Abstract
Autoimmunity has been associated with vitamin D deficiency and resistance, with gene polymorphisms related to vitamin D metabolism frequently described in affected patients. High doses of vitamin D3 may conceivably compensate for inherited resistance to its biological effects. This study aimed to assess the efficacy and safety of prolonged high-dose vitamin D3 treatment of patients with psoriasis and vitiligo. Nine patients with psoriasis and 16 patients with vitiligo received vitamin D3 35,000 IU once daily for six months in association with a low-calcium diet (avoiding dairy products and calcium-enriched foods like oat, rice or soya “milk”) and hydration (minimum 2.5 L daily). All psoriasis patients were scored according to “Psoriasis Area and Severity Index” (PASI) at baseline and after treatment. Evaluation of clinical response of vitiligo patients required a quartile grading scale. All patients presented low vitamin D status (serum 25(OH)D3 ≤ 30 ng/mL) at baseline. After treatment 25(OH)D3 levels significantly increased (from 14.9 ± 7.4 to 106.3 ± 31.9 ng/mL and from 18.4 ± 8.9 to 132.5 ± 37.0 ng/mL) and PTH levels significantly decreased (from 57.8 ± 16.7 to 28.9 ± 8.2 pg/mL and from 55.3 ± 25.0 to 25.4 ± 10.7 pg/mL) in patients with psoriasis and vitiligo respectively. PTH and 25(OH)D3 serum concentrations correlated inversely. The PASI score significantly improved in all nine patients with psoriasis. Fourteen of 16 patients with vitiligo had 25–75% repigmentation. Serum urea, creatinine and calcium (total and ionized) did not change and urinary calcium excretion increased within the normal range. High-dose vitamin D3 therapy may be effective and safe for vitiligo and psoriasis patients.
Keywords: vitiligo, psoriasis, vitamin D, 25(OH)D3, high dose, calcium, treatment, toxicity, autoimmunity
Introduction
The steroid hormone known as the active form of vitamin D (“calcitriol,” “1,25-dihydroxy vitamin D” or “1,25(OH)2D”) generates a wide range of biological responses. The human genome possesses 2,776 positions occupied by the VDR; about 10% of the human genes are, therefore, directly and/or indirectly responsive to vitamin D.1 Similarly, diverse human cells (including bone, colon, breast, prostate, skin, muscle, blood vessel, brain and immune cells) express the enzyme 25-hydroxyvitamin D-1 α-hydroxylase (CYP27B1), indicating that the extra-renal intracrine and paracrine 1,25(OH)2D3 synthesis may critically affect the activities of many tissues and organs.2-4 Accordingly, cumulative data have associated low vitamin D status (as assessed by measuring “25-hydroxyvitamin D3” or “25(OH)D3”—the main circulating form of vitamin D) not only to rickets and osteoporosis, but also to an increasing number of prevalent health disorders including autoimmune, infectious and cardiovascular diseases, hypertension, diabetes and deadly cancers.2,5-7
Contrasting with the pathophysiological importance of 1,25(OH)2D3, vitamin D deficiency is a poorly recognized worldwide epidemic among both children and adults.2 Regular and moderate sun exposure (the only significant natural source of vitamin D for most subjects) is currently uncommon due to indoor lifestyle, sun avoidance and indiscriminate sunscreen use.7 Even though there is mounting evidence indicating that physiologic doses should be closer to those achieved through a few minutes of daily skin exposure to sunlight,2 the RDA upgraded (from 200 to 600 IU per day) by the Institute of Medicine in 20108 remains much lower than that,7 and are lower than the daily doses required to correct vitamin D deficiency in most subjects.9
Higher daily doses may be particularly critical for controlling the activity of autoimmune disorders. Cumulative data over the past 30 y have established the regulatory effects of vitamin D on both innate and adaptive immune responses,10,11 and circulating levels of 25(OH)D3 inversely relate to the activity of autoimmune disease.12-19 Intracrine and paracrine actions of 1,25(OH)2D3 synthesized within immune cells may control inappropriate activation of interleukin-17-producing cells20-25—the Th17 aberrant response, which may play a major pathogenic role in multiple inflammatory and autoimmune disorders.26 Cumulative evidence also indicates that vitamin D promotes—by both direct and indirect mechanisms—regulatory T cells (Treg) that inhibit a variety of inappropriate (both innate and adaptive) immune responses underlying autoimmune disease.21,27
Estimations of daily requirements of vitamin D3 for patients with autoimmune disorders should take account of the functional consequences of genetic polymorphisms related to vitamin D metabolism. For instance, the polymorphic changes of the enzyme CYP27B1 associated with autoimmunity28,29 predictably originates a relative resistance to vitamin D, i.e., a higher level of circulating 25(OH)D3 required to achieve normal 1,25(OH)2D3 concentrations within the immune cells. While the in vivo Km of the normal enzyme exceeds the physiologic range of 25(OH)D3 concentrations,30,31 a polymorphic variant may have a higher Km (decreased affinity for substrate) and/or a lower Vmax, requiring higher concentrations of substrate to achieve a physiologic rate of product formation (Fig. 1). Frequent genetic polymorphisms and wide (ten to a hundred fold) variability in enzyme expression of the cytochrome P450 superfamily of enzymes (CYPs) are common and may considerably modify enzyme activity, causing large interindividual variability in the rate of product formation.32-35 Therefore, a subject bearing a CYP27B1 polymorphism may be prone to develop autoimmunity for sustaining concentrations of 1,25(OH)2D3 within the immune cells that are insufficient for full intracrine and paracrine tolerogenic effects of this powerful steroid molecule. High doses of vitamin D3 leading to supraphysiologic range of circulating 25(OH)D3 may compensate for this genetic-related status of relative vitamin D deficiency, thereby establishing tolerance to auto-antigens.
