Abstract
Novel pregna-5, 7-dienes were synthesized and subjected to UVB irradiation to generate the corresponding pre-D intermediates, tachysterol and lumisterol analogs. The kinetics of the conversion from each of the pre-D intermediates to the corresponding novel D analogs was investigated by using real time 1H NMR measurements inside the NMR magnet. Both the length and composition of the side chains were found to affect the rate of the kinetic conversion from pre-D intermediates to the thermodynamically more stable D analogs. Compound 7cc which has both a long side chain and a tertiary alcohol moiety showed the highest conversion rate, while compound 4a-S which has a very short side chain without the tertiary alcohol had the lowest conversion rate among the 13 tested compounds. We also determined product distributions for these 5,7-dienes upon UVB irradiation followed by thermodynamic equilibration. No clear correlations between product distribution and side chain length or composition were identifiable under the current experimental conditions, suggesting there are other factors affecting the kinetics during the photochemical reactions for these 5,7-dienes. To the best of our knowledge, this is the first time the influences of side chain length and composition on the real time conversion kinetics from pre-D to D are studied. This study could serve as step-stones in future kinetic studies of novel biologically active 5,7-dienes and their corresponding D analogs under more physiologically relevant ex vivo or in vivo conditions, as well as providing important insights into optimizing yields of the desired active products during their organic syntheses.
Keywords: kinetics, pre-D, vitamin D, UVB, real-time NMR
Introduction
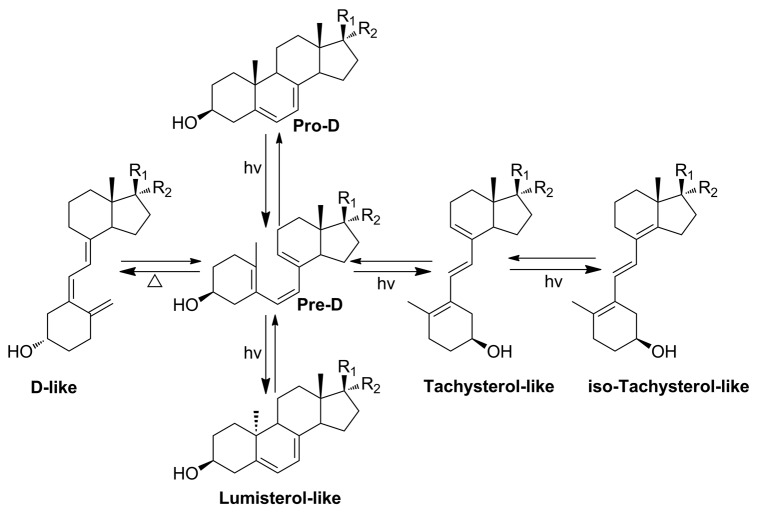
The UVB-driven conversion of cholesta-5, 7-diene-3β-ol (7-dehydrocholesterol, 7DHC) to (3β, 5Z, 7E)-9, 10-secocholesta-5, 7, 10(19)-trien-3-ol (cholecalciferol, vitamin D3) is one of the most fundamental reactions in photobiology.1-3 In addition to vitamin D3, the reaction also generates tachysterol (6E-9, 10-secocholesta-5(10), 6, 8-trien-3β-ol, T), lumisterol (9β, 10α-cholesta-5, 7-diene-3β-ol, L), as well as iso-tachysterol. The conversion of 7DHC to its analogs has been demonstrated by the Holick group as a two-step process (Fig. 1).1 The first step is the formation of pre-D (unless specified, the term “D” used in this paper refer to the D3-like, D2-like, or pD analogs with short chains). The second step is the conversion of pre-D to the D, T, and L analogs. Tremendous efforts have been made to investigate the photosynthesis and biological activity of many D analogs with different side chains on the 17-position.4-12 The effect of potential interactions of previtamin D3 with intracellular proteins and/or lipids in the skin on the kinetics and thermodynamics of the thermal isomerization to form vitamin D3 has been established.2,13-15 However, to the best of our knowledge, the effects of the sidechain length and composition on the real time conversion kinetics from pre-D to D have not been reported in the literature.
Figure 1. UVB induced transformation of 5,7-dienes to the D, T, and L-like analogs.
To determine the kinetics of the conversion of pre-D to D and to search for additional biologically active 5,7-dienes, a series of pregna-5, 7-dienes with different side chains were synthesized and subjected to UVB irradiation. The conversion from pre-D to D after UV initiation was monitored in real time by proton NMR at 37°C inside the NMR magnet. In addition, the distribution of each product from the UVB irradiation of novel 5,7-dienes synthesized was also described in this report to provide potential insights in optimizing yields of future organic synthesis for desired secosteroids and minimize undesired side products.
