Abstract
Vitamin D is the sunshine vitamin that has been produced on this earth for more than 500 million years. During exposure to sunlight 7-dehydrocholesterol in the skin absorbs UV B radiation and is converted to previtamin D3 which in turn isomerizes into vitamin D3. Previtamin D3 and vitamin D3 also absorb UV B radiation and are converted into a variety of photoproducts some of which have unique biologic properties. Sun induced vitamin D synthesis is greatly influenced by season, time of day, latitude, altitude, air pollution, skin pigmentation, sunscreen use, passing through glass and plastic, and aging. Vitamin D is metabolized sequentially in the liver and kidneys into 25-hydroxyvitamin D which is a major circulating form and 1,25-dihydroxyvitamin D which is the biologically active form respectively. 1,25-dihydroxyvitamin D plays an important role in regulating calcium and phosphate metabolism for maintenance of metabolic functions and for skeletal health. Most cells and organs in the body have a vitamin D receptor and many cells and organs are able to produce 1,25-dihydroxyvitamin D. As a result 1,25-dihydroxyvitamin D influences a large number of biologic pathways which may help explain association studies relating vitamin D deficiency and living at higher latitudes with increased risk for many chronic diseases including autoimmune diseases, some cancers, cardiovascular disease, infectious disease, schizophrenia and type 2 diabetes. A three-part strategy of increasing food fortification programs with vitamin D, sensible sun exposure recommendations and encouraging ingestion of a vitamin D supplement when needed should be implemented to prevent global vitamin D deficiency and its negative health consequences.
Keywords: 25-hydroxyvitamin D, UV lamp, UV radiation, UVB, autoimmune, cancer, latitude, rickets, sunlight, vitamin D
Prehistoric Perspective
Life forms began to evolve in the oceans over 1 billion years ago. They took advantage of sunlight and used it as an energy source to generate carbohydrates. Curiously some of the earliest phytoplankton including Emiliania huxleyi (which is a coccolithophore, i.e., has a calcium carbonate exoskeleton) which has existed unchanged in the Sargasso Sea (Atlantic Ocean) for more than 500 million years when exposed to sunlight not only photosynthesized glucose but also produced vitamin D2 (Fig. 1).1 This phytoplankton produces a large amount of ergosterol that when exposed to sunlight absorbs ultraviolet B (UVB) radiation and undergoes a photolysis reaction to form previtamin D2. Once formed this thermodynamically unstable isomer is transformed into vitamin D2. Similarly yeast and fungi also contain high amounts of ergosterol and when exposed to sunlight produce vitamin D2.1-4
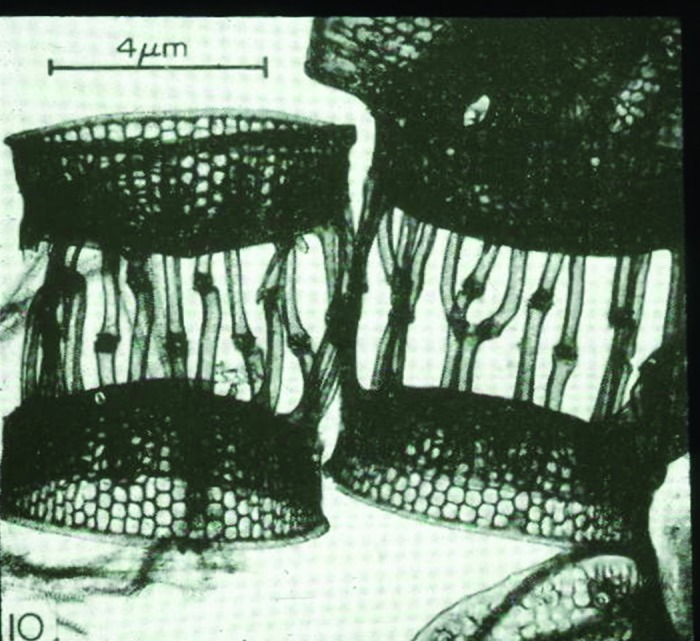
Figure 1. Microscopic picture of Emiliana huxleyi, which is a cocolithophore i.e., has a calcium carbonate exoskeleton. Holick, copyright 2013. Reproduced with permission.
