Abstract
Solar ultraviolet (UV) radiation is the main source of vitamin D production and is also the most important environmental risk factor for cutaneous malignant melanoma (CMM) development. In the present study the relationships between daily or seasonal UV radiation doses and vitamin D status, dietary vitamin D intake and CMM incidence rates at different geographical latitudes were investigated. North-South gradients of 25-hydroxyvitamin D (25(OH)D) generation and CMM induction were calculated, based on known action spectra, and compared with measured vitamin D levels and incidence rates of CMM. The relative roles of UVA and UVB in CMM induction are discussed. Latitudinal dependencies of serum 25(OH)D levels and CMM incidence rates can only partly be explained by ambient UV doses. The UV sensitivity is different among populations with different skin color. This is well known for CMM, but seems also to be true for vitamin D status. The fact that UV-induced vitamin D may reduce the risk of CMM complicates the discussion. To some extent high dietary vitamin D intake seems to compensate low UV doses.
Keywords: vitamin D, solar ultraviolet radiation, UVA and UVB ratio, photoimmunosuppression, cutaneous malignant melanoma
Introduction
For decades the beneficial (synthesis of vitamin D) and the adverse (induction of skin cancer) effects of solar UV radiation have been known and discussed. Nevertheless, no consensus exists concerning the optimal balance between positive and negative effects of UV radiation. Vitamin D can be obtained through UVB exposure or diet. Inadequate sun exposure or too low intake of vitamin D can lead to vitamin D deficiency. Deficiency has been reported for different latitudes and seasons.1 Higher UVB radiation doses are obtained by humans in the South than in the North,2 and one might suppose that people in southern regions have a better vitamin D status than people in northern regions. In contrast to such expectations, the vitamin D status is better in Scandinavia than in south Europe.3 This phenomenon has to be explained by other factors than ambient UVB, such as differences in skin color, diet, genetics or vitamin D supplementation. Such factors may play more important roles for the serum 25-hydroxyvitamin D (25(OH)D) levels than thought before.
Solar UV radiation can induce direct (UVB) and indirect (UVA) oxidative DNA damage and can lead to carcinogenesis. Non-melanoma skin cancers (NMSC) have different sun exposure patterns. As suggested from etiologic studies, UVB is the most important spectral region in causing squamous cell carcinoma (SCC), while both UVB and UVA may be related to basal cell carcinoma (BCC).4,5 The role of UVA in initiation of CMM is more controversial.6 The risk of skin cancer is very high for xeroderma pigmentosum variant patients with defective excision repair of UVB-type DNA damage, e.g., of cyclobutane pyrimidine dimers (CPD).7 Epidemiological evidences suggest that UVA may be involved in melanomagenesis.8 The newest experimental data obtained by use of mouse models indicate that not only UVB, but also UVA can induce melanoma.9 Human response to UVA radiation cannot be fully elucidated by animal models, and humans may respond differently. However one might expect strong similarities.
CMM is more common among indoor workers than among outdoor workers.10 This may have several reasons among them elastosis and skin wrinkling caused by chronic UV exposure and differences in vitamin D status. Epidemiological evidence support the hypothesis that skin aging has a protective effect on melanomagenesis.11 The role of vitamin D in CMM induction has been reviewed and discussed.12-14 It has been demonstrated that vitamin D has anti-proliferative effects on melanoma cells, and that CMM patients with high vitamin D status have thinner lesions and better survival.15 Case-control studies from some European countries, indicate no association of serum 25(OH)D and melanoma, but there is no reason to believe that a good status of vitamin D is disadvantageous.16
Photoimmunosuppression contributes to the adverse effects of UV radiation.17,18 UV radiation suppresses immunity,19,20 while vitamin D improves it.21 Organ transplant patients with long-term immunosuppression often develop NMSC, and human papillomavirus infection is an important risk factor.22 The risk of developing CMM seems also to be associated with immunosuppression.23,24 The immunosuppressive effectiveness of UVA is 3-fold higher than that of UVB at standard conditions of noon solar exposure.19 Peaks in both the UVB (300 nm) and UVA (370 nm) regions in the action spectrum of photoimmosuppression suggest that different chromophores and mechanisms are involved in the induction of photoimmunosuppression in these regions. This is important since the ratio of UVB to UVA varies with the latitude and with the time of the day. It is unclear how these variations will affect photoimmunosuppression. In addition to the harmful effect of UVA on the immune system, UVA-formation of free radicals should be taken into account as an important factor in skin carcinogenesis. Not only primary actions of reactive oxygen species in melanoma development and progression is involved,25 but also “bystander effects” may play an important role,26 where stress-free cells can be stressed by nearby stressed cells.
To better understand the impact of UV on vitamin D production, erythema induction, DNA damage and CMM induction, it is necessary to look at the separate roles of UVA and UVB for these processes. A good option for such a study is to investigate countries at different latitudes where ratio of UVA to UVB is different. UVB is more scattered and absorbed in the atmosphere than UVA.27 Thus, the latitude gradient of UVB is much greater than that of UVA. However, the latitudinal UVA gradient is more important for melanoma than for non-melanomas.28 For epidemiological evaluations, the place of residence can be used in approximations of UV exposures and their impacts.29
The aim of the present study is to determine the relationship between daily or seasonal UV radiation doses, CMM incidence rates and 25(OH)D levels in blood at different geographical latitudes. Dietary vitamin D intake will also be taken into account. North-south gradients of 25(OH)D levels and CMM incidence rates will be calculated based on epidemiological data, and calculations of biological effectiveness depending on action spectra and worldwide data of vitamin D status. The importance of UVA and UVB in CMM induction will be discussed.
Results
Daily UV doses
Calculations of daily integrated biological effective UV doses for different latitudes are shown in Figure 1. Daily relative erythema UV doses, doses for photoimmunosuppression and vitamin D production are dependent on the transmission of UV to the ground at different geographical regions. Longer days during the summer at higher latitudes tend to reduce north-south differences (Fig. 1). The ratios between summer UV doses at 20°N and 70°N are about 2 for both erythema induction (Fig. 1A) and vitamin D production (Fig. 1B), and about 1.3 for photoimmunosuppression (Fig. 1C). Vitamin D effective daily doses (Fig. 1B) and erythema doses (Fig. 1A) are comparable. At high latitudes (Scandinavia) production of vitamin D and induction of erythema is significant only from April to October, whereas in the tropics the variations during a year are small (Fig. 1A and B).