Results and Discussion
Longer chain and the presence of a tertiary alcohol moiety on the side chain facilitate the conversion from pre-D to D
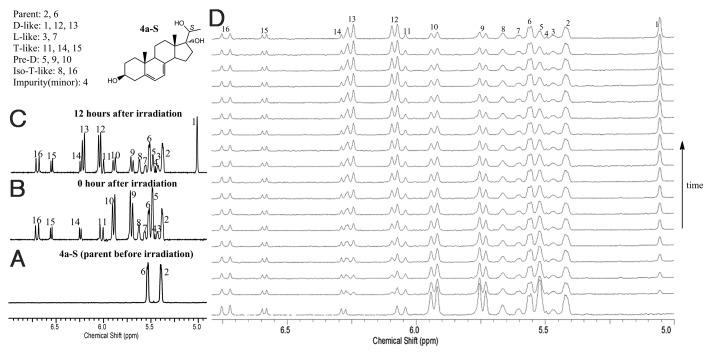
The kinetics of the conversion from pre-D to D is shown in Figure 2 using compound 4a-S as an example. Before irradiation, only proton NMR peaks corresponding to the 5,7-diene were observed (peaks 2 and 6, Figure 2A). After UVB irradiation for 5 min, many new peaks appeared corresponding to different initial photolysis products (Fig. 2B). Intensities of proton NMR peaks (peak number 5, 9 and 10, Figure 2B and C) corresponding to pre-D decreased over time, while intensities of peaks (peak number 1,12,13, Figure 2B and C) corresponding to D increased over time, clearly indicating that the thermodynamically unstable pre-D isomerized to the more stable D structure. There were no changes in the amounts for all other products (T, isoT, and L) based on the peak intensities corresponding to those products once UVB irradiation was terminated. Figure 2D shows the real-time monitoring of changes of pre-D and D over time at 40 time points (30 min/time point). The rate of conversion was calculated by fitting the corresponding peaks at those time points. Results for all compounds are summarized in Table 1.
Figure 2. 1.8 mM of each compound solution in methanol-d4 was irradiated for 5 min by UVB (280–315 nm). Each irradiated sample was monitored by proton NMR at 37°C immediately after the irradiation in a time course manner. Data was recorded for each time point of 0 min, 10 min, and every 30 min thereafter.
Table 1. Relative rates of conversion for the irradiated products (pre-D-like to D-like).
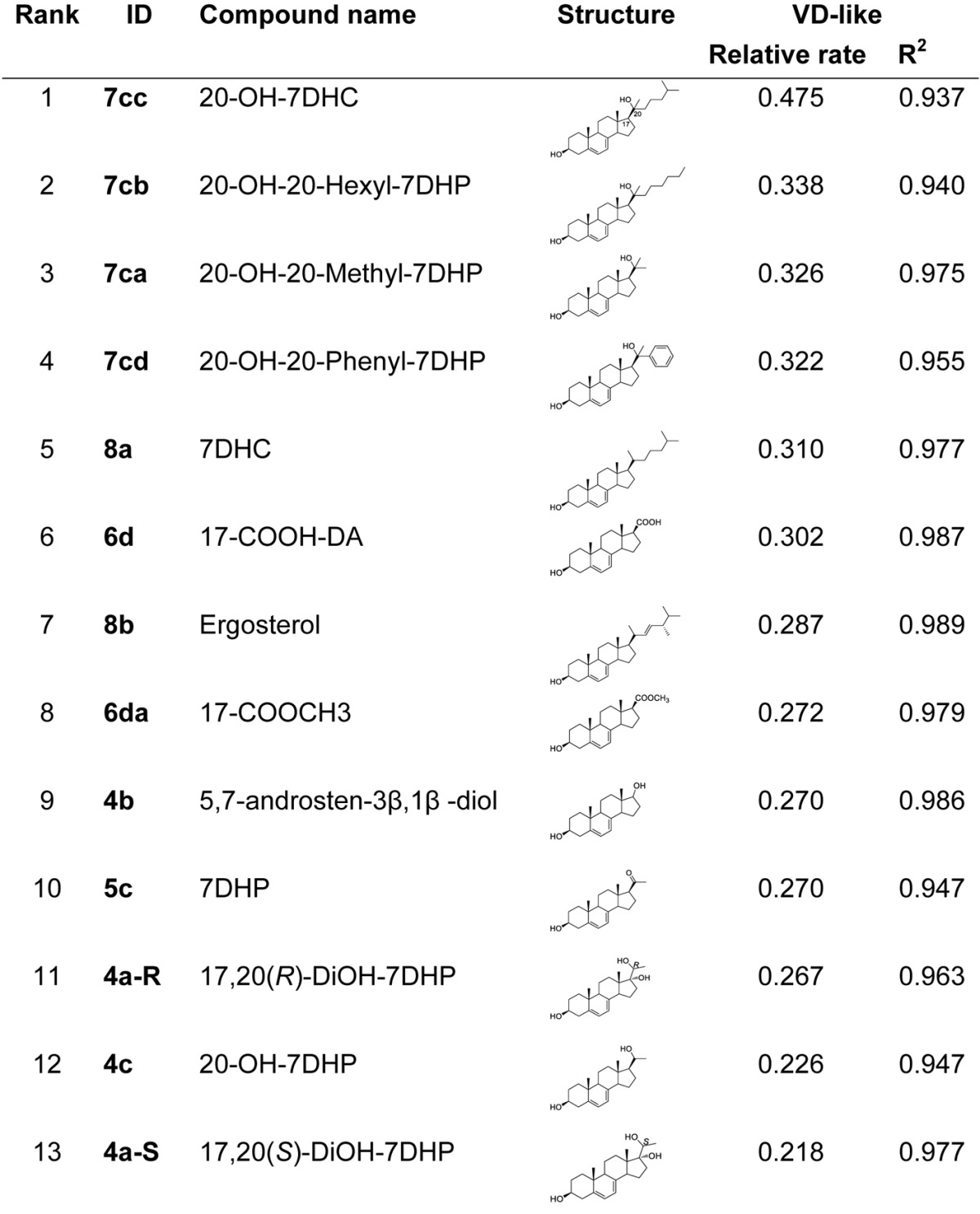
Relative rates of conversion for the irradiated products (pre-D-like to D-like) based on the slope (a) of the equation: Y = aX+b; Solvent: CD3OD; Concentration: 1.8 mMol. Temperature: 37°C; monitoring the changes by NMR every 30 min.
Analysis of the compound structures and their relative conversion rate reveal two interesting trends associated with side chain length and composition. First, with a few exceptions, compounds with a longer (or larger) side chain (e.g., 7cc, 7cb, 7cd, 8a) generally have a faster conversion rate than compounds with a shorter (or smaller) side chain (e.g., 4a-S, 4c, 4a-R, 5c and 4b). Second, all