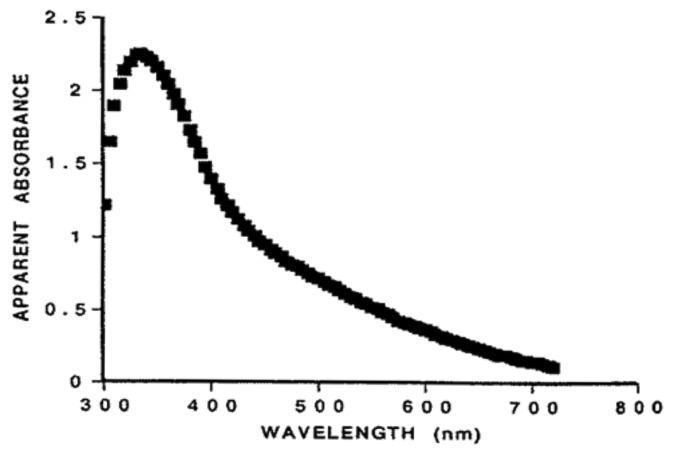
Although the functions of ergosterol and vitamin D2 are unknown in these primitive unicellular photosynthesizing factories there are at least three possible functions that have been proposed. Ergosterol can efficiently absorb UVB radiation, which would make it an ideal natural sunscreen to protect UVB sensitive macromolecules in the organism including its proteins, RNA and DNA (Fig. 2).1,2
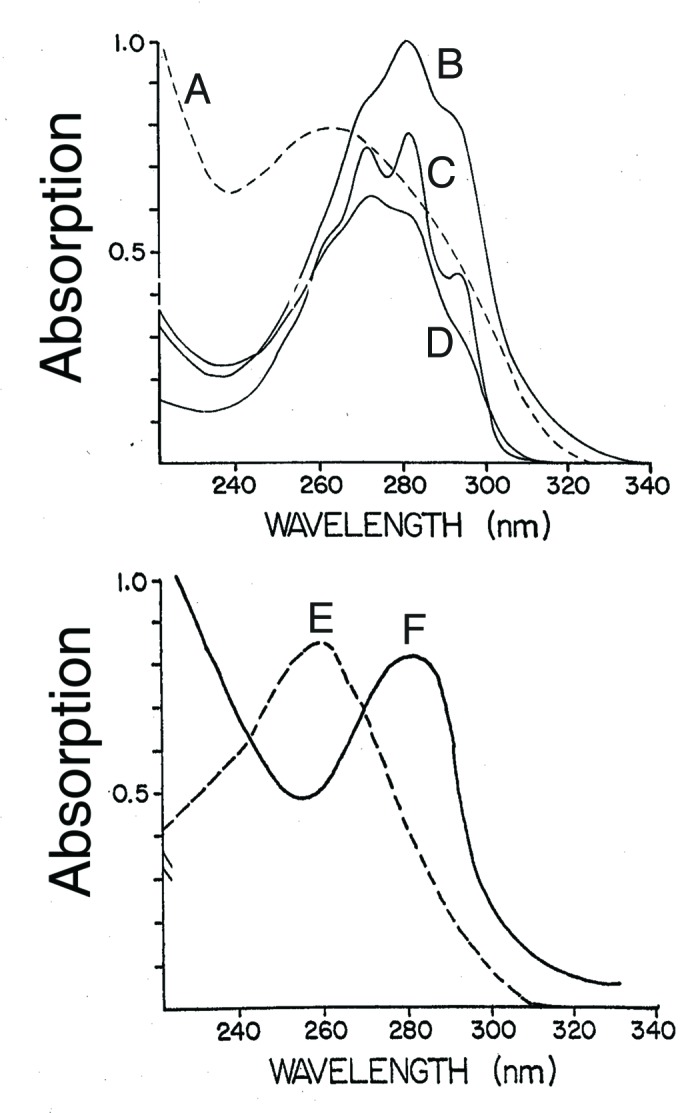
Figure 2. UV absorption spectra for (A) previtamin D3, (B) tachysterol, (C) provitamin D3 (7-dehydrocholesterol), (D) lumisterol, (E) DNA, and (F) albumin. Holick, copyright 2007. Reproduced with permission.