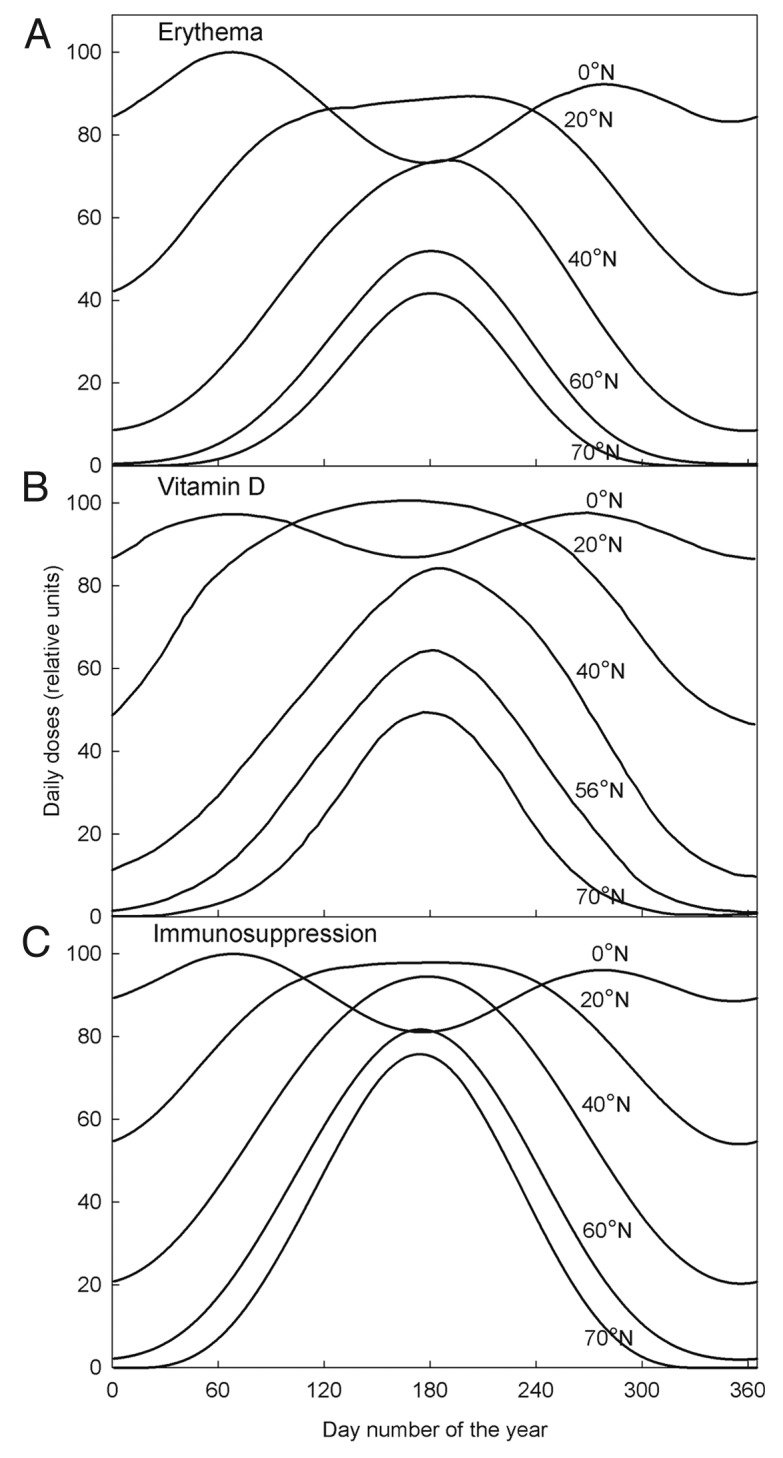
Figure 1. UV doses per day at different latitudes on the northern hemisphere for erythema induction (A), vitamin D production (B) and induction of immunosuppression (C).
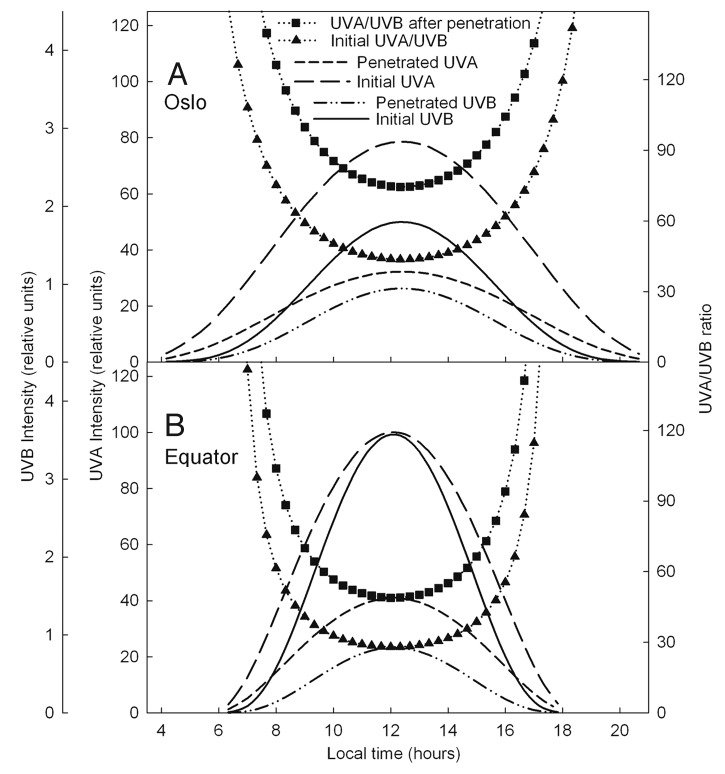
UV penetration
The ratio of UVA to UVB on the skin surface for a typical summer day is about 45 at 60°N latitudes and about 25 at the Equator (Fig. 2). At noon both ratios are about 1.7 times larger below than above the epidermis (Fig. 2). The ratio of UVA to UVB in the middle of a summer day is more stable and smaller at the Equator than in Oslo and Stockholm (Fig. 2).
Figure 2. UVA and UVB intensities (normalized to the same value at the Equator) before and after penetration of epidermis in Oslo (A) and in the Equator (B).
Annual UV doses
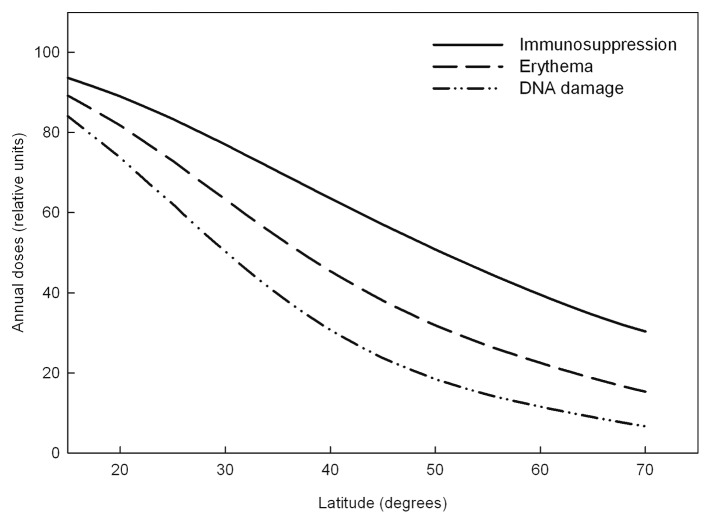
North-south gradients of annual biological effective UV doses were calculated using action spectra for DNA damage,30 for erythema31 and immunosuppression inductions19 (Fig. 3). An action spectrum with only a small peak in the UVA region gives a much smaller north- south gradient than does the UVB weighted action spectra.