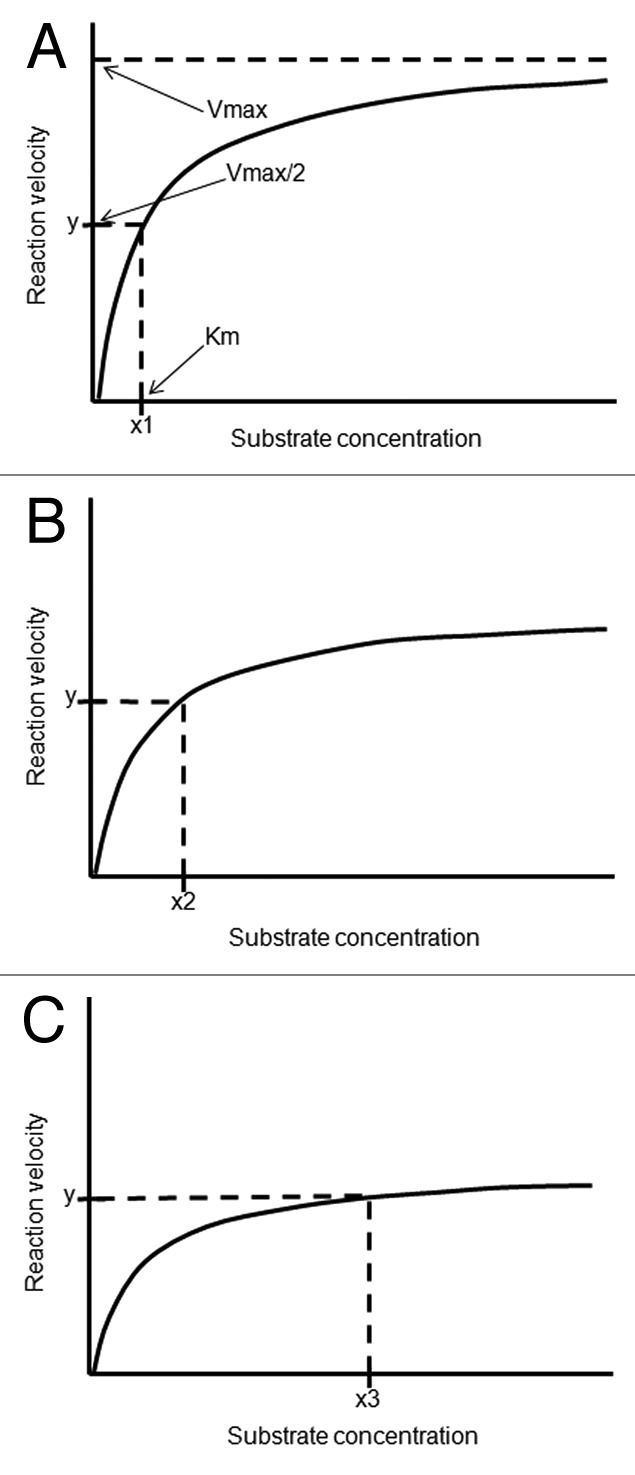
Figure 1. Enzyme kinetics of three hypothetical polymorphic variants of 25-hydroxyvitamin D-1 α-hydroxylase (CYP27B1), “A,” “B” and “C.” Some variants predictably exhibit decreased affinity for the substrate (increased Km) and/or decreased Vmax, requiring requiring a supra-physiologic substrate concentration to achieve a physiologic rate of substrate transformation. The polymorphic variants “B” and “C” require higher plasma concentrations of 25-hydroxyvitamin D (“x2” and “x3” respectively) to achieve the rate “y” of 1,25-dihydroxy vitamin D synthesis than the Km (“x1”) of the enzyme variant “A.” Compared with “A” or “B,” the polymorphic variant “C” requires a much higher plasma concentration of 25-hydroxyvitamin D to compensate for a reduced intracellular activity and achieve physiologic concentrations of 1,25-dihydroxy vitamin D within the immune cells.
Doses ranging up to 40,000 IU/day of vitamin D3 are probably safe for healthy individuals,36,37 and enzyme polymorphisms affecting vitamin D metabolism may conceivably increase tolerability in patients with autoimmune disorders. This study aimed to assess the therapeutic efficacy and safety of a daily dose of 35,000 IU of vitamin D3 administered with a low-calcium diet for 6 mo to patients with psoriasis and vitiligo.
Results
Subjects
The physical characteristics of the 25 patients who participated in this study are shown in Table 1.
Table 1. Characteristics of study subjects.
| z | Vitiligo | Psoriasis |
|---|---|---|
| Age (y ± SD) | 49.2 ± 11.9 | 45.3 ± 12.2 |
|
Sex (N) M F |
3 13 |
4 5 |
| BMI ± SD (Kg/m2) | 26.6 ± 4.4 | 27.0 ± 4.0 |
|
Fritzpatrick skin phototype I II III IV V VI |
(N) 0 0 11 4 1 0 |
(N) 1 2 3 3 0 0 |
BMI, body mass index; y, years; N, number of subjects; SD, standard deviation
Laboratory parameters
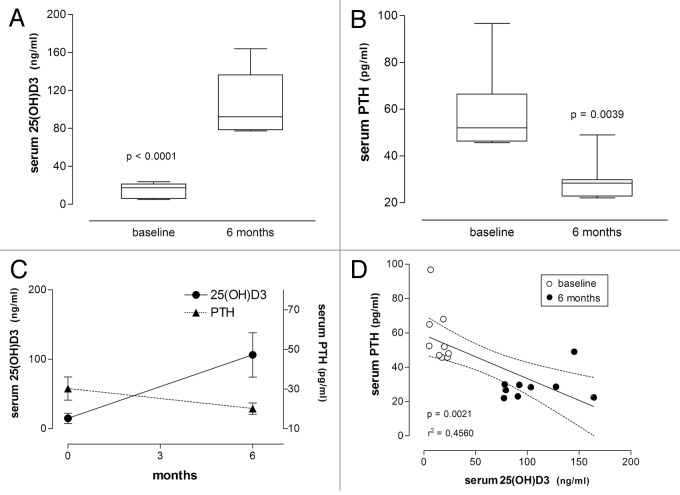
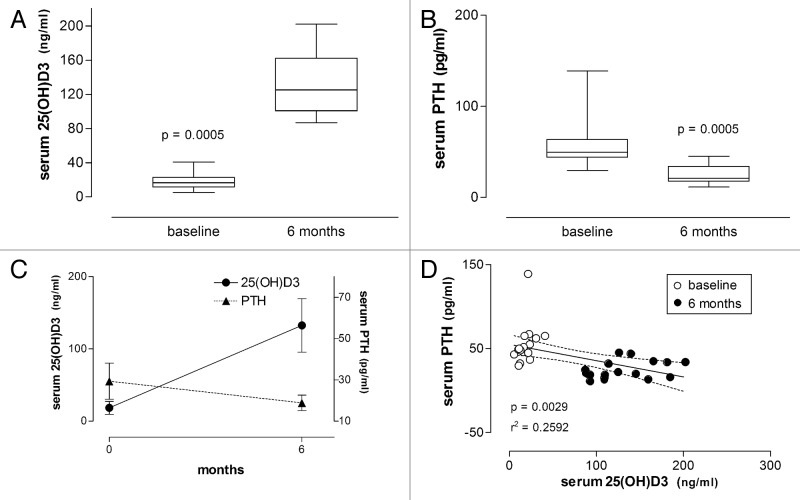
As shown in Table 2 and 3 and Figures 2 and 5, all patients presented low baseline levels of 25(OH)D3 (≤ 30ng/mL or ≤ 75 nmol/L), with a mean of 14.9 ± 7.4 ng/mL in the psoriasis group and a mean of 18.4 ± 8.9 ng/mL in vitiligo patients. After 6 mo of treatment with vitamin D3 (35,000 IU per day), 25(OH)D3 levels increased significantly, to 106.3 ± 31.9 ng/mL in the psoriasis group (p < 0.0001, Wilcoxon signed rank test) and to 132.5 ± 37.0 ng/mL in vitiligo patients (p < 0.0005, Wilcoxon signed rank test).
Table 2. Serum concentrations of 25(OH)D3, PTH, total calcium, ionized calcium, urea and creatinine and clinical status of nine patients with psoriasis at baseline and after treatment with vitamin D (35,000 IU per day for 6 mo).
| Parameter (normal range) |
Baseline (mean ± SD) |
6 mo (mean ± SD) |
p value * |
|---|---|---|---|
|
25(OH)D3 (30–100 ng/mL) |
14.9 ± 7.4 | 106.3 ± 31.9 | < 0.0001 |
|
PTH (8–74 pg/mL) |
57.8 ± 16.7 | 28.9 ± 8.2 | 0.0039 |
|
Serum Calcium (8.4 – 11 mg/dL) |
9.7 ± 0.7 | 9.4 ± 0.7 | 0.1641 |
|
Ionized Calcium (1.10 – 1.40 mmol/L) |
1.4 ± 0.3 | 1.2 ± 0.2 | 0.1641 |
|
Urinary Calcium (100–300mg/24h) |
123.6 ± 60.0 | 226.8 ± 41.6 | 0.0039 |
|
Urea (< 50 mg/dL) |
35.8 ± 8.3 | 28.9 ± 9.8 | 0.1289 |
|
Creatinine (0.7 – 1.5 mg/dL) |
0.9 ± 0.2 | 0.8 ± 0.2 | 0.6406 |
Wilcoxon signed rank test
Table 3. Serum concentrations of 25(OH)D3, PTH, total calcium, ionized calcium, urea and creatinine and clinical status of 16 patients with vitiligo at baseline and after treatment with vitamin D (35,000 IU per day for 6 mo).