compounds having a tertiary alcohol moiety at the C20 position (e.g., the top four ranked compounds 7cc, 7cb, 7ca, and 7cd) have higher conversion rate than those not having this tertiary alcohol moiety. This may suggest that pre-D compounds with tertiary alcohol side chains are more prone to undergo isomerization to form D analogs, therefore leading to the higher conversion rate than the non-tertiary moieties. Figure 3 shows the comparison of changes of D-like analogs over time using compounds 4a-R, 8a, and 7cc as examples. Overall, compound 7cc has the highest conversion rate while compound 4a-S showed the lowest conversion rate.
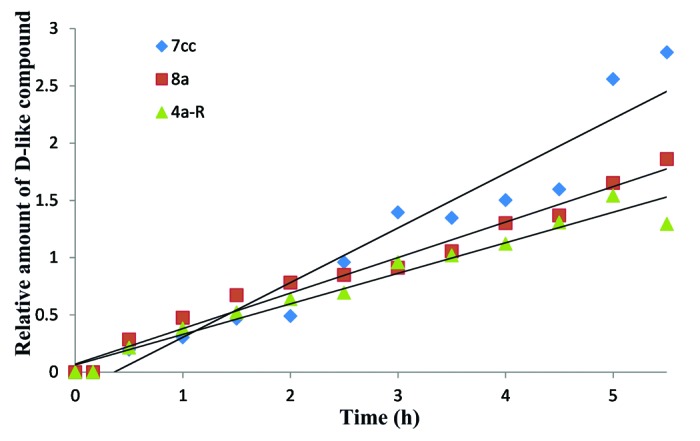
Figure 3. Comparison of conversion from pre-D analogs to D-like analogs over time for 4a-R, 8a, and 7cc.
These results are somewhat unexpected. The isomerization of pre-D to form D is an antarafacial [1,7]-sigmatropic hydrogen shift from C19 to C9,16,17 one of processes defined by the Woodward-Hoffmann rule.18 This process requires a large rotation around C6-C7 bond which intuitively means a pre-D analog with a larger sidechain (e.g., 7cc) may convert to the D analog slower than an analog with a shorter sidechain (e.g., 4a). This seems to contradict to what we observed in this study. However, several previous report have shown that addition of a single hydroxyl group either on the sidechain (e.g., 25-hydroxylation14) or on the ring systems (e.g., 11-hydroxylation19) can significantly affect the kinetics of this conversion process. Since most of the analogs reported in the current study have a hydroxyl group at C20, it is possible that 20-hydroxylation also exerts its influence to the overall kinetics. It may also be worthwhile to point out that the only reaction observed in this process (proton NMR monitoring after irradiation) is the conversion of pre-D to D analogs. The characteristic peaks for D kept increasing over time while the characteristic peaks for pre-D kept decreasing. Proton NMR peaks corresponding to other molecules in the solution remains unchanged. This observation is consistent with the fact that the conversion from pre-D to D is a thermodynamic process, while the conversion from the 5,7-diene precursors to pre-D and from pre-D to tachysterol, lumisterol and iso-T is a photochemical process which requires UVB irradiation.