Figure 3. Latitudinal dependency of annual UV doses on the northern hemisphere for immunosuppression, erythema and DNA damage.
Latitude gradient of CMM incidence rates
Countries located in a wide latitudinal range (Norway, Sweden, Finland, New Zealand and Australia) have similar latitudinal gradients of CMM incident rates (Table 1; Fig. 4). However, in Germany there is a 'negative' gradient for CMM rates. Furthermore, Australia’s and Norway’s incident rates for females do not follow a linear approximation (Fig. 4). The incidence rates in Norway are larger than those in the other Scandinavian countries. Finally, it seems that incidence rates are lower in the southern part of Germany than in the northern part. The slopes of the incidence rates of CMM in Australia are smaller (being 0.23 ± 0.14, p = 0.12 for females) than that of CMM rates when northern countries are taken into the picture. For females these slopes are around 0.59 ± 0.06 (p < 0.0001) while for males they are around 0.90 ± 0.06 (p < 0.0001). CMM incidence rates for males in Norway are larger (0.94 ± 0.12, p < 0.0001) than the rates when other countries are included in the analysis (0.90 ± 0.06, p < 0.0001).
Table 1. Characteristics of CMM incidence rates.
| Country | Slopes (Males) | P (Males) | Slopes (Females) | P (Females) |
|---|---|---|---|---|
| Sweden | 0.65 ± 0.14 | < 0.001 | 0.55 ± 0.15 | < 0.01 |
| Norway | 0.94 ± 0.12 | < 0.0001 | 1.01 ± 0.17 | < 0.0001 |
| Denmark | 1.24 ± 1.13 | 0.33 | 0.66 ± 1.32 | 0.64 |
| Finland | 0.63 ± 0.16 | 0.03 | 0.66 ± 0.08 | < 0.01 |
| Scotland | 0.02 ± 0.54 | 0.97 | 0.31 ± 1.58 | 0.88 |
| Germany | -0.94 ± 0.39 | 0.10 | -1.05 ± 0.54 | 0.15 |
| Australia | 0.77 ± 0.16 | < 0.01 | 0.23 ± 0.14 | 0.12 |
| All countries | 0.90 ± 0.06 | < 0.0001 | 0.59 ± 0.06 | < 0.0001 |
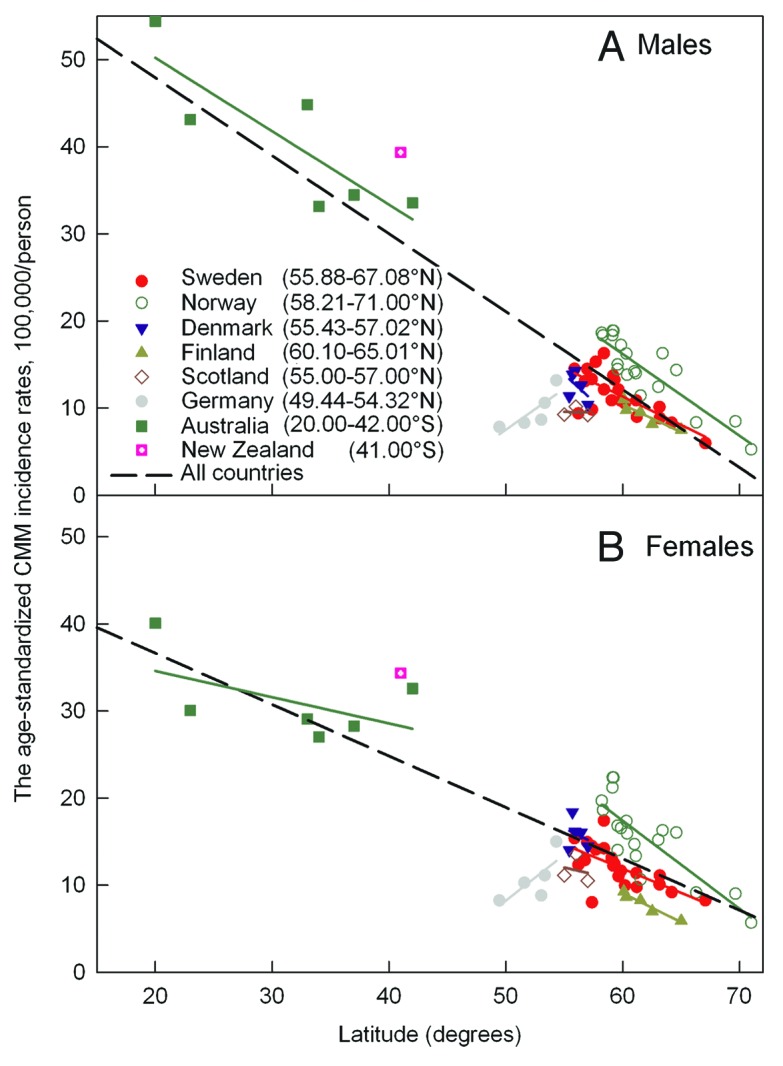
Figure 4. The age-standardized incidence rates (ASIR) according to the world standard population (W) per 100,000 males (A) and females (B) for CMM in different countries.