| Parameter (normal range) |
Baseline (mean ± SD) |
6 mo (mean ± SD) |
p value * |
|---|---|---|---|
|
25(OH)D3 (30–100 ng/mL) |
18.4 ± 8.9 | 132.5 ± 37.0 | 0.0005 |
|
PTH (8 – 74 pg/mL) |
55.3 ± 25.0 | 25.4 ± 10.7 | 0.0005 |
|
Serum Calcium (8.4 – 11.0 mg/dL) |
9.2 ± 0.3 | 9.2 ± 0.3 | 0.7615 |
|
Ionized Calcium (1.10 – 1.40 mmol/L) |
1.2 ± 0.2 | 1.2 ± 0.1 | 0.5417 |
|
Urinary Calcium (100–300mg/24h) |
158.3 ± 73.6 | 230.1 ± 81.4 | 0.0239 |
|
Urea (< 50 mg/dL) |
35.5 ± 7.2 | 33.9 ± 9.9 | 0.7148 |
|
Creatinine (0.7 – 1.5 mg/dL) |
0.9 ± 0.2 | 0.9 ± 0.2 | 0.5016 |
Wilcoxon signed rank test
Figure 2. Serum concentrations of 25(OH)D3 and PTH in patients with psoriasis before and after treatment with vitamin D (35,000 IU per day for 6 mo). (A) Box plot showing serum concentrations of 25(OH)D3 before and after treatment. (B) Same for the respective serum PTH concentrations. Significance level (Wilcoxon signed rank test) indicated in (A) and (B). (C) Serum concentrations of 25(OH)D3 and PTH respectively increased and decreased during treatment. (D) Linear regression of serum PTH on serum 25(OH)D3 concentrations is significant (significance level and r2 value are shown; dashed lines represent the 95% CIs for the linear regression line; baseline and 6-mo values are respectively shown as empty and filled circles).
Figure 5. Serum concentrations of 25(OH)D3 and PTH in patients with vitiligo before and after treatment with vitamin D (35,000 IU per day for 6 mo). (A) Box plot showing serum concentrations of 25(OH)D3 before and after treatment. (B) Same for the respective serum PTH concentrations. Significance level (Wilcoxon signed rank test) indicated in (A) and (B). (C) Serum concentrations of 25(OH)D3 and PTH respectively increased and decreased during treatment. (D) Linear regression of serum PTH on serum 25(OH)D3 concentrations is significant (significance level and r2 value are shown; dashed lines represent the 95% CIs for the linear regression line; baseline and 6-mo values are respectively shown as empty and filled circles).
Baseline concentrations of PTH were 57.8 ± 16.7 pg/mL in the psoriasis group and 55.3 ± 25.0 pg/mL in vitiligo patients. After 6 mo of treatment with vitamin D3 PTH levels decreased significantly to 28.9 ± 8.2 pg/mL in the psoriasis group (p = 0.0039, Wilcoxon signed rank test) and to 25.4 ± 10.7 pg/mL in vitiligo patients (p = 0.0005, Wilcoxon signed rank test) (Tables 2 and 3, Fig. 2 and 5).
Statistical analysis revealed a negative correlation between 25(OH)D3 and PTH levels (r = -0.6753, p = 0.0153 in the psoriasis group; r = -0.5091, p = 0.0015 in vitiligo patients, Pearson’s correlation). Linear regression also revealed negative correlation between these two parameters using data collected at baseline and 6-mo outcome (Fig. 2 and 5, r2 and p values indicated).
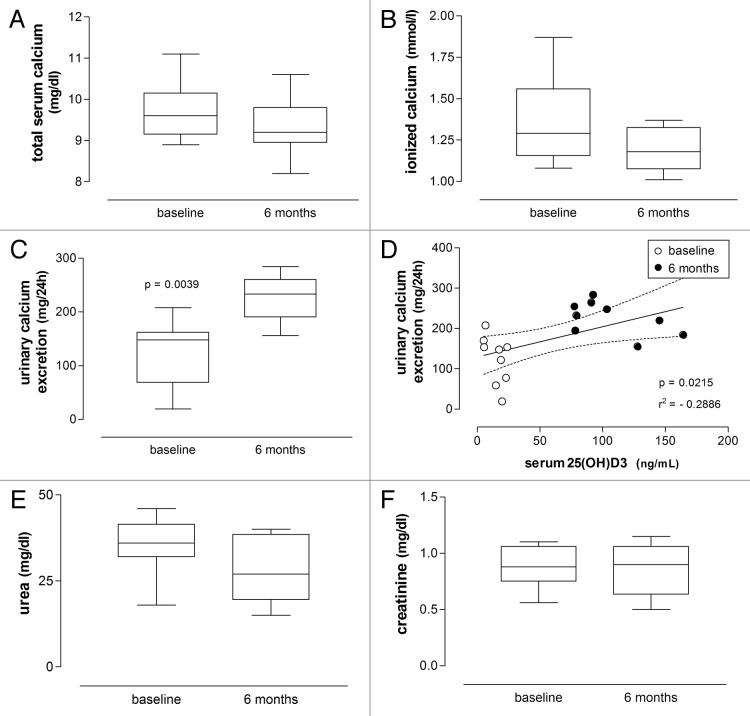
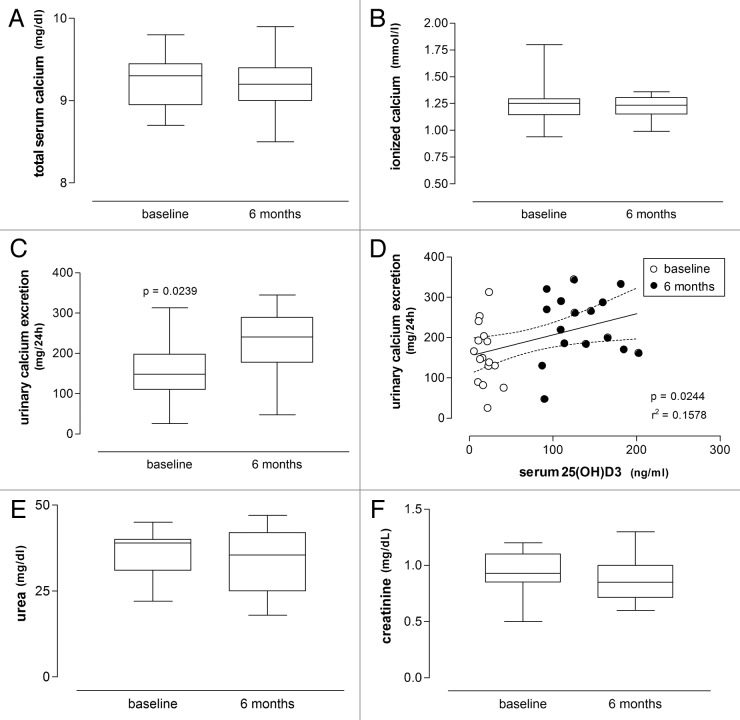
The concentrations of (total and ionized) serum calcium, urea and creatinine did not differ significantly from baseline values after 6 mo of high-dose vitamin D3 treatment (Tables 2 and 3; Fig. 3 and 6; Wilcoxon signed rank test).