Effects of side chains on the 5,7-dienes on their photochemical product distributions
The distribution of the products after 5.5 h post UVB irradiation is shown in Table 2. We selected the 5.5 h as the ending point because after this time the kinetic curves from pre-D analogs to D analogs have significant deviation from linearity, although after a long time (5~7 d), virtually all pre-D analogs are converted to their corresponding D analogs. As we discussed earlier, except D and pre-D, the amounts of other products from UVB irradiation of the 5,7-dienes remains unchanged during the proton NMR monitoring process. The percentage of the parent compounds is generally reversely proportional to the relative conversion rate, although there are some exceptions. This can be confirmed by 4a-S, which had the lowest conversion rate but with the highest percentage of its parent compound (30%). Compounds with the highest conversion rates (7cc, 7cb, 7ca ranked 1st, 2nd, and 3rd, respectively) had relatively lower percentages of their parent compounds (15%, 14%, 10% for 7cc, 7cb, 7ca, respectively). Compounds 4b, 5c had both low percentages of their parent compounds (17%, 9% for 4b, 5c, respectively) and low conversion rates (4b, 5c ranked 9th, 10th, respectively). There does not appear to have an identifiable correlations between photochemical product distributions and the length or composition of the side chains in these 5,7-dienes, suggesting these two factors are not the dominant in this photochemical process. Percentages of lumisterol-like (L-like) and iso-tachesterol-like (iso-T-like) compounds are similar for all tested compounds (4–9% and 6–11% for L-like and iso-T-like compounds, respectively). It should be of particular interest to note that several compounds tend to form more T-like compounds (48%, 30%, 35%, 43%, 37% for 7cc, 7cb, 7ca, 4b, 5c, respectively) during photolysis. This may suggest that the side chain of these molecules may accelerate the conversion of pre-D to T-like compounds. On the other hand, it might be attributed to the high stability of the T-like analogs of these compounds under UVB irradiation. The fact that compound 8a had only 1% of T-like remaining but with a relatively higher amount of iso-T (11%) may indicate that the T-like analog of 8a is extremely unstable in the presence of UVB irradiation and converted to the corresponding iso-T analog very quickly.
Table 2. Distribution of products 5.5 h after irradiation.
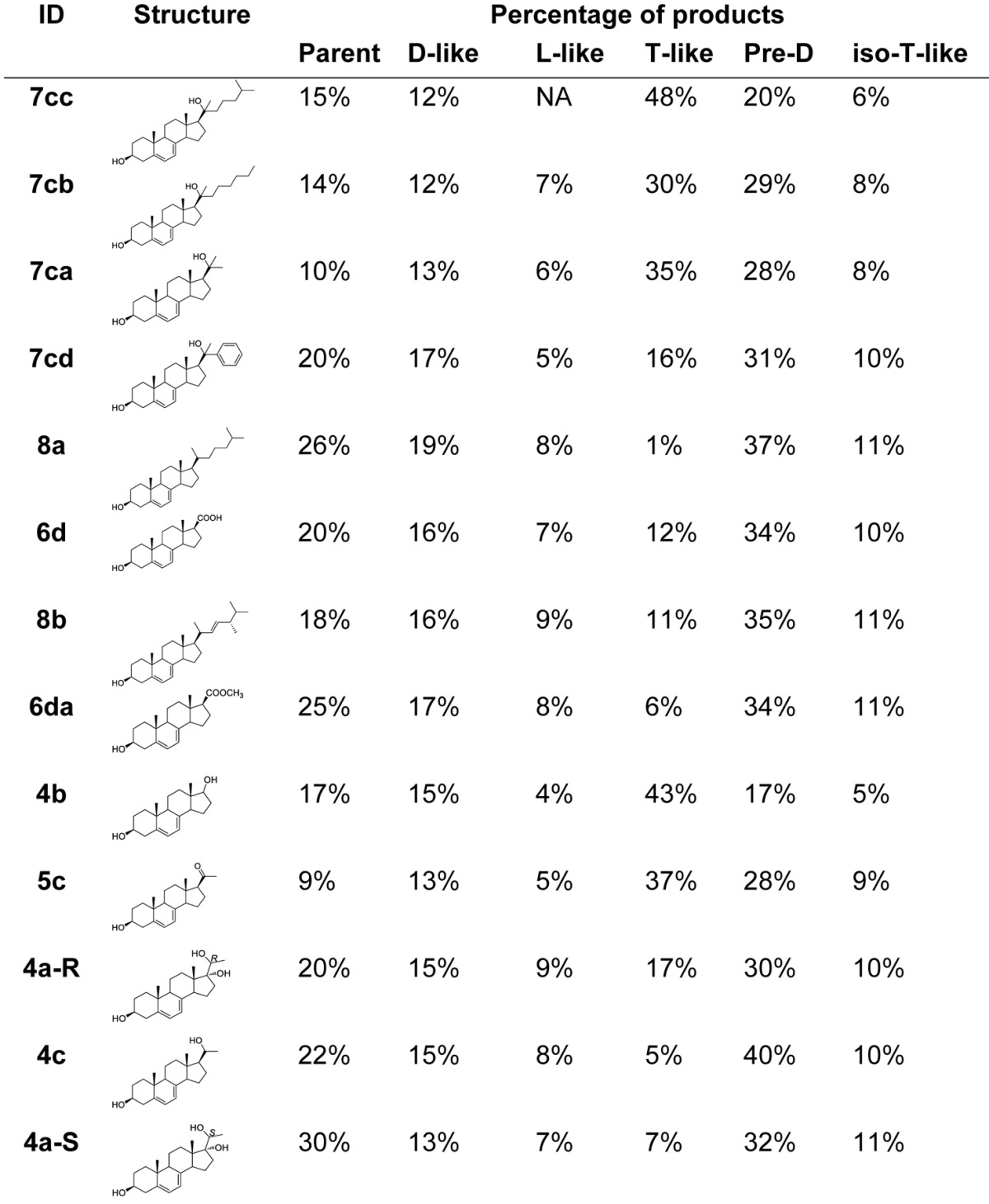
Distribution of products 5.5 h after irradiation. The characteristic peak of each specific product (D, T, and L) was integrated 5.5h and divided by the sum of integrations of all products. NA, not available; D, Vitamin D; L, Lumisterol; T, Tachysterol; Pre-D, Pre-vitamin D; iso-T, iso-Tachysterol.