Vitamin D at different latitudes
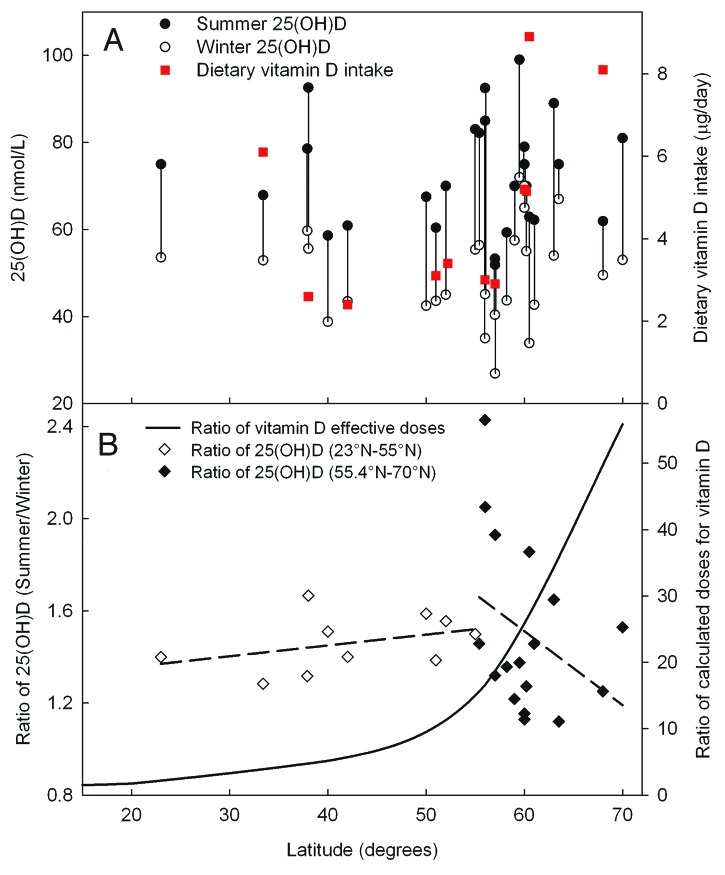
More vitamin D is synthesized in summer than in winter (Fig. 5A) due to higher UVB doses in the summer. Around 1.5 times higher serum 25(OH)D levels were observed during the summer than during the winter in most of the countries (Fig. 5). This observation is worthy of being remarked, and, therefore, we expanded on this finding in Figure 5B. After integration of summer- and winter UV doses for vitamin D production at different latitudes, the summer to winter ratios were compared with reported summer to winter ratios of 25(OH)D levels. Such a procedure will minimize the role of different baseline levels and focus on the role of the sun. The absence of any correlation between theoretical and experimental ratios is remarkable and will be discussed.
Figure 5. Summer and winter levels of 25(OH)D and dietary intake of vitamin D in populations living at different latitudes (A). Theoretically estimated relative summer to winter ratios of vitamin D photosynthesis (according to the effective UV doses, Fig. 1B) and the summer to winter ratios of measured 25(OH) D levels (B). 25(OH) D levels are taken from panel (A).
Discussion
The annual fluence of ambient UV varies strongly with geographical localization. Furthermore, personal sunbathing habits are important for health effects. Self-reported sun exposures are difficult to obtain and unreliable, which introduces large uncertainties in evaluations and predictions. Therefore, we decided to study the crude latitudinal dependency. Place of residence can be used as approximation for UV exposures and their impact at given locations.29 Thus, mathematical modeling, using relevant action spectra, is a valuable tool for estimations of health effects of solar radiation.
The seasonal variation of erythemal exposures are similar to the seasonal variation of vitamin D generating exposures of solar radiation at all latitudes (Fig. 1). This is to be expected in view of the similarity of the corresponding action spectra,31,32 both being strongly UVB-weighted. Shapes and relative amplitudes of vitamin D production (Fig. 1B) are similar to previously published observations regarding photosynthesis of vitamin D at different latitudes.33 The action spectrum of photoimmunosuppression has a significant UVA contribution19 which explains the relatively high midsummer photoimmunosuppression exposures at high latitudes (Fig. 1C).
In agreement with the above data, at all latitudes UVA radiation lasts much longer in the afternoon than the UVB radiation (as here exemplified by the data for Oslo or Stockholm and for the Equator) (Fig. 1). Thus, if photoimmunosuppression plays a role for CMM induction, the afternoon is not a good time for sun exposure, since at that time the sun gives minimally of vitamin D but still gives much UVA which may be melanomagenic. The “danger and benefit” ratio is certainly related to the UVA/UVB ratio which increases strongly with decreasing solar elevation, i.e., with time before and after noon (Fig. 2).
Since vitamin D generation is mostly caused by UVB, just as DNA damage and erythema are, while melanomagenesis is caused by UVB and also by UVA radiation, we expect the latitudinal gradient of CMM incidence rates to be smaller than that of vitamin D generation. However, it is known that vitamin D generation is related to skin color34 (which is generally darker at low latitudes), and CMM risk is also related to skin color under similar exposure conditions.
We have calculated the latitudinal dependency of annual ambient exposures of solar radiation leading to immunosuppression, erythema and DNA damage (Fig. 3). In view of the similarity of the action spectra the latitudinal dependency of generation of vitamin D, erythema and DNA damage would be similar. When comparing latitudinal gradients of UVA and CMM incidence rates, similarities are found.35 Latitudinal comparisons of UVB, BCC and SCC gradients are more complicated to carry out. This is due to the fact that routine use of sunscreens has been shown to be relatively ineffective in reducing the rates of BCC, while SCC rates did statistically decrease in populations using sunscreens.36 In addition, the latitudinal gradient in Europe of CMM incidence rates for males is opposite that for SCC, while the gradient of BCC is between those of CMM and SCC.28,37 Thus, the UVB impact on BCC and SCC rates may be different.