Figure 3.(A), (B), (C), (D), (F) Box plots respectively showing concentrations of serum calcium, 24-h urinary calcium excretion, serum urea and serum creatinine in patients with psoriasis before and after treatment with vitamin D (35,000 IU per day for 6 mo), with a significant pre- and post-treatment difference only found for 24-h urinary calcium excretion (Wilcoxon signed rank test). (D) Linear regression of 24-h urinary calcium excretion on serum 25(OH)D3 concentrations is significant (significance level and r2 value are shown; dashed lines represent the 95% CIs for the linear regression line; baseline and 6-mo values are respectively shown as empty and filled circles).
Figure 6.(A), (B), (C), (D), (F) Box plots respectively showing concentrations of serum calcium, 24-h urinary calcium excretion, serum urea and serum creatinine in patients with vitiligo before and after treatment with vitamin D (35,000 IU per day for 6 mo), with a significant pre- and post-treatment difference only found for 24-h urinary calcium excretion (Wilcoxon signed rank test). (D) Linear regression of 24-h urinary calcium excretion on serum 25(OH)D3 concentrations is significant (significance level and r2 value are shown; dashed lines represent the 95% CIs for the linear regression line; baseline and 6-mo values are respectively shown as empty and filled circles).
Urinary calcium excretion significantly increased at 6 mo of vitamin D3 treatment in both groups (from 123.6 ± 60.0 mg/24h to 226.8 ± 41.6 mg/24h in psoriasis group (p = 0.0039, Wilcoxon signed rank test) and from 158.3 ± 73.6 mg/24h to 230.1 ± to 81.4 mg/24h in vitiligo group (p = 0.0239, Wilcoxon signed rank test), but remained within the normal range (Tables 2 and 3; Fig. 3 and 6). Statistical analysis revealed a positive correlation between urinary calcium excretion and 25(OH)D3 levels in both groups of patients (r = 0.5372, p = 0.0108 in the psoriasis group; r = 0.3972, p = 0.0122 in vitiligo patients, Pearson’s correlation). Linear regression also revealed a negative correlation between these parameters (Fig. 3 and 6, r2 and p values indicated).
Clinical outcome
The clinical condition of all patients with psoriasis (n = 9) significantly improved during the treatment (Fig. 4 and 8; p = 0.0023, Wilcoxon signed rank test). Statistical analysis revealed a negative correlation between the PASI score and 25(OH)D3 levels (r = -0.5614, p = 0.0011, Pearson’s correlation). Linear regression also demonstrated correlation between these parameters (Fig. 4, r2 and p values indicated).
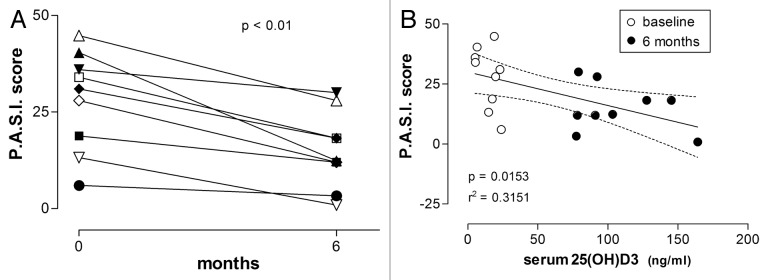
Figure 4. PASI scores in patients with psoriasis before and after treatment with vitamin D (35,000 IU per day for 6 mo). (A) Individual temporal profiles of the P.A.S.I. score during the treatment showing clinical improvement in all patients. (B) Linear regression of P.A.S.I. on 25(OH)D3 concentration is significant (significance level and r2 value are shown; dashed lines represent the 95% CIs for the linear regression line; baseline and 6-mo values are respectively shown as empty and filled circles).
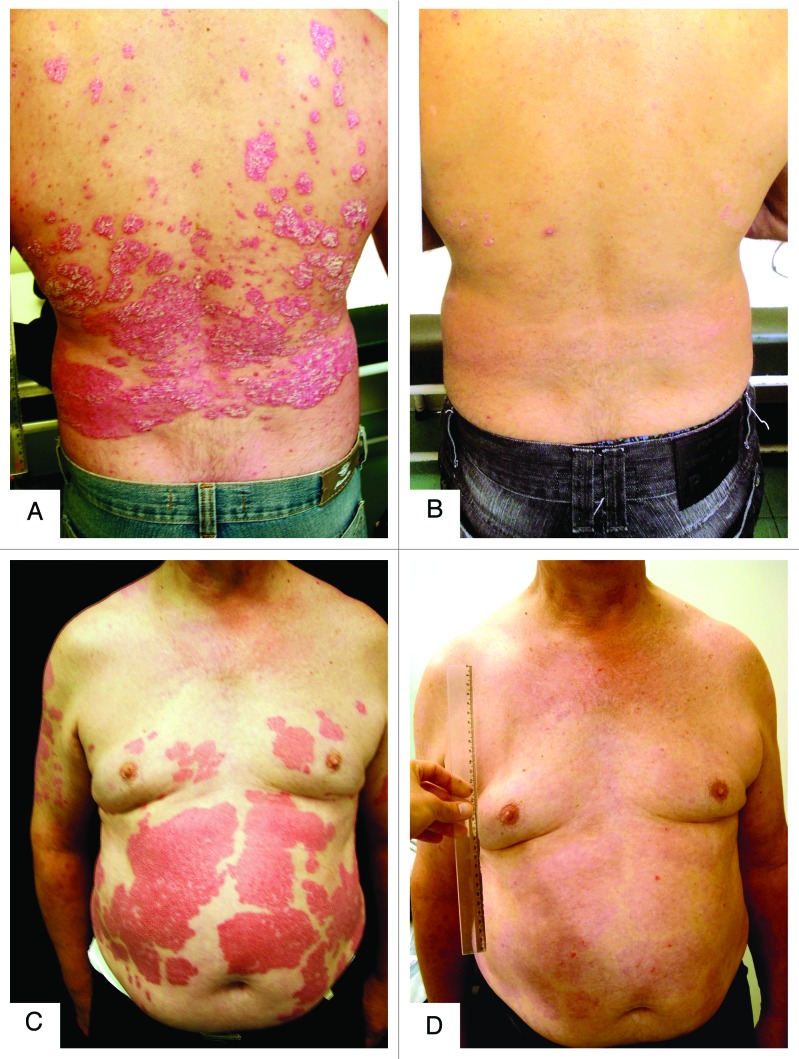
Figure 8. Photographs of two male patients with psoriasis before (A and C) and after (B and D) six months of treatment with vitamin D (35,000 IU per day). (A and B) A 59 y-old patient with BMI of 24.8 presenting a PASI score of 31 before treatment and achieving score of 18.2 after six months of treatment; his serum concentration of 25(OH)D3 was 22.8 ng/mL at baseline, reaching 127.5 ng/mL after 6 mo of treatment. (C and D) 60 y-old patient with BMI of 33.6 presenting a PASI score of 40.4 at baseline, achieving score of 12.4 after six months; his serum concentration of 25(OH)D3 was 5.6 ng/mL, reaching 103.2 ng/mL after six months of treatment.
Two out of 16 vitiligo patients showed no repigmentation of the affected areas; four patients showed 1‒25% repigmentation, five patients showed 26–50% repigmentation, five patients showed 51‒75% repigmentation and none showed more than 75% repigmentation of the affected areas (Fig. 7 and 9).