Even though no obvious correlation between the side chain and product distribution can be identified at this stage, these studies do suggest that they have certain influences on the product distribution. Accumulating evidence in the literature clearly indicated that unique nonhypercalcemic D analogs possessing beneficial biological activities (e.g., antiproliferation and anti-inflammation) exist either endogenously produced or newly synthesized.4-7,9,12,20-32 Such compounds likely have significantly improved toxicity profiles compared with 1,25-dihydroxy vitamin D3 (the biologically active form of D3). At least currently, making these compounds using the classical photochemistry method10,12 is much easier than total organic synthesis.33-35 Therefore, understanding and proper use of the effects of the side chain and other factors on the photochemical product distributions may provide very useful controls when optimizing the yields of desired products while minimizing the undesired side products.
Conclusion
We have synthesized several novel 5,7-dienes and examined the real-time conversion kinetics from pre-D analogs to their corresponding D analogs. The rate of this conversion was found to be correlated with side chain length and composition. Photochemical product distributions from these 5,7-dienes are also documented under the experimental conditions, although no significant correlations to the side chain was observed. To the best of our knowledge this is the first time that the kinetics for conversion in real time from pre-D to D is described. We are well aware the limitation of our current study performed in an isotropic solution environment that is too simple compared with the complex biological environment existing in the human skin. The interactions between pre-vitamin D3 and the surrounding biological environment have profound effects to the kinetics and thermodynamics of vitamin D3 formation.2,13-15 Therefore, the future direction of this study will be to evaluate this thermal isomerization process of pre-D to D under a more physiologically relevant condition by using either human skin or its mimic such as a liposomal model.13,14 Nevertheless, the current study could still serve as step-stones for future kinetic studies of novel 5,7-dienes under more physiologically relevant ex vivo or in vivo conditions, as well as providing insights in controlling yields of the biologically active products such as nonhypercalcemic D analogs from the photochemical transformation of novel 5,7-dienes.