Two features should be remarked: (1) In Europe, notably in Germany, CMM is more common in the north than in the south (Fig. 4); (2) Migration to sunnier countries leads to an increase in CMM risk.38 For the populations of Scandinavia and Australia the rates follow almost the same latitudinal gradient, although for females the gradient in Australia is uncertain (Fig. 4). These populations, as well as those in the other analyzed countries, have similar Caucasian skin types, mostly types I−III. It is known that in Germany and in central Europe, the skin type is different in north and south, with increasing skin darkness in the south.39 Skin pigmentation attenuates penetration of UVB, also UVA radiation and, thus, a dark skin type may protect against CMM.40 This is probably the reason for the inverse latitudinal gradient found for CMM in Germany (Fig. 4). In addition to the fact that there may be inconsistencies between different cancer registries concerning recording of incidence rates, 'negative' latitudinal gradients of CMM incidence rates may also be related to genetic differences in sensitivities to UVB and UVA. UVB-induced synthesis of previtamin D3 and UVA-induced effects on the deeper skin layers depend on skin pigmentation.41
Sunnier countries have smaller and more stable UVA to UVB ratios during most of the daytime of vitamin D generation (Fig. 2). Interestingly, melanoma mortality rates seem to increase with increasing UVA to UVB ratios.28 For a complete evaluation of the relationship between vitamin D photosynthesis in the skin and measured 25(OH)D levels in different countries, skin pigmentation need to be taken into account. An intake of vitamin D rich food leads to lower concentrations of 25(OH)D in humans with dark skin than with white skin.42 This fits with the evolutionary hypothesis for skin lightening at higher latitudes.43 In order to elucidate how the fluence rate of UV varies at the bottom of the epidermis during a day, we used relevant skin transmission coefficients. To generate the same amount of vitamin D, dark skin needs about six times more UVB than light skin.34 Regardless of this fact, Bogh et al. suggested that skin pigmentation is only a secondary factor for limitation of vitamin D production in darker skin, the baseline levels of vitamin D and total cholesterol being more important.44
Vitamin D intake is probably of significant importance for winter vitamin D status in populations with similar genetic constitutions. This suggestion is reflected, not only by decreasing summer/winter ratio of 25(OH)D, but also by shifts from the winter level at 60°N. Daily effective vitamin D doses (Fig. 1) are about twice as large at 20°N as at 70°N latitudes. Due to UVB differences in summer and winter there should be significant differences between vitamin D photosynthesis in the skin during summer and winter months at northern latitudes. However, the calculated effective doses of vitamin D-generating radiation do not correlate with the measured levels of vitamin D (Fig. 5B). In Norway the vitamin D intake is 10–20% larger in the north than in the south.45 In the late 90s it was reported that the highest fish to meat ratios (0.50–1.26) in food was found in the northwestern region of the Nordic countries, (i.e., in Denmark, Finland, Iceland, Norway and Sweden).46 In the rest of the region the ratio was only 0.07–0.28.46 Nutrition is likely to be the most reasonable explanation why the best vitamin D status in Europe47 is observed in the Nordic countries. The latitudinal variation of multiple sclerosis indicates a beneficial effect of high oral vitamin D intake in northern Europe, where the prevalence decreases with increasing latitude.48 However, Zitterman et al. used all age groups in his search for a latitudinal gradient of the 25(OH)D level, and found that the level decreased with increasing latitude.49 Others researchers have found the opposite.50 Our review of 25(OH)D levels shows no significant latitudinal gradients, neither for vitamin D status in the summer nor for summer/winter ratios, and nor for dietary vitamin D intake (Fig. 5A). A high dietary intake of vitamin D, especially in winter, may mask the effect of seasonal variation in UV-exposure. However, the vitamin D status exhibits clear seasonal variation at all northern latitudes, being high in late summer and low in late winter. We find no latitudinal trend neither for the winter nor for the summer vitamin D status (Fig. 5), in spite of the fact that the annual doses of vitamin D-generating UVB increase strongly with decreasing latitude (Fig. 4). Several possible explanations of this discrepancy can be mentioned: First, the sun seeking behavior of the investigated populations may be more pronounced in the north than in the south. Second, vacations to southern latitudes may play a role.51 Third, the average genetic constitution may be latitudinally dependent, with darker skin types more frequent in the south than in the north. As mentioned above, it is well known that under similar conditions people with a dark skin tend to have lower serum 25(OH)D levels, even when food is the main vitamin D source.42
The summer/winter ratio of vitamin D-generating doses has a strong latitudinal dependency (Fig. 5B), being two times larger in north Norway than in Australia. On the other hand, the published 25(OH)D levels for summer and winter show no latitudinal dependency of the summer/winter ratio, which is about 1.2 to 1.8 in most countries (Fig. 5B). The ratio is about 1.3 at latitudes between 20°N and 40°N, while a ratio of only 1.1 is found for the theoretical vitamin D-generating sun doses (Fig. 5B).
The CMM rates are significantly higher in Norway than in the other Scandinavian countries (Fig. 4). This is probably related to skin types, since historically the contact with- and immigration from Europe and/or Russia is larger in Finland, Sweden and Denmark than in Norway, which has had a closer contact with England and Ireland where the skin types are light.
We may conclude that 25(OH)D levels in the countries we have studied, depend on vitamin D intake, solar UVB doses, skin color and other genetic properties. Significant variations of the UVA/UVB ratio (mainly due to UVB variations) do not correlate with the lack of a summer and winter latitudinal independency of the 25(OH)D level. In the Nordic countries there is a clear latitudinal gradient for CMM incidence rates. This indicates that UV plays a major role for CMM induction which is of particular importance for the Nordic countries, where the seasonal- and latitudinal UVA and UVB variations are particularly large.
Methods and data
Radiative transfer calculations
In the calculations for erythema and photoimmunosuppression effective doses (Fig. 1A and C) we used a zonal seasonal total ozone column climatology for each latitude based on ozone measurements with the TOMS instrument on the Nimbus 7 satellite in the time period 1979 – 1992. For more UVB sensitive vitamin D effective doses (Fig. 1B) monthly averaged ozone levels that were obtained until 1989's were used.52 Our accurate multiple scattering radiative transfer model uses the radiative transfer equation solver DISORT.53 The calculations were done for exposures on horizontal surface.
The fluence rate of healthily or carcinogenically effective solar radiation is defined by the expression: E(t) = ∫I(λ,t) φ(λ) dλ. The integration being performed over the wavelength (λ) region of the solar spectrum. I(λ,t) is the solar irradiance at earth’s surface, φ(λ) is the action spectrum that describes the relative effectiveness of energy at different wavelengths in producing a particular biological response, and t is time. The daily effective doses from the sun are: D = ∫E(t)dt.
The same zonal seasonal climatology, for each latitude, was used to calculate annual UV doses (Fig. 3). In the present work we have used CIE proposed action spectrum for UV induced erythema in human skin,31 action spectra for imunnosuppression induction,19 DNA damage30 and vitamin D production.32
UVA and UVB intensities during the day at the Equator (0°) and in Oslo (60°) initial and after penetration of epidermis (Fig. 2) were calculated with FastRT simulation tool.54 FastRT is based on the pseudospherical approximation (SDISORT)55 and is able to ensure high levels of accuracy even for low solar elevation. It was chosen cloudless 2011’s 197-th Julian day (the middle of summer’s season) in Oslo for variation of solar elevation during the day. Total ozone column 250 Dobson units (DU) was set for The Equator and 330 DU for Oslo. For penetration of white Caucasian skin by UV rays total transmission coefficients was used (directly transmitted light plus that scattered forward).56
CMM incidence rates
The age-standardized CMM incidence rates among Caucasians in different countries (Fig. 4) (according to the world standard population (ASIR, W) were retrieved from the online database of International Agency for Research of Cancer (IARC)57,58 and published articles.59-61 Epidemiological data for Norway were obtained from the Cancer Registry of Norway. From “Association of Population-based Cancer Registries in Germany” were achieved data for Germany.62 Data for Australia and New Zealand were obtained from the Australian Institute of Health and Welfare,63 The New Zealand Cancer Registry,64 and published articles.65-68 Epidemiological data for Scotland are based on the Scottish Cancer Registry data.69
Vitamin D data
25(OH)D levels and vitamin D intake in different countries were retrieved from published articles.3,70-95
Statistical analysis
The data were analyzed using SigmaPlot 11.0 software from Systat Software, Inc. (Richmond, CA, USA).