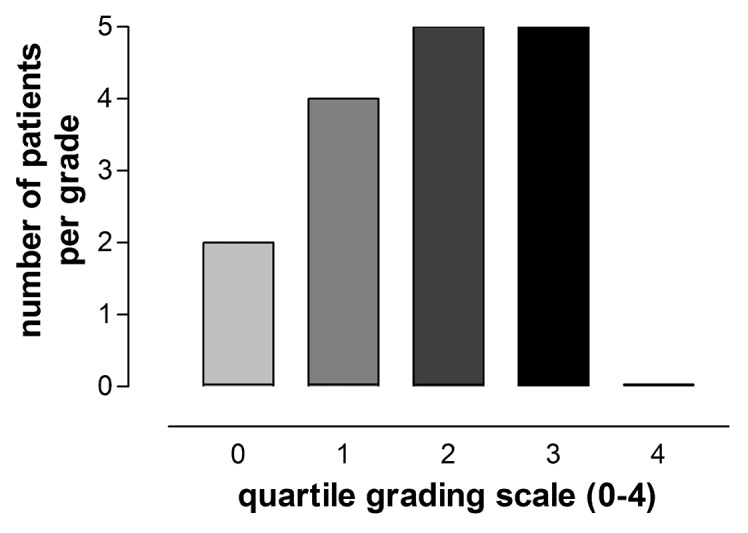
Figure 7. Clinical response of 16 patients with vitiligo after treatment with vitamin D (35,000 IU per day for six months). Two patients showed no repigmentation (quartile 0); four patients showed 1‒25% repigmentation (quartile 1), five patients showed 26–50% repigmentation (quartile 2), five patients showed 51‒75% repigmentation (quartile 3) and none showed more than 75% repigmentation (quartile 4).
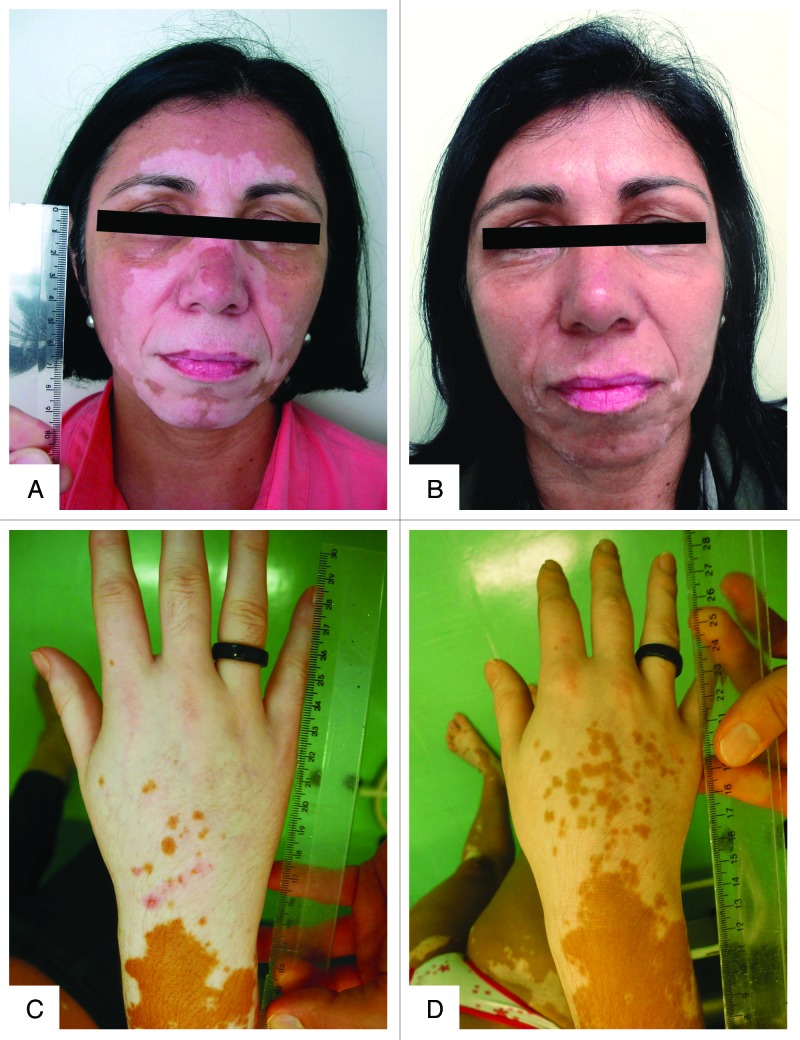
Figure 9. Photographs of two female patients with vitiligo before (A and C) and after (B and D) six months of treatment with vitamin D (35,000 IU per day). (A and B) A 50 y-old patient with BMI of 28.3 achieving between 51 and 75% of repigmentation (quartile 3) after six months of treatment; her serum concentration of 25(OH)D3 was 12.5 ng/mL, at baseline, reaching 92.4 ng/mL after 6 mo of treatment. (C and D) A 36 y-old patient with BMI of 22.7 achieving between 1 and 25% of repigmentation (quartile 1) after six months of treatment; her serum concentration of 25(OH)D3 was 12.0 ng/mL, at baseline, reaching 92.5 ng/mL after 6 mo of treatment.
Discussion
This is an open-label study where all patients with psoriasis or vitiligo received oral treatment with 35,000 IU of vitamin D per day for six months associated with preventive measures (partial dietary calcium restriction and a daily hydration of at least 2.5 L). The treatment provided benefit to 9 out of 9 patients with psoriasis and to 14 out of 16 patients vitiligo.
A placebo-controlled approach was avoided for ethical reasons. Cumulative data have implicated vitamin D deficiency in the pathophysiology of vitiligo38,39 and psoriasis.39-41 Vitamin D deficiency (deficient levels of a potent steroid hormone that modulates hundreds of human genes) also increases the risk of developing or aggravating a myriad of serious health disorders,1,5-7 including cancer42 or death from cardiovascular events.43 Administering placebo to vitamin D-deficient patients for the sake of randomization may not be ethically acceptable.44,45 Potential research participants would not take the chances of being assigned to the control group if informed of the association of vitamin D levels and disease activity. On the other hand, intentionally omitting essential information to facilitate consent to placebo treatment may imply disregard for patient autonomy46 and violation of the principle of beneficence on the part of the physician.47 Conversely, restoring the physiologic intracellular levels of a potent steroid hormone (by increasing the availability of substrate to its polymorphic activating enzyme) should be regarded as good medical practice.
Laboratory or clinical signs of toxicity (hypercalcemia, hypercalciuria or kidney dysfunction) were not observed in any of the 25 participants, including a patient with vitiligo who reached a serum concentration of 25(OH)D3 of 202.2 ng/mL (504.49 mmol/L). Considering that 25(OH)D3 has a half-life of 15 d48 those high concentrations of 25(OH)D3 were achieved at two months and sustained for the last four months of treatment without side effects. To the best of our knowledge, this is the highest dose of vitamin D3 administered therapeutically to patients with autoimmune disorders on a regular daily basis for several months. These findings are consistent with the viewpoint that serum 25(OH)D concentrations lower than 750 nmol/L (300 ng/mL) are unlikely to cause toxicity.49 Enhancing both innate and adaptive immunity50 is a significant advantage of high-dose vitamin D3 therapy for autoimmune disorders over the current treatment with immunosuppressive drugs.