After absorbing UVB radiation previtamin D2 is produced. Its absorption spectrum with a wavelength maximum at 260 nm overlaps the UV absorption spectrum for both DNA and RNA and thus would be able to protect DNA and RNA from photodamage (Fig. 2).2 When previtamin D2 is exposed to UVB radiation it is converted to tachysterol2 which has a UV absorption spectrum with a wavelength maximum at 282 nm which overlaps the UV absorption spectrum for amino acids in proteins that have conjugated double bonds including tryptophan and tyrosine (Fig. 1 and 2).1-4 Thus early in evolution as organisms began to utilize solar energy for photosynthesis they needed a sun protection factor to absorb solar UVB radiation to minimize damage to UVB sensitive molecules. Ergosterol, previtamin D2, and its photoproducts could have acted as an ideal UVB sunscreen since they could absorb UVB radiation and dissipate its energy by the rearrangement of the double bonds.2
The amount of previtamin D2 and photoproducts produced during sun exposure could also have been a photochemical signal (actinometer) to tell the organism that it has been exposed to enough solar UVB radiation and to signal it to leave the surface into deeper water where it would no longer be exposed to UVB radiation due to the ocean’s ability to absorb this solar energy.2
It has also been speculated that if ergosterol was principally present in the plasma membrane and contained within the lipid bilayer that this rigid planar structure after exposure to solar UVB radiation would be transformed into a more flexible vitamin D2 molecule that would likely be released into the extracellular space. This process could alter membrane permeability and possibly open up a pore to permit the entrance and exit of ions including calcium. This could be the connection for why vertebrates including humans have depended on sun exposure for the maintenance of their calcium metabolism.2,5-7
Historical Perspective
As the industrial revolution swept across Northern Europe in the 1600s resulting in buildings built in close proximity and coal burning causing a pall of air pollution (Fig. 3) so too appeared a bone deforming disease rickets in children that had devastating health consequences (Fig. 4).8,9

Figure 3. Photograph from Glasgow, Great Britain, in about 1870 showing that the buildings are built in close proximity to each other. Holick, copyright 1994. Reproduced with permission.
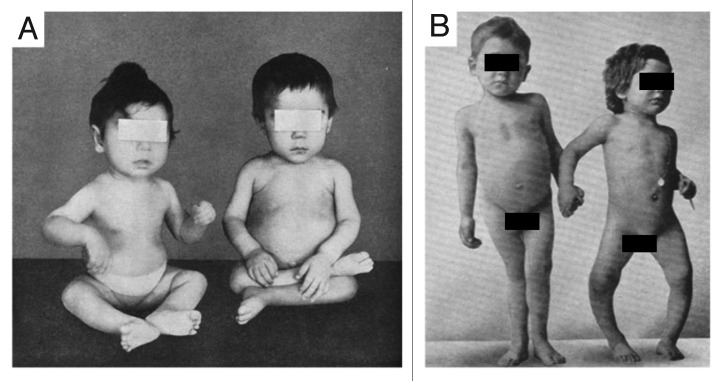
Figure 4. Skeletal deformities observed in rickets. (A) Photograph from the 1930s of a sister (left) and brother (right), aged 10 mo and 2.5 y, respectively, showing enlargement of the ends of the bones at the wrist, carpopedal spasm, and a typical “Taylorwise” posture of rickets. (B) The same brother and sister 4 y later, with classic knock-knees and bow legs, growth retardation, and other skeletal deformities. Holick, copyright 2006. Reproduced with permission.
The first insight into the possible relationship between the industrialization of Northern Europe and rickets was made by Śniadecki10 (Fig. 5A) in 1822 when he concluded that children who lived in the inner city of Warsaw had a high incidence of rickets because of their lack of sun exposure. This was based on his clinical observations that children living in rural areas outside of Warsaw did not suffer from rickets while children born and raised in Warsaw were plagued with the disease. More than 70 y later Palm11 came to the same conclusion based on reports from his colleagues in third world countries including India and China that rickets was uncommon compared with the high prevalence of the disease in children living in London. Another 30 y would pass before Huldschinsky12,13 (Fig. 5B) would report that rachitic children exposed to a mercury arc lamp had dramatic radiologic improvement of their rickets several months later. He cleverly also realized that exposing one arm of a child with rickets had the same dramatic radiologic improvement in the forearm of the arm not exposed to the mercury arc lamp. He therefore correctly concluded that it was likely that something was being made in the skin and entered into the circulation to improve the global bone health of the child (Fig. 6).12,13 Finally in 1921 Hess and Unger8,14 (Fig. 5C) exposed rachitic children to sunlight on the roof of their hospital in New York City and demonstrated significant radiologic improvement in the children’s rickets. These physicians also realized that children of color were at much higher risk for rickets and concluded that they needed longer exposure to sunlight to both treat and prevent rickets.