Materials and Methods
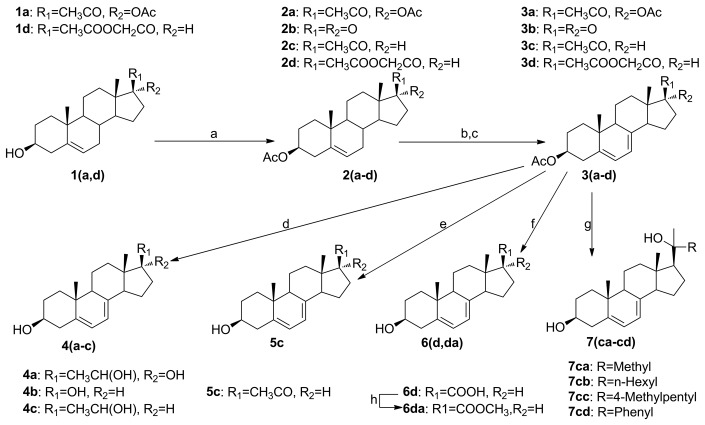
Compound 8a (7DHC) was purchased from Sigma-Aldrich and recrystallized from methanol to remove impurities. Compound 8b (ergosterol) was also purchased from Sigma-Aldrich and used for NMR experiment without further purification. The synthesis of other compounds is outlined in Figure 4. Compounds 4a-c, 5c, 7cc, 6d and 6da were generated following a previously reported procedure.7,8,11,12 Generally, compound 1 was protected by an acetyl group at the 3-position to give compound 2. Compound 2 was then brominated at the 7-position followed by dehydrobromination to afford compound 3 with an extra double bond at the 7 position. Compound 3 was used for the generation of the subsequent products under various conditions. Compounds 7ca, 7cb, and 7cd are new molecules that have not been reported previously. The synthesis of 7ca-cd was performed by reacting 3c with appropriate alky lithium or Grignard reagents to build up the tertiary alcohol moiety in the structure (Fig. 4).36-38 Alkyl lithium reagents for the synthesis of 7ca and 7cd were commercially available from Sigma-Aldrich. Grignard reagents used for the synthesis of 7cb and 7cc were freshly prepared by reacting the corresponding alkyl bromide with magnesium in anhydrous THF.39 NMR and Mass spec data: 20-OH-20-Methyl-7DHP (7ca): 1H NMR (500 MHz, CD3OD): δ 5.55–5.58 (m, 1 H), 5.39–5.42 (m, 1 H), 3.49–3.57 (m, 1 H), 2.40–2.46 (m, 1 H), 2.20–2.31 (m, 2 H), 1.31–2.01 (m, 24 H), 1.30 (s, 3 H), 1.23 (s, 3 H), 0.97 (s, 3 H), 0.80 (s, 3 H). ESI-MS: calculated for C22H34O2, 330.3, found 353.3 [M+Na]+. 20-OH-20-Hexyl-7DHP (7cb): 1H NMR (500 MHz, CD3OD): δ 5.54–5.59 (m, 1 H), 5.38–5.42 (m, 1 H), 3.48–3.57 (m, 1 H), 2.40–2.47 (m, 1 H), 2.25–2.30 (m, 1 H), 2.16–2.25 (m, 1 H), 1.30–2.00 (m, 24 H), 1.28 (s, 3 H), 0.97 (s, 3 H), 0.93 (t, J = 6.0 Hz, 3 H), 0.82 (s, 3 H). ESI-MS: calculated for C27H44O3, 400.3, found 423.3 [M+Na]+. 20-OH-20-Phenyl-7DHP (7cd): 1H NMR (500 MHz, CD3OD): δ 7.46 (d, J = 8.0 Hz, 2 H), 7.29 (t, J = 8.0 Hz, 2 H), 7.18 (t, J = 7.5 Hz, 1 H), 5.53–5.56 (m, 1 H), 5.35–5.39 (m, 1 H), 3.49–3.56 (m, 1 H), 2.39–2.45 (m, 1 H), 2.22–2.30 (m, 1 H), 1.68–2.06 (m, 8 H), 1.66 (s, 3 H), 1.25–1.65 (m, 7 H), 0.96 (s, 3 H), 0.83 (s, 3 H). ESI-MS: calculated for C27H36O2, 392.3, found 415.3 [M+Na]+. The synthesis and chromatographic data for 7cc,12 6d,11 6da,11 4b,8 4c,8 5c,8 4a-R,7 4a-S7 were described in our previous publications.
Figure 4. Synthesis of 5,7-dienes. Step a is for 1a, 1d only; step d is for 3(a–c) only; step 3 is for 3c only; step f is for 3d only; step g is for 3c only. Reagents and conditions: (a) Ac2O, microwave, p-toluenesulfonic acid monohydrate; (b) dibromantin, 2,2'-azobisisobutyronitrile, benzene/hexane (1:1), 100°C, reflux; (c) Bu4NBr, Bu4NF, THF, rt; (d) LiAlH4, THF, 0°C; (e) K2CO3, MeOH-THF, Argon bubbling, rt; (f) K2CO3, MeOH-THF, air, rt; (g) R-Li (7ca,7cd) or R-MgBr (7cb-mL), THF; (h) DBU, THF, MeI.