Acknowledgments
The present work was supported by the South-Eastern Norway Regional Health Authority and by Oslo University Hospital. The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- UV
ultraviolet
- CMM
cutaneous malignant melanoma
- SCC
squamous cell carcinoma
- BCC
basal cell carcinoma
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/22941
References
- 1.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 2.Munakata N, Cornain S, Kanoko M, Mulyadi K, Lestari S, Wirohadidjojo W, et al. Biological monitoring of solar UV radiation at 17 sites in Asia, Europe and South America from 1999 to 2004. Photochem Photobiol. 2006;82:689–94. doi: 10.1562/2005-07-07-RA-602. [DOI] [PubMed] [Google Scholar]
- 3.Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol. 2007;103:620–5. doi: 10.1016/j.jsbmb.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher RP, Hill GB, Bajdik CD, Fincham S, Coldman AJ, McLean DI, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995;131:157–63. doi: 10.1001/archderm.1995.01690140041006. [DOI] [PubMed] [Google Scholar]
- 5.Rosso S, Zanetti R, Martinez C, Tormo MJ, Schraub S, Sancho-Garnier H, et al. The multicentre south European study ‘Helios’. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996;73:1447–54. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanavy HE, Gerstenblith MR. Ultraviolet radiation and melanoma. Semin Cutan Med Surg. 2011;30:222–8. doi: 10.1016/j.sder.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 7.DiGiovanna JJ, Kraemer KH. Shining a light on xeroderma pigmentosum. J Invest Dermatol. 2012;132:785–96. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autier P, Doré JF, Eggermont AM, Coebergh JW. Epidemiological evidence that UVA radiation is involved in the genesis of cutaneous melanoma. Curr Opin Oncol. 2011;23:189–96. doi: 10.1097/CCO.0b013e3283436e5d. [DOI] [PubMed] [Google Scholar]
- 9.Noonan FP, Zaidi MR, Wolnicka-Glubisz A, Anver MR, Bahn J, Wielgus A, et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat Commun. 2012;3:884. doi: 10.1038/ncomms1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang YM, Barrett JH, Bishop DT, Armstrong BK, Bataille V, Bergman W, et al. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int J Epidemiol. 2009;38:814–30. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant WB. Skin aging from ultraviolet irradiance and smoking reduces risk of melanoma: epidemiological evidence. Anticancer Res. 2008;28(6B):4003–8. [PubMed] [Google Scholar]
- 12.Volkovova K, Bilanicova D, Bartonova A, Letašiová S, Dusinska M. Associations between environmental factors and incidence of cutaneous melanoma. Review. Environ Health. 2012;11(Suppl 1):S12. doi: 10.1186/1476-069X-11-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field S, Newton-Bishop JA. Melanoma and vitamin D. Mol Oncol. 2011;5:197–214. doi: 10.1016/j.molonc.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson PE, Osborne JE, Pringle JH. Higher serum 25-hydroxy vitamin D3 levels at presentation are associated with improved survival from melanoma, but there is no evidence that later prevailing levels are protective. J Clin Oncol. 2010;28:e492–3, author reply 494-5. doi: 10.1200/JCO.2010.29.6095. [DOI] [PubMed] [Google Scholar]
- 15.Newton-Bishop JA, Beswick S, Randerson-Moor J, Chang YM, Affleck P, Elliott F, et al. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–44. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major JM, Kiruthu C, Weinstein SJ, Horst RL, Snyder K, Virtamo J, et al. Pre-diagnostic circulating vitamin D and risk of melanoma in men. PLoS ONE. 2012;7:e35112. doi: 10.1371/journal.pone.0035112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domingo DS, Baron ED. Melanoma and nonmelanoma skin cancers and the immune system. Adv Exp Med Biol. 2008;624:187–202. doi: 10.1007/978-0-387-77574-6_15. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Damian DL, Matthews YJ, Phan TA, Halliday GM. An action spectrum for ultraviolet radiation-induced immunosuppression in humans. Br J Dermatol. 2011;164:657–9. doi: 10.1111/j.1365-2133.2010.10161.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz T. Mechanisms of UV-induced immunosuppression. Keio J Med. 2005;54:165–71. doi: 10.2302/kjm.54.165. [DOI] [PubMed] [Google Scholar]
- 21.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–39. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leigh IM, Glover MT. Skin cancer and warts in immunosuppressed renal transplant recipients. Recent Results Cancer Res. 1995;139:69–86. doi: 10.1007/978-3-642-78771-3_6. [DOI] [PubMed] [Google Scholar]
- 23.Kubica AW, Brewer JD. Melanoma in immunosuppressed patients. Mayo Clin Proc. 2012;87:991–1003. doi: 10.1016/j.mayocp.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:253–61, quiz 262. doi: 10.1016/j.jaad.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 25.Wittgen HG, van Kempen LC. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007;17:400–9. doi: 10.1097/CMR.0b013e3282f1d312. [DOI] [PubMed] [Google Scholar]
- 26.Nishiura H, Kumagai J, Kashino G, Okada T, Tano K, Watanabe M. The bystander effect is a novel mechanism of UVA-induced melanogenesis. Photochem Photobiol. 2012;88:389–97. doi: 10.1111/j.1751-1097.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 27.Godar DE. UV doses worldwide. Photochem Photobiol. 2005;81:736–49. doi: 10.1562/2004-09-07-IR-308R.1. [DOI] [PubMed] [Google Scholar]
- 28.Garland CF, Garland FC, Gorham ED. Epidemiologic evidence for different roles of ultraviolet A and B radiation in melanoma mortality rates. Ann Epidemiol. 2003;13:395–404. doi: 10.1016/S1047-2797(02)00461-1. [DOI] [PubMed] [Google Scholar]
- 29.Diffey BL, Gibson CJ, Haylock R, McKinlay AF. Outdoor ultraviolet exposure of children and adolescents. Br J Dermatol. 1996;134:1030–4. doi: 10.1111/j.1365-2133.1996.tb07937.x. [DOI] [PubMed] [Google Scholar]
- 30.Setlow RB. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci USA. 1974;71:3363–6. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinlay AF, Diffey BL. A reference action spectrum for ultraviolet induced erythyema in human skin. CIE J. 1978;6:17–22. [Google Scholar]
