothreitol) and washing buffer (10mM HEPES (pH 7.1), 0.1 mM ethylenediaminetetraacetic acid, 1 mM dithiothreitol).
DNA binding assay
The assay was performed as described3 using TransAM NF-κB p65 assay kit (catalog no. 40096; Active Motif, Carlsbad, CA, USA).
Obtaining conditioned medium and enzyme-linked immunosorbent assay
Conditioned media were obtained from PC3 cell cultures as described.35 Cell number from each well was counted to normalize data for differences. MCP-1 concentration was measured by MCP-1 ELISA kit (R&D System, Minneapolis, MN, USA) following the manufacturer’s protocol.
The Gene Expression Omnibus analysis
Normalized PPM1A expression data were downloaded from Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/projects/geo/) with citations for individual studies. Gene expressions were analyzed and plotted with average ± s.d. in Prism 5.0 (GraphPad, La Jolla, CA, USA).
In vivo tumor metastasis assay and bioluminescent imaging ex vivo analysis
The 1 × 105 PC3-LUC cells infected with control or PPM1A-expressing retrovirus suspended in 0.1 ml of phosphate-buffered saline were inoculated into the left cardiac ventricle of male nude mice. Metastases were monitored by bioluminescent imaging weekly (Xenogen IVIS 200 Imaging System, Alameda, CA, USA). Mice with bioluminescent signal in the chest cavity 1 h after injection were excluded from the study since this indicated leakage of tumor cells during injection.56 Four weeks after injection, mice were radiographed (Faxitron X-ray System, Tucson, AZ, USA), and long bones and spines were decalcified and paraffin-embedded. Sections were stained with hematoxylin and eosin.
Statistical analysis
Statistical analyses were performed using R version 2.14.1 for Windows, and data plotted and analyzed using Student′s t-test, Fisher′s exact test, Wilcoxon′s rank-sum test or Kruskal–Wallis χ2 test. *, **, *** represents P-values <.05, 0.01 and 0.001, respectively, unless otherwise noted.
Supplementary Material
ACKNOWLEDGEMENTS
We thank W Sun, LA Donehower, N Perkins, Reuven Agami and IM Verma for providing constructs and cell lines. We are extremely grateful to A Richmond, R Matusik, J McLean, A Weaver, D Webb and to Baker Lab members for insightful advice. This work was supported by Grant NIH R01 DE013173 (to WGY) and by funds provided through an endowment from Barry and Amy Baker to the Barry Baker Laboratory for Head & Neck Oncology, from the Vanderbilt Ingram Cancer Center, from the Department of Otolaryngology at Vanderbilt University and from the Vanderbilt Bill Wilkerson Center.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orlowski RZ, Baldwin J, Albert S. NF-[kappa]B as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, An H, Mayo MW, Baldwin AS, Yarbrough WG. LZAP a putative tumor suppressor, selectively inhibits NF-kappaB. Cancer Cell. 2007;12:239–251. doi: 10.1016/j.ccr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Arun P, Brown MS, Ehsanian R, Chen Z, Van Waes C. Nuclear NF-kappaB p65 phosphorylation at serine 276 by protein kinase A contributes to the malignant phenotype of head and neck cancer. Clin Cancer Res. 2009;15:5974–5984. doi: 10.1158/1078-0432.CCR-09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Karin M. NF-kappaB in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003;8:215–223. doi: 10.1023/a:1025905008934. [DOI] [PubMed] [Google Scholar]
- 6.Wu JT, Kral JG. The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J Surg Res. 2005;123:158–169. doi: 10.1016/j.jss.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. 2007. [DOI] [PubMed] [Google Scholar]
- 8.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 9.Min J, Zaslavsky A, Fedele G, McLaughlin SK, Reczek EE, De Raedt T, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16:286–294. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Patel L, Pienta KJ. Targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci. 2010;95:31–53. doi: 10.1016/B978-0-12-385071-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen L-F, Green WC. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 13.Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J. 2007;21:2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, et al. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 15.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 16.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Fan GH, Wadzinski BE, Sakurai H, Richmond A. Protein phosphatase 2A interacts with and directly dephosphorylates RelA. J Biol Chem. 2001;276:47828–47833. doi: 10.1074/jbc.M106103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chew J, Biswas S, Shreeram S, Humaidi M, Wong ET, Dhillion MK, et al. WIP1 phosphatase is a negative regulator of NF-kappaB signalling. Nat Cell Biol. 2009;11:659–666. doi: 10.1038/ncb1873. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell. 2006;125:915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammers T, Lavi S. Role of type 2C protein phosphatases in growth regulation and in cellular stress signaling. Crit Rev Biochem Mol Biol. 2007;42:437–461. doi: 10.1080/10409230701693342. [DOI] [PubMed] [Google Scholar]
- 21.Shohat M, Ben-Meir D, Lavi S. Protein phosphatase magnesium dependent 1A (PPM1A) plays a role in the differentiation and survival processes of nerve cells. PLoS ONE. 2012;7:e32438. doi: 10.1371/journal.pone.0032438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Zhou Z, Lin H, Lv X, Fu J, Lin P, et al. Protein phosphatase 1A (PPM1A) is involved in human cytotrophoblast cell invasion and migration. Histochem Cell Biol. 2009;132:169–179. doi: 10.1007/s00418-009-0601-5. [DOI] [PubMed] [Google Scholar]