High-dose vitamin D3 supplementation to patients with autoimmune disorders is conceivably advantageous over 1,25(OH)2D3 treatment concerning lower calcemic effects and more efficient control of autoimmunity. Administration of 1,25(OH)2D3 or 1,25(OH)2D3 analogs overpasses critical regulatory mechanisms related to the calciotropic effects of vitamin D by directly stimulating intestinal VDR and calcium absorption. In contrast, administration of vitamin D3 increases circulating concentrations of 25(OH)D3, which then faces different renal and extra-renal control mechanisms for expression and activity of the enzyme 1 α-hydroxylase.41 Renal 1 α-hydroxylase undergoes feedback downregulation (associated with 24-hydroxylase upregulation) by 1,25(OH)2D3 and 1,25(OH)2D3 production is also under strict control of other calcium- and phosphate-regulating hormones (PTH and FGF23).51 Conversely, the availability of 25(OH)D3 to immune cells (the production of which is not tightly controlled by the liver)37 may be the primary determinant of the amount of 1,25(OH)2D3 produced for intracrine and paracrine effects at sites of inflammation,50 where the local expression of cytokines may instead facilitate the conversion of 25(OH)D3 by inducing the expression of 1 α-hydroxylase.51,52
Hypervitaminosis D is associated with upregulation of intestinal VDR and increased absorption of dietary calcium.53 A low calcium diet protects against vitamin D toxicity, not only by reducing the availability of calcium for gastrointestinal absorption, but also by facilitating vitamin D inactivation at sites related to calcium metabolism.37 Reduced intestinal calcium by dietary restriction of milk, dairy products and calcium-enriched foods (like oat, rice or soya “milk”) has contributed to minimize the calciotropic effects of high daily doses of vitamin D3 in the current study. Increased gastrointestinal absorption of calcium is partly responsible for the hypercalcemia in vitamin D intoxication and a low dietary calcium intake gradually reduces serum calcium in such patients.54 Preliminary data (not shown) obtained from patients treated with progressively higher doses of vitamin D3 up to 35,000 IU daily showed that the adoption of such easily understandable dietary recommendations reduced urinary calcium from borderline elevated levels (around 400 mg or 10 mmol per day, with serum calcium sustained at the upper normal range) to values within the normal range without changing vitamin D daily dose. Further restriction of dietary calcium (by also avoiding foods prepared with milk, such as smashed potatoes, bread, cakes and cookies) dropped urinary calcium to levels below the lower limit of the normal range adopted by the local laboratory (100 mg or 2.5 mmol per day) while serum calcium remained around the lower limit (8.6 mg/dL or 2.15 mmol/L).
Taken together, those data suggest that partial dietary calcium restriction efficiently prevents hypercalcemia and hypercalciuria by controlling the gastrointestinal availability of calcium under the calciotropic effect of the treatment paradigm employed in patients with psoriasis and vitiligo in this study. Potentiation of osteoclastic activity in bone by pharmacologically high levels of 25(OH)D3 competing for binding to VDR is a recognized mechanism of hypercalcemia in vitamin D intoxication.37,55 The high daily doses of vitamin D3 administered for 6 mo in this study, however, do not seem to have significantly enhanced osteoclastic activity in patients with psoriasis and vitiligo, since partial restriction of dietary calcium efficiently prevented hypercalcemia and hypercalciuria.
Compared with this study, longer-term follow up research should look for yet unknown potential side-effects related to high-dose vitamin D3 treatment of a larger sample of subjects with autoimmune disorders. Future studies should also aim to develop a method for setting the particular daily dose that compensates for genetic polymorphisms and other distinctive features, providing the highest therapeutic effect against autoimmunity without causing side effects like hypercalcemia / hypercalciuria due to enhanced osteoclastic activity. The individual dose needed to achieve optimal biological effects of vitamin D might be related not only to a single but also to multiple gene polymorphisms affecting vitamin D hydroxylases, DBP and/or VDR,48 as well as to body weight, body fat, age, skin color, season, latitude and sunning habits;56 optimal therapeutic effect in autoimmunity, in addition, should conceivably require pharmacological doses, much higher than those necessary for preventive measures. All interventions using high-dose vitamin D3 so far applied to treat autoimmune disorders, including the current one, have used arbitrarily selected doses, without taking into account the potentially large variability of resistance to the benefits and side-effects of vitamin D among the patients participating in the clinical trial. Such uniform research approach precludes the development of a rational protocol for establishing the optimal daily dose of vitamin D for each patient, which would improve both safety and therapeutic effectiveness in the clinical setting. Ultimately resulting from all these multiple variables interacting in the same organism, the serum concentration of PTH may be the best biological indicator for the individual setting of the optimal therapeutic dose of vitamin D3 for the treatment of autoimmune disorders. An isolated measurement of serum PTH concentration does not provide a reliable ancillary parameter to estimate the dose of vitamin D3 required for optimal control of autoimmunity. The enhancing effect of PTH on renal 1,25(OH)2D3 production declines with age,57 so that young patients may sustain efficient gastrointestinal absorption of calcium without raising PTH to the upper normal range or above in contrast to mature or old adults. Conversely, the magnitude of the decrease in serum PTH concentration, from the basal value to the level achieved after a period of treatment, may provide a reasonable estimation of how much the initial daily dose of vitamin D3 should be increased to reduce serum PTH levels to the lower reference range. A period of at least 2 mo should be allowed between the two serum PTH measurements, considering that 25(OH)D3 has a half-life of 15 d.48 Using the PTH level as an ancillary index of therapeutic response requires a diet only partially restricted in calcium (like the one described in this study) since excessive restriction of calcium intake would maintain increased bone resorption to preserve normocalcemia, thereby limiting or preventing vitamin D3-induced PTH drop. Avoiding excessively high doses of vitamin D3 capable of suppressing PTH and periodically measuring bone density, on the other hand, may conceivably indicate that 25(OH)D3 probably have not reached circulating concentrations capable of increasing osteoclastic activity. According to this view, the PTH reductions observed with sustained normal serum and urinary calcium after 6 mo of treatment either in patients with psoriasis (from 57.8 ± 16.7 pg/mL to 28.9 ± 8.2 pg/mL) or vitiligo (from 55.3 ± 25.0 pg/mL to 25.4 ± 10.7 pg/mL) did not reach the lower limit of the reference range (8–74 pg/mL), suggesting that they could obtain additional therapeutic benefit from even higher doses of vitamin D3 than those used in the current study without compromising the safety of treatment.
In summary, the present study suggests that, at least for patients with autoimmune disorders like vitiligo and psoriasis, a daily dose of 35,000 IU of vitamin D is a safe and effective therapeutic approach for reducing disease activity. Dietary calcium limited by avoiding dairy products and calcium-enriched foods – like oat, rice or soya “milk” and minimum hydration (2.5 L daily) ensures safety. Further research should investigate whether the magnitude of PTH reduction may be a valuable marker for the individual setting of maximal daily doses of vitamin D in autoimmune diseases.
Materials and Methods
Subjects
The Ethics Committee of the Hospital Heliópolis (Sao Paulo Brazil) and the Ethics Committee of the Universidade Federal de São Paulo approved this study protocol (registered under the CONEP number 0356/08). All patients gave spoken and written informed consent before inclusion in the study.This study included nine psoriasis patients and 16 vitiligo patients from two outpatient clinics in São Paulo, Brazil—the Dermatology Clinic of the Hospital Heliópolis and the Internal Medicine Clinic of Universidade Federal de São Paulo. Subjects were men or women over 18 y of age. The study protocol excluded (1) patients under treatment with thiazide diuretics or lithium to avoid drug-vitamin D interactions on calcium metabolism;58 (2) patients taking psoriasis- or vitiligo-inducing drugs;59,60 (3) patients with cognitive impairment; (d) patients with renal insufficiency and (4) patients with elevated calcium/calciuria or primary hyperparathyroidism.
Design
This is an open-label study where all patients received oral treatment with 35,000 IU of vitamin D (1.75 ml of a 20,000 IU/mL sunflower oil solution) per day. Whenever present, standard treatments were not changed. Following instructions, all patients excluded milk and dairy products (as well as calcium-fortified foods like soy, oat or rice milk) from their diet and ingested at least 2.5 L of fluid per day to prevent, respectively, excessive absorption of intestinal calcium and concentrated urinary calcium. Calcium supplementation was not allowed. The onset of symptoms suggestive of hypercalcemia (increased thirst, constipation, nausea, vomiting) would require performing extra laboratory tests. Regular medical follow-up appointments ensured compliance with dietary restriction and fluid intake.