Each of these compounds was dissolved in methanol-d4 to make a 1.8 mM solution and subsequently transferred to a quartz NMR tube. A standard proton NMR at 37þC was run for each sample before irradiation by UVB. Each sample was then irradiated for 5 min using a Biorad UV Transilluminator 2000 (Biorad, Hercules, CA). The spectral characteristics of the UVB (280–320 nm) source were published previously40 and its strength (4.8 ± 0.2 mW cm−1) was measured routinely using a digital UVB Meter Model 6.0 (Solartech Inc., Harrison Twp,MI). Each irradiated sample was monitored by proton NMR at 37°C immediately after the irradiation in a time course manner. Data was recorded for each time point of 0 min, 10 min, and every 30 min thereafter. Analysis of data was accomplished by using ACD software (Advanced Chemistry Development, Toronto, ON, Canada). The percentage of products (D, T, L, iso-T) at each time point is determined by the peak areas calculated from the proton NMR spectra. The characteristic peak of each specific product (D, T, and L) was integrated at each time point and plotted against time to produce a kinetic curve (supporting information). The equation generated from this curve is expressed as follows: Y = aX+b, where a stands for the slope, b stands for the intercept of the curve in Y-axis. The slope a of the curve was taken as the relative conversion rate. The relative accuracy of the equation is judged by R2 (The closer to 1, the better).
Acknowledgments
This work was supported by NIH grant 1RO1 AR052190–01A2 (to AS), 1S10RR026377–01 and 1S10OD010678–01 (both to WL) and R21AR063242-01A1 (to WL and DDM). Additional support comes from the Department of Pharmaceutical Sciences, College of Pharmacy, the University of Tennessee Health Science Center. The authors want to express their sincere gratitude to the two expert reviewers who provided very insightful comments and advice that greatly improves the quality of this manuscript.
Glossary
Abbreviations:
- NMR
Nuclear Magnetic Resonance
- UVB
Ultra-Violet B
- 7DHC
7-Dehydrocholesterol
- 7DHP
7-Dehydropregnenolone
- D
Vitamin D (D3, D2, and pD for short chains)
- T
Tachysterol
- L
Lumisterol
- Iso-T
iso-Tachysterol
- RT
room temperature
- THF
Tetrahydrofuran
- DBU
Diazabicycloundecene
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/24339
Reference
- 1.Holick MF, Clark MB. The photobiogenesis and metabolism of vitamin D. Fed Proc. 1978;37:2567–74. [PubMed] [Google Scholar]
- 2.Holick MF, Tian XQ, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc Natl Acad Sci U S A. 1995;92:3124–6. doi: 10.1073/pnas.92.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Li W, Zbytek B, Tuckey RC, Zjawiony J, Nguyen MN, et al. Enzymatic production or chemical synthesis and uses for 5,7-dienes and UVB conversion products thereof. US2011/0118228A1, 2011.
- 5.Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011;131:1167–9. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, et al. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–28. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, et al. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci. 2008;7:1570–6. doi: 10.1039/b809005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, et al. Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76:193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y, Chen J, Janjetovic Z, Michaels P, Tang EK, Wang J, et al. Design, synthesis, and biological action of 20R-hydroxyvitamin D3. J Med Chem. 2012;55:3573–7. doi: 10.1021/jm201478e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim T-K, Chen J, Li W, Zjawiony J, Miller D, Janjetovic Z, et al. A new steroidal 5,7-diene derivative, 3β-hydroxyandrosta-5,7-diene-17β-carboxylic acid, shows potent anti-proliferative activity. Steroids. 2010;75:230–9. doi: 10.1016/j.steroids.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, et al. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–35. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian XQ, Chen TC, Matsuoka LY, Wortsman J, Holick MF. Kinetic and thermodynamic studies of the conversion of previtamin D3 to vitamin D3 in human skin. J Biol Chem. 1993;268:14888–92. [PubMed] [Google Scholar]
- 14.Tian XQ, Holick MF. A liposomal model that mimics the cutaneous production of vitamin D3. Studies of the mechanism of the membrane-enhanced thermal isomerization of previtamin D3 to vitamin D3. J Biol Chem. 1999;274:4174–9. doi: 10.1074/jbc.274.7.4174. [DOI] [PubMed] [Google Scholar]
- 15.Tian XQ, Holick MF. Catalyzed thermal isomerization between previtamin D3 and vitamin D3 via beta-cyclodextrin complexation. J Biol Chem. 1995;270:8706–11. doi: 10.1074/jbc.270.15.8706. [DOI] [PubMed] [Google Scholar]