- 23.Sun W, Yu Y, Dotti G, Shen T, Tan X, Savoldo B, et al. PPM1A and PPM1B act as IKKbeta phosphatases to terminate TNFalpha-induced IKKbeta-NF-kappaB activation. Cell Signal. 2009;21:95–102. doi: 10.1016/j.cellsig.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buss H, Dorrie A, Schmitz ML, Frank R, Livingstone M, Resch K, et al. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem. 2004;279:49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- 25.Schwabe RF, Sakurai H. IKKbeta phosphorylates p65 at S468 in transactivaton domain 2. FASEB J. 2005;19:1758–1760. doi: 10.1096/fj.05-3736fje. [DOI] [PubMed] [Google Scholar]
- 26.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Rho B, Lee TH, Lee JM, Kim SJ, Park JH. The interaction of hepatitis B virus X protein and protein phosphatase type 2 Calpha and its effect on IL-6. Biochem Biophys Res Commun. 2006;351:253–258. doi: 10.1016/j.bbrc.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, et al. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- 29.Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 31.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 33.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Patel L, Pienta KJCC. chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, et al. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 36.Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W, et al. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 2007;7:64. doi: 10.1186/1471-2407-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 38.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887–894. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl 1):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 40.Gardam S, Beyaert R. The kinase NIK as a therapeutic target in multiple myeloma. Expert Opin Ther Targets. 2011;15:207–218. doi: 10.1517/14728222.2011.548861. [DOI] [PubMed] [Google Scholar]
- 41.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: ′one size does not fit all′. Oncogene. 2011;30:1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi H, Durell SR, Chatterjee DK, Anderson CW, Appella E. The Wip1 phosphatase PPM1D dephosphorylates SQ/TQ motifs in checkpoint substrates phosphorylated by PI3K-like kinases. Biochemistry. 2007;46:12594–12603. doi: 10.1021/bi701096s. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi H, Minopoli G, Demidov ON, Chatterjee DK, Anderson CW, Durell SR, et al. Substrate specificity of the human protein phosphatase 2Cdelta, Wip1. Biochemistry. 2005;44:5285–5294. doi: 10.1021/bi0476634. [DOI] [PubMed] [Google Scholar]
- 44.Browning DD, Pan ZK, Prossnitz ER, Ye RD. Cell type- and developmental stage-specific activation of NF-kappaB by fMet-Leu-Phe in myeloid cells. J Biol Chem. 1997;272:7995–8001. doi: 10.1074/jbc.272.12.7995. [DOI] [PubMed] [Google Scholar]
- 45.Smale ST. Hierarchies of NF-kappaB target-gene regulation. Nat Immunol. 2011;12:689–694. doi: 10.1038/ni.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azevedo A, Cunha V, Teixeira AL, Medeiros R. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World J Clin Oncol. 2011;2:384–396. doi: 10.5306/wjco.v2.i12.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. 2012;22:91–105. doi: 10.1016/j.ccr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Mueller L, Seggern LV, Schumacher J, Goumas F, Wilms C, Braun F, et al. TNF-alpha similarly induces IL-6 and MCP-1 in fibroblasts from colorectal liver metastases and normal liver fibroblasts. Biochem Biophys Res Commun. 2010;397:586–591. doi: 10.1016/j.bbrc.2010.05.163. [DOI] [PubMed] [Google Scholar]
- 49.Sosnoski DM, Krishnan V, Kraemer WJ, Dunn-Lewis C, Mastro AM. Changes in cytokines of the bone microenvironment during breast cancer metastasis. Int J Breast Cancer. 2012;2012:160265. doi: 10.1155/2012/160265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zollo M, Di Dato V, Spano D, De Martino D, Liguori L, Marino N, et al. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin Exp Metastasis. 2012;29:585–601. doi: 10.1007/s10585-012-9473-5. [DOI] [PubMed] [Google Scholar]
- 51.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–6096. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lammers T, Peschke P, Ehemann V, Debus J, Slobodin B, Lavi S, et al. Role of PP2Calpha in cell growth, in radio- and chemosensitivity, and in tumorigenicity. Mol Cancer. 2007;6:65. doi: 10.1186/1476-4598-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu X, Ma O, Nguyen TA, Jones SN, Oren M, Donehower LA. The Wip1 phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell. 2007;12:342–354. doi: 10.1016/j.ccr.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 54.An H, Lu X, Liu D, Yarbrough WG. LZAP inhibits p38 MAPK (p38) phosphorylation and activity by facilitating p38 association with the wild-type p53 induced phosphatase 1 (WIP1) PLoS ONE. 2011;6:e16427. doi: 10.1371/journal.pone.0016427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drake JM, Gabriel CL, Henry MD. Assessing tumor growth and distribution in a model of prostate cancer metastasis using bioluminescence imaging. Clin Exp Metastasis. 2005;22:674–684. doi: 10.1007/s10585-006-9011-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.