- 32.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–3. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF, Vitamin D. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 34.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–6. doi: 10.1016/S0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 35.Moan J, Dahlback A, Setlow RB. Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation. Photochem Photobiol. 1999;70:243–7. doi: 10.1111/j.1751-1097.1999.tb07995.x. [DOI] [PubMed] [Google Scholar]
- 36.Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27:58–67. doi: 10.1111/j.1600-0781.2011.00557.x. [DOI] [PubMed] [Google Scholar]
- 37.Boniol M, Doré JF, Autier P, Smans M, Boyle P. Descriptive epidemiology of skin cancer incidence and mortality. In: Ringborg U, Brandberg Y, Breitbart EW, Greinert R, eds. Skin Cancer Prevention. NY: Informa Health Care, 2007:203-23. [Google Scholar]
- 38.Moan J, Porojnicu AC, Dahlback A. Ultraviolet radiation and malignant melanoma. Adv Exp Med Biol. 2008;624:104–16. doi: 10.1007/978-0-387-77574-6_9. [DOI] [PubMed] [Google Scholar]
- 39.Crombie IK. Variation of melanoma incidence with latitude in North America and Europe. Br J Cancer. 1979;40:774–81. doi: 10.1038/bjc.1979.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of california cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246–52. doi: 10.1023/A:1018432632528. [DOI] [PubMed] [Google Scholar]
- 41.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 42.Signorello LB, Williams SM, Zheng W, Smith JR, Long J, Cai Q, et al. Blood vitamin d levels in relation to genetic estimation of African ancestry. Cancer Epidemiol Biomarkers Prev. 2010;19:2325–31. doi: 10.1158/1055-9965.EPI-10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juzeniene A, Setlow R, Porojnicu A, Steindal AH, Moan J. Development of different human skin colors: a review highlighting photobiological and photobiophysical aspects. J Photochem Photobiol B. 2009;96:93–100. doi: 10.1016/j.jphotobiol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol. 2010;130:546–53. doi: 10.1038/jid.2009.323. [DOI] [PubMed] [Google Scholar]
- 45.Johansson L, Solvoll K.National survey for nutrition and physical activity. 45 1999 [Google Scholar]
- 46.Ackefors H. A regional survey of the aquaculture sector in Eastern and Northwestern Europe. 1-54. 1989. Rome: FAO. [Google Scholar]
- 47.Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121:297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Kurtzke JF. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–92. doi: 10.1079/BJN20051544. [DOI] [PubMed] [Google Scholar]
- 50.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–21. doi: 10.1210/jc.86.3.1212. [DOI] [PubMed] [Google Scholar]
- 51.Agredano YZ, Chan JL, Kimball RC, Kimball AB. Accessibility to air travel correlates strongly with increasing melanoma incidence. Melanoma Res. 2006;16:77–81. doi: 10.1097/01.cmr.0000195696.50390.23. [DOI] [PubMed] [Google Scholar]
- 52.Moan J, Dahlback A. The relationship between skin cancers, solar radiation and ozone depletion. Br J Cancer. 1992;65:916–21. doi: 10.1038/bjc.1992.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamnes K, Tsay SC, Wiscombe W, Jayaweera K. Numerically stable algorithm for discrete-ordinate-method radiative transfer in multiple scattering and emitting layered media. Appl Opt. 1988;27:2502–9. doi: 10.1364/AO.27.002502. [DOI] [PubMed] [Google Scholar]
- 54.Engelsen O, Kylling A. Fast simulation tool for ultraviolet radiation at the Earth's surface. Opt Eng. 2005;44:1–7. [Google Scholar]
- 55.Dahlback A, Stamnes K. A new spherical model for computing the radiation field available for photolysis and heating at twilight. Planet Space Sci. 1991;39:671–83. doi: 10.1016/0032-0633(91)90061-E. [DOI] [Google Scholar]
- 56.Everett MA, Yeargers E, Sayre RM, Olson RL. Penetration of epidermis by ultraviolet rays. Photochem Photobiol. 1966;5:533–42. doi: 10.1111/j.1751-1097.1966.tb09843.x. [DOI] [PubMed] [Google Scholar]
- 57.Engholm G, Ferlay J, Christensen N, Johannesen TB, Klint Å, Køtlum JE, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 5.1. 2012. Available: http://www.ancr.nu/ [accessed 9 May 2012].
- 58.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Available: http://globocan.iarc.fr/ [accessed 1 April 2012].
- 59.Steding-Jessen M, Birch-Johansen F, Jensen A, Schüz J, Kjær SK, Dalton SO. Socioeconomic status and non-melanoma skin cancer: a nationwide cohort study of incidence and survival in Denmark. Cancer Epidemiol. 2010;34:689–95. doi: 10.1016/j.canep.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Birch-Johansen F, Jensen A, Mortensen L, Olesen AB, Kjær SK. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978-2007: Rapid incidence increase among young Danish women. Int J Cancer. 2010;127:2190–8. doi: 10.1002/ijc.25411. [DOI] [PubMed] [Google Scholar]
- 61.Dal H, Boldemann C, Lindelöf B. Trends during a half century in relative squamous cell carcinoma distribution by body site in the Swedish population: support for accumulated sun exposure as the main risk factor. J Dermatol. 2008;35:55–62. doi: 10.1111/j.1346-8138.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 62.Association of Population-based Cancer Registries in Germany. Available: http://www. gekid. de/ [accessed 1 April 2012].
- 63.AIHW. (Australian Institute of Health and Welfare) 2007. ACIM (Australian Cancer Incidence and Mortality) Books. Available: http://www.aihw.gov.au/ [accessed 1 April 2012].
- 64.New Zealand Ministry of Health. 2011. Cancer: New registrations and deaths 2007-1997. Available: http://www.health.govt.nz/ [accessed 4 April 2012].
- 65.Richmond-Sinclair NM, Pandeya N, Ware RS, Neale RE, Williams GM, van der Pols JC, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323–8. doi: 10.1038/jid.2008.234. [DOI] [PubMed] [Google Scholar]
- 66.Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust. 2006;184:6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 67.Richardson A, Fletcher L, Sneyd M, Cox B, Reeder AI. The incidence and thickness of cutaneous malignant melanoma in New Zealand 1994-2004. N Z Med J. 2008;121:18–26. [PubMed] [Google Scholar]
- 68.Brougham ND, Dennett ER, Tan ST. Changing incidence of non-melanoma skin cancer in New Zealand. ANZ J Surg. 2011;81:633–6. doi: 10.1111/j.1445-2197.2010.05583.x. [DOI] [PubMed] [Google Scholar]
- 69.The Scottish Cancer Registry. Available: http://www.isdscotlandarchive.scot.nhs.uk/ [accessed 3 March 2012].