After clinical diagnosis, all patients had clinical scoring, photo documentation of affected skin areas, laboratory tests performed before, at three and six mo of treatment. Laboratory tests included basal vitamin D status (25(OH)D3 levels), total and ionized serum calcium, total 24-h urinary calcium excretion, parathormone (PTH), serum urea and creatinine levels. By collecting all data from March to October 2011 and from March to October 2012, the authors attempted to minimize seasonal variations in 25(OH)D3 levels and related effects.
Analytical methods
25(OH)D3 levels were determined by HPLC (LC 20AT) with UV detection at 265 nm (UV/VIS model SPD-20A). 25(OH)D3 standard, solvents and other reagents were of analytical grade (all purchased from Sigma-Aldrich).
An internal standard (IS) of 25(OH)D3 compensated the reduced HPLC sensitivity to detect very low levels of 25(OH)D3. Stock solution of IS was prepared in methanol at 100 µg/mL and kept at -20°C in amber tube. The HPLC conditions were LiChrosorb RP-18 5 um, 12,5 × 0,44 mm column (Merck), 40°C, acetonitrile-methanol-water (90:4:6, v/v) as the mobile phase, 3.2 min for retention time and flow rate of 1 ml/min. The calibration curve was linear over the concentration range of 9.8 ng/mL to 1250.0 ng/mL (r2 = 0,9994).
Serum samples were immediately stored at -80°C until analysis. The following steps were performed under light protection. Each serum sample of 100 µL was thawed, added to 70 µL of methanol-2-propanol mixture (80:20) and then vortex-mixed for 30 sec. Subsequently, 400 µL of hexane was added, vortex-mixed again for 60 sec and centrifuged under 2500 g for 10 min at 10°C. A volume of 100 µL of the resulting organic phase was submitted to evaporation in a Concentrator model 5301 for 10 min at room temperature. The resulting dried residue was then resuspended in 50µL of methanol plus 50µL of IS (working solution of 200 ng/mL) and vortex-mixed for 2 min prior to injection into HPLC. The final result was obtained by deducting the amount corresponding to added IS from the ultimate figure released from HPLC. The IS method appropriately correlated with assessments of standard 25(OH)D3 concentrations and serum samples.
PTH was essayed by chemiluminescence, while other laboratory tests (total and ionized serum calcium, urea and creatinine) were analyzed by standard methods at the Central Laboratory of the University Hospital (Federal University of São Paulo).
Clinical parameters of vitiligo and psoriasis
The PASI score used for assessment of severity and extent of psoriasis61 enabled the evaluation of the response to treatment. Pre- and post-treatment photographic documentation of the affected skin under Wood's light (UVA 351 nm) and a quartile grading scale62 enabled the evaluation of the clinical response to the treatment in patients with vitiligo. The percentage of the affected skin surface that achieved repigmentation at 6 mo of treatment yielded five categories of clinical response. These included “0” for no repigmentation; “1” for 1 to 25% repigmentation, “2” for 26 to 50% repigmentation, “3” for 51 to 75% repigmentation and “4” for repigmentation above 75% of the total baseline area of affected skin.
Statistics
Results are expressed in tables as mean ± standard deviation. The Wilcoxon assigned rank test was employed to assess the effect of treatment with vitamin D (50,000 IU daily for six months). The relationship between 25(OH)D3 and continuous variables (PTH and urinary calcium excretion) was evaluated using a Pearson correlation coefficient. Curves relating serum 25(OH)D3 and PTH or urinary calcium levels were fitted using linear regression models. Statistical analyses and graphical presentation were performed using the GraphPad Prism (Version 3.02, San Diego, USA).
Acknowledgments
C.G.C., R.S.G., D.C.F. and A.C.L. designed the study; D.C.F. and R.S.C. conducted the research; M.G. provided and prepared vitamin D supplement, R.S.C., L.C.M.N., L.D.T., J.J.S. and F.S. performed the analytical method for 25(OH)D3 measurements; N.F.N. and Y.J. performed statistical analysis; C.G.C., R.S.C., D.C.F., L.C.M.N., D.J.N. and A.C.L. analyzed data; C.G.C., R.S.C. and D.C.F. wrote the paper. All authors have approved the manuscript. This project was supported by grants from CAPES, Institute for Investigation and Treatment of Autoimmune Disorders (I.I.T.A.) and UNIMED do ABC, Brazil. DCF was a CAPES fellow.
Glossary
Abbreviations:
- BMI
body mass index
- CIs
confidence intervals’ CYP, cytochrome P450 superfamily
- CYP27B1
25-hydroxyvitamin D-1 alpha-hydroxylase
- DBP
vitamin D-binding protein
- VDR
vitamin D receptor
- FGF23
fibroblast growth factor 23
- HPLC
high performance liquid chromatography
- IOM
Institute of Medicine
- IS
internal standard
- Km
Michaelis–Menten constant
- PASI
psoriasis area and severity index
- PTH
parathormone
- RDA
recommended dietary allowance
- Treg
regulatory T cells
- VDR
vitamin D receptor
- Vmax
maximal velocity of enzymatic reaction
- UV
ultraviolet
- UVA
ultraviolet A
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/24808
References
- 1.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–60. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29:361–8. doi: 10.1016/j.mam.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012;523:95–102. doi: 10.1016/j.abb.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315–25. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Evidence-based D-bate on health benefits of vitamin D revisited. Dermatoendocrinol. 2012;4:183–90. doi: 10.4161/derm.20015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IOM (Institute of Medicine). Dietary reference intakes for calcium and vitamin D. Committee to Review Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press. 2011. [PubMed] [Google Scholar]
- 9.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 10.Lagishetty V, Liu NQ, Hewison M. Vitamin D metabolism and innate immunity. Mol Cell Endocrinol. 2011;347:97–105. doi: 10.1016/j.mce.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterling KA, Eftekhari P, Girndt M, Kimmel PL, Raj DS. The immunoregulatory function of vitamin D: implications in chronic kidney disease. Nat Rev Nephrol. 2012;8:403–12. doi: 10.1038/nrneph.2012.93. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56:2143–9. doi: 10.1002/art.22722. [DOI] [PubMed] [Google Scholar]
- 13.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. 2008;14:1220–4. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 14.Amital H, Szekanecz Z, Szücs G, Dankó K, Nagy E, Csépány T, et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis. 2010;69:1155–7. doi: 10.1136/ard.2009.120329. [DOI] [PubMed] [Google Scholar]
- 15.Haque UJ, Bartlett SJ. Relationships among vitamin D, disease activity, pain and disability in rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:745–7. [PubMed] [Google Scholar]
- 16.Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol. 2010;67:618–24. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- 17.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308–16. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 18.Weinstock-Guttman B, Zivadinov R, Qu J, Cookfair D, Duan X, Bang E, et al. Vitamin D metabolites are associated with clinical and MRI outcomes in multiple sclerosis patients. J Neurol Neurosurg Psychiatry. 2011;82:189–95. doi: 10.1136/jnnp.2010.227942. [DOI] [PubMed] [Google Scholar]
- 19.Runia TF, Hop WC, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79:261–6. doi: 10.1212/WNL.0b013e31825fdec7. [DOI] [PubMed] [Google Scholar]