- 16.Okamura WH, Midland MM, Hammond MW, Abd Rahman N, Dormanen MC, Nemere I, et al. Chemistry and conformation of vitamin D molecules. J Steroid Biochem Mol Biol. 1995;53:603–13. doi: 10.1016/0960-0760(95)00107-B. [DOI] [PubMed] [Google Scholar]
- 17.Dauben WG, Funhoff DJH. Theoretical evaluation of the conformation of previtamin D3. J Org Chem. 1988;53:5070–5. doi: 10.1021/jo00256a031. [DOI] [Google Scholar]
- 18.Woodward RB, Hoffmann R. Stereochemistry of Electrocyclic Reactions. J Am Chem Soc. 1965;87:395–7. doi: 10.1021/ja01080a054. [DOI] [Google Scholar]
- 19.Enas JD, Palenzuela A, Okamura WH. Studies on vitamin D (calciferol) and its analogs. 37. A-Homo-11-hydroxy-3-deoxy vitamin D: ring size and. pi.-facial selectivity effects on the [1,7]-sigmatropic hydrogen shift of previtamin D to vitamin D. J Am Chem Soc. 1991;113:1355–63. doi: 10.1021/ja00004a043. [DOI] [Google Scholar]
- 20.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012;44:2003–18. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slominski AT, Kim T-K, Shehabi HZ, Semak I, Tang EKY, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D₃ metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–15. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, et al. High basal NF-κB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–84. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr., Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slominski AT, Janjetovic Z, Kim T-K, Wright AC, Grese LN, Riney SJ, et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–42. [PMC free article] [PubMed] [Google Scholar]
- 26.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–41. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, et al. 20-hydroxyvitamin D₃ inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–46. [PMC free article] [PubMed] [Google Scholar]
- 29.Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmun Rev. 2007;7:59–64. doi: 10.1016/j.autrev.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Field S, Newton-Bishop JA. Melanoma and vitamin D. Mol Oncol. 2011;5:197–214. doi: 10.1016/j.molonc.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine. 2010;77:552–7. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Na S, Ma Y, Zhao J, Schmidt C, Zeng QQ, Chandrasekhar S, et al. A Nonsecosteroidal Vitamin D Receptor Modulator Ameliorates Experimental Autoimmune Encephalomyelitis without Causing Hypercalcemia. Autoimmune Dis. 2011;2011:132958. doi: 10.4061/2011/132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carballa DM, Seoane S, Zacconi F, Pérez X, Rumbo A, Alvarez-Díaz S, et al. Synthesis and biological evaluation of 1α,25-dihydroxyvitamin D₃ analogues with a long side chain at C12 and short C17 side chains. J Med Chem. 2012;55:8642–56. doi: 10.1021/jm3008272. [DOI] [PubMed] [Google Scholar]
- 34.Flores A, Sicinski RR, Grzywacz P, Thoden JB, Plum LA, Clagett-Dame M, et al. A 20S combined with a 22R configuration markedly increases both in vivo and in vitro biological activity of 1α,25-dihydroxy-22-methyl-2-methylene-19-norvitamin D3. J Med Chem. 2012;55:4352–66. doi: 10.1021/jm300187x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plonska-Ocypa K, Sibilska I, Sicinski RR, Sicinska W, Plum LA, DeLuca HF. 13,13-Dimethyl-des-C,D analogues of (20S)-1α,25-dihydroxy-2-methylene-19-norvitamin D₃ (2MD): total synthesis, docking to the VDR, and biological evaluation. Bioorg Med Chem. 2011;19:7205–20. doi: 10.1016/j.bmc.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 36.McNaughton-Smith GA, Burns JF, Stocker JW, Rigdon GC, Creech C, Arrington S, et al. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J Med Chem. 2008;51:976–82. doi: 10.1021/jm070663s. [DOI] [PubMed] [Google Scholar]
- 37.Theodorou V, Skobridis K, Karkatsoulis A. Base-induced rearrangement of tritylamines to imines:discovery and investigation of the mechanism. Tetrahedron. 2007;63:4284–9. doi: 10.1016/j.tet.2007.03.055. [DOI] [Google Scholar]
- 38.Mijares A, Cargill DI, Glasel JA, Lieberman S. Studies on the C-20 epimers of 20-hydroxycholesterol. J Org Chem. 1967;32:810–2. doi: 10.1021/jo01278a066. [DOI] [PubMed] [Google Scholar]
- 39.van Summeren RP, Moody DB, Feringa BL, Minnaard AJ. Total synthesis of enantiopure beta-D-mannosyl phosphomycoketides from Mycobacterium tuberculosis. J Am Chem Soc. 2006;128:4546–7. doi: 10.1021/ja060499i. [DOI] [PubMed] [Google Scholar]
- 40.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–6. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]