- 70.Birgisdottir BE, Brantsaeter AL, Kvalem HE, Knutsen HK, Haugen M, Alexander J, et al. Fish liver and seagull eggs, vitamin D-rich foods with a shadow: results from the Norwegian Fish and Game Study. Mol Nutr Food Res. 2012;56:388–98. doi: 10.1002/mnfr.201100395. [DOI] [PubMed] [Google Scholar]
- 71.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sørensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(Suppl 1):S97–103. doi: 10.1079/BJN2001345. [DOI] [PubMed] [Google Scholar]
- 72.Brustad M, Alsaker E, Engelsen O, Aksnes L, Lund E. Vitamin D status of middle-aged women at 65-71 degrees N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 2004;7:327–35. doi: 10.1079/PHN2003536. [DOI] [PubMed] [Google Scholar]
- 73.Burgaz A, Akesson A, Michaëlsson K, Wolk A. 25-hydroxyvitamin D accumulation during summer in elderly women at latitude 60 degrees N. J Intern Med. 2009;266:476–83. doi: 10.1111/j.1365-2796.2009.02125.x. [DOI] [PubMed] [Google Scholar]
- 74.Christensen MH, Lien EA, Hustad S, Almås B. Seasonal and age-related differences in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone in patients from Western Norway. Scand J Clin Lab Invest. 2010;70:281–6. doi: 10.3109/00365511003797172. [DOI] [PubMed] [Google Scholar]
- 75.Hanwell HE, Vieth R, Cole DE, Scillitani A, Modoni S, Frusciante V, et al. Sun exposure questionnaire predicts circulating 25-hydroxyvitamin D concentrations in Caucasian hospital workers in southern Italy. J Steroid Biochem Mol Biol. 2010;121:334–7. doi: 10.1016/j.jsbmb.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 76.Kull M, Jr., Kallikorm R, Tamm A, Lember M. Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European country. BMC Public Health. 2009;9:22. doi: 10.1186/1471-2458-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamberg-Allardt C. Vitamin D intake, sunlight exposure and 25-hydroxyvitamin D levels in the elderly during one year. Ann Nutr Metab. 1984;28:144–50. doi: 10.1159/000176796. [DOI] [PubMed] [Google Scholar]
- 78.Lamberg-Allardt CJ, Outila TA, Kärkkainen MU, Rita HJ, Valsta LM. Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J Bone Miner Res. 2001;16:2066–73. doi: 10.1359/jbmr.2001.16.11.2066. [DOI] [PubMed] [Google Scholar]
- 79.Macdonald HM, Mavroeidi A, Fraser WD, Darling AL, Black AJ, Aucott L, et al. Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: a major cause for concern? Osteoporos Int. 2011;22:2461–72. doi: 10.1007/s00198-010-1467-z. [DOI] [PubMed] [Google Scholar]
- 80.Mavroeidi A, O’Neill F, Lee PA, Darling AL, Fraser WD, Berry JL, et al. Seasonal 25-hydroxyvitamin D changes in British postmenopausal women at 57 degrees N and 51 degrees N: a longitudinal study. J Steroid Biochem Mol Biol. 2010;121:459–61. doi: 10.1016/j.jsbmb.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 81.Moan J, Lagunova Z, Cicarma E, Aksnes L, Dahlback A, Grant WB, et al. Sunbeds as vitamin D sources. Photochem Photobiol. 2009;85:1474–9. doi: 10.1111/j.1751-1097.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 82.Mowé M, Bøhmer T, Haug E. Vitamin D-mangel hos eldre sykehusinnlagte og hjemmeboende i Oslo. Tidsskr Nor Laegeforen. 1998;118:3929–31. [PubMed] [Google Scholar]
- 83.Nakamura K, Nashimoto M, Yamamoto M. Summer/winter differences in the serum 25-hydroxyvitamin D3 and parathyroid hormone levels of Japanese women. Int J Biometeorol. 2000;44:186–9. doi: 10.1007/s004840000067. [DOI] [PubMed] [Google Scholar]
- 84.Nanri A, Foo LH, Nakamura K, Hori A, Poudel-Tandukar K, Matsushita Y, et al. Serum 25-hydroxyvitamin d concentrations and season-specific correlates in Japanese adults. J Epidemiol. 2011;21:346–53. doi: 10.2188/jea.JE20100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nowson CA, Margerison C. Vitamin D intake and vitamin D status of Australians. Med J Aust. 2002;177:149–52. doi: 10.5694/j.1326-5377.2002.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 86.Overgaard K, Nilas L, Johansen JS, Christiansen C. Lack of seasonal variation in bone mass and biochemical estimates of bone turnover. Bone. 1988;9:285–8. doi: 10.1016/8756-3282(88)90011-7. [DOI] [PubMed] [Google Scholar]
- 87.Reusch J, Ackermann H, Badenhoop K. Cyclic changes of vitamin D and PTH are primarily regulated by solar radiation: 5-year analysis of a German (50 degrees N) population. Horm Metab Res. 2009;41:402–7. doi: 10.1055/s-0028-1128131. [DOI] [PubMed] [Google Scholar]
- 88.Scharla SH. Prevalence of subclinical vitamin D deficiency in different European countries. Osteoporos Int. 1998;8(Suppl 2):S7–12. doi: 10.1007/PL00022726. [DOI] [PubMed] [Google Scholar]
- 89.Thieden E, Philipsen PA, Heydenreich J, Wulf HC. Vitamin D level in summer and winter related to measured UVR exposure and behavior. Photochem Photobiol. 2009;85:1480–4. doi: 10.1111/j.1751-1097.2009.00612.x. [DOI] [PubMed] [Google Scholar]
- 90.Thuesen B, Husemoen L, Fenger M, Jakobsen J, Schwarz P, Toft U, et al. Determinants of vitamin D status in a general population of Danish adults. Bone. 2012;50:605–10. doi: 10.1016/j.bone.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 91.Tjellesen L, Christiansen C. Vitamin D metabolites in normal subjects during one year. A longitudinal study. Scand J Clin Lab Invest. 1983;43:85–9. doi: 10.3109/00365518309168226. [DOI] [PubMed] [Google Scholar]
- 92.van der Mei IA, Ponsonby AL, Engelsen O, Pasco JA, McGrath JJ, Eyles DW, et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007;115:1132–9. doi: 10.1289/ehp.9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Taylor BV, Kilpatrick T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254:581–90. doi: 10.1007/s00415-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 94.Vik T, Try K, Strømme JH. The vitamin D status of man at 70 degrees north. Scand J Clin Lab Invest. 1980;40:227–32. doi: 10.3109/00365518009095571. [DOI] [PubMed] [Google Scholar]
- 95.Zgaga L, Theodoratou E, Farrington SM, Agakov F, Tenesa A, Walker M, et al. Diet, environmental factors, and lifestyle underlie the high prevalence of vitamin D deficiency in healthy adults in Scotland, and supplementation reduces the proportion that are severely deficient. J Nutr. 2011;141:1535–42. doi: 10.3945/jn.111.140012. [DOI] [PMC free article] [PubMed] [Google Scholar]