- 20.Correale J, Ysrraelit MC, Gaitán MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–60. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 21.Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23:519–28. doi: 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 23.Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285:38751–5. doi: 10.1074/jbc.C110.185777. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegyi Z, Zwicker S, Bureik D, Peric M, Koglin S, Batycka-Baran A, et al. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 “alarmins” psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J Invest Dermatol. 2012;132:1416–24. doi: 10.1038/jid.2011.486. [DOI] [PubMed] [Google Scholar]
- 26.Waite JC, Skokos D. Th17 response and inflammatory autoimmune diseases. Int J Inflam. 2012;2012:819467. doi: 10.1155/2012/819467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11:29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 28.Pani MA, Regulla K, Segni M, Krause M, Hofmann S, Hufner M, et al. Vitamin D 1alpha-hydroxylase (CYP1alpha) polymorphism in Graves’ disease, Hashimoto’s thyroiditis and type 1 diabetes mellitus. Eur J Endocrinol. 2002;146:777–81. doi: 10.1530/eje.0.1460777. [DOI] [PubMed] [Google Scholar]
- 29.Sundqvist E, Bäärnhielm M, Alfredsson L, Hillert J, Olsson T, Kockum I. Confirmation of association between multiple sclerosis and CYP27B1. Eur J Hum Genet. 2010;18:1349–52. doi: 10.1038/ejhg.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vieth R, Fraser D. Kinetic behavior of 25-hydroxyvitamin D-1-hydroxylase and -24-hydroxylase in rat kidney mitochondria. J Biol Chem. 1979;254:12455–60. [PubMed] [Google Scholar]
- 31.Vieth R, McCarten K, Norwich KH. Role of 25-hydroxyvitamin D3 dose in determining rat 1,25-dihydroxyvitamin D3 production. Am J Physiol. 1990;258:E780–9. doi: 10.1152/ajpendo.1990.258.5.E780. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Antona C, Donato MT, Pareja E, Gómez-Lechón MJ, Castell JV. Cytochrome P-450 mRNA expression in human liver and its relationship with enzyme activity. Arch Biochem Biophys. 2001;393:308–15. doi: 10.1006/abbi.2001.2499. [DOI] [PubMed] [Google Scholar]
- 33.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–62. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 34.Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
- 36.Vieth R. Toxicity of Vitamin D. In: Holick MF, ed. Vitamin D. New York, NY: Humana Press, 2010:603-612. [Google Scholar]
- 37.Cusano NE, Thys-Jacobs S, Bilezikian JP. Hypercalcemia due to vitamin D toxicity. In: Feldman D, Pike JW, Adams JS, ed. Vitamin D. San Diego, CA: Academic Press, 2011:1381–1402. [Google Scholar]
- 38.Silverberg JI, Silverberg AI, Malka E, Silverberg NB. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937–41. doi: 10.1016/j.jaad.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Benrashid M, Moyers K, Mohty M, Savani BN. Vitamin D deficiency, autoimmunity, and graft-versus-host-disease risk: Implication for preventive therapy. Exp Hematol. 2012;40:263–7. doi: 10.1016/j.exphem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Gisondi P, Rossini M, Di Cesare A, Idolazzi L, Farina S, Beltrami G, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505–10. doi: 10.1111/j.1365-2133.2011.10699.x. [DOI] [PubMed] [Google Scholar]
- 41.Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, Ruiz JC, Arias-Santiago S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931–8. doi: 10.1016/j.jaad.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 42.Byers SW, Rowlands T, Beildeck M, Bong YS. Mechanism of action of vitamin D and the vitamin D receptor in colorectal cancer prevention and treatment. Rev Endocr Metab Disord. 2012;13:31–8. doi: 10.1007/s11154-011-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muscogiuri G, Sorice GP, Ajjan R, Mezza T, Pilz S, Prioletta A, et al. Can vitamin D deficiency cause diabetes and cardiovascular diseases? Present evidence and future perspectives. Nutr Metab Cardiovasc Dis. 2012;22:81–7. doi: 10.1016/j.numecd.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Rothman KJ, Michels KB. The continuing unethical use of placebo controls. N Engl J Med. 1994;331:394–8. doi: 10.1056/NEJM199408113310611. [DOI] [PubMed] [Google Scholar]
- 45.Kavanaugh A. Ethical and practical issues in conducting clinical trials in psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii46–8. doi: 10.1136/ard.2004.030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resnik DB. Ethical dilemmas in communicating medical information to the public. Health Policy. 2001;55:129–49. doi: 10.1016/S0168-8510(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 47.Simon R. Are placebo-controlled clinical trials ethical or needed when alternative treatment exists? Ann Intern Med. 2000;133:474–5. doi: 10.7326/0003-4819-133-6-200009190-00017. [DOI] [PubMed] [Google Scholar]
- 48.Zhang F, Moayyeri A, Spector TD. Genetic influences on circulating vitamin D level: a review. Curr Cardiovasc Risk Rep. 2012;6:549–55. doi: 10.1007/s12170-012-0278-5. [DOI] [Google Scholar]
- 49.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–6S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 50.Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012;71:50–61. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 51.Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011;25:531–41. doi: 10.1016/j.beem.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Bikle DD. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep. 2009;7:58–63. doi: 10.1007/s11914-009-0011-6. [DOI] [PubMed] [Google Scholar]
- 53.Beckman MJ, Horst RL, Reinhardt TA, Beitz DC. Up-regulation of the intestinal 1,25-dihydroxyvitamin D receptor during hypervitaminosis D: a comparison between vitamin D2 and vitamin D3. Biochem Biophys Res Commun. 1990;169:910–5. doi: 10.1016/0006-291X(90)91979-3. [DOI] [PubMed] [Google Scholar]
- 54.Buckle RM, Gamlen TR, Pullen IM. Vitamin D intoxication treated with porcine calcitonin. Br Med J. 1972;3:205–7. doi: 10.1136/bmj.3.5820.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selby PL, Davies M, Marks JS, Mawer EB. Vitamin D intoxication causes hypercalcaemia by increased bone resorption which responds to pamidronate. Clin Endocrinol (Oxf) 1995;43:531–6. doi: 10.1111/j.1365-2265.1995.tb02916.x. [DOI] [PubMed] [Google Scholar]
- 56.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13:6–20. [PubMed] [Google Scholar]
- 57.Tsai KS, Wahner HW, Offord KP, Melton LJ, 3rd, Kumar R, Riggs BL. Effect of aging on vitamin D stores and bone density in women. Calcif Tissue Int. 1987;40:241–3. doi: 10.1007/BF02555255. [DOI] [PubMed] [Google Scholar]
- 58.Hariri A, Mount DB, Rastegar A. Disorders of calcium, phosphate, and magnesium metabolism. In: Mount DB, Sayegh MH, Singh AK, ed. Core Concepts in the Disorders of Fluid, Electrolytes and Acid-Base Balance. New York, NY: Springer US, 2013:103-146. [Google Scholar]
- 59.Duhra P, Foulds IS. Persistent vitiligo induced by diphencyprone. Br J Dermatol. 1990;123:415–6. doi: 10.1111/j.1365-2133.1990.tb06306.x. [DOI] [PubMed] [Google Scholar]
- 60.Basavaraj KH, Ashok NM, Rashmi R, Praveen TK. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351–61. doi: 10.1111/j.1365-4632.2010.04570.x. [DOI] [PubMed] [Google Scholar]
- 61.Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 1978;157:238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 62.Shin J, Lee JS, Hann SK, Oh SH. Combination treatment by 10 600 nm ablative fractional carbon dioxide laser and narrowband ultraviolet B in refractory nonsegmental vitiligo: a prospective, randomized half-body comparative study. Br J Dermatol. 2012;166:658–61. doi: 10.1111/j.1365-2133.2011.10723.x. [DOI] [PubMed] [Google Scholar]