Figure 5. Photographs of researchers who made crucial contributions to vitamin D and rickets research. (A) Jędrzej Śniadecki, (B) Kurt Huldschinsky, (C) Alfred Hess, (D) Harry Steenbock. Holick, copyright 2013. Reproduced with permission.
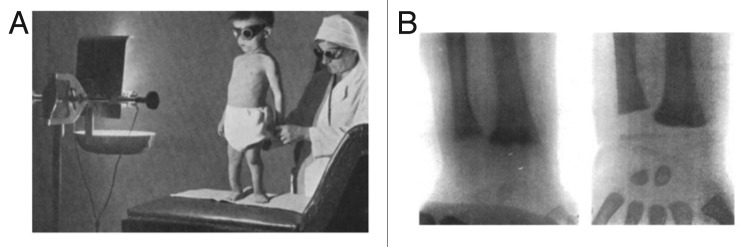
Figure 6. UV radiation therapy for rickets. (A) Photograph from the 1920s of a child with rickets being exposed to UV radiation. (B) Radiographs demonstrating florid rickets of the hand and wrist (left) and the same wrist and hand taken after treatment with 1 h UV radiation 2 times a week for 8 weeks. Note mineralization of the carpal bones and epiphyseal plates (right). Holick, copyright 2006. Reproduced with permission.
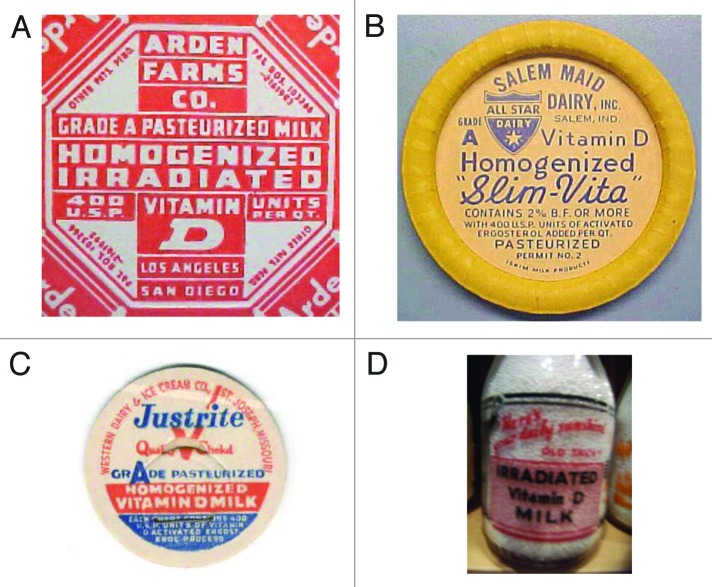
By turn of the 20th century it was estimated that upwards of 80–90% of children living in Northern Europe and in Northeastern United States had evidence of rickets.8 Steenbock and Black15 (Fig. 5D) and Hess and Weinstock16 exposed various foods including cotton seed oil, corn oil, and milk to UVB radiation and demonstrated that this process imparted antirachitic activity for rodents. This led to the addition of ergosterol to milk followed by UVB irradiation or the addition of ergosterol that had been previously exposed to UVB radiation or to the addition of vitamin D2 to the milk (Fig. 7).8
Figure 7. (A) Seal of a milk bottle that denoted that the milk was irradiated with UV radiation and contained vitamin D. (B) Cap of a milk bottle stating that activated ergosterol has been added to the milk. (C) Cap of milk bottle stating that the milk had been fortified with vitamin D. (D) Seal of a bottle of milk that denoted that the milk had been irradiated and contained vitamin D. Holick, copyright 2013. Reproduced with permission.
This process was quickly embraced by the dairy processors and in the early 1930s essentially all milk in the United States and in most industrialized countries including Great Britain and other European countries had vitamin D fortified milk. The United States government also established an agency in 1931 whose goal was to promote sensible sun exposure of young children to prevent rickets and improve their bone health (Fig. 8).9,14 Within a few years these interventions essentially eradicated rickets.8,17-19
Figure 8. Brochure of the US. Department of Labor promoting sensible sun exposure in children in 1931.
Vitamin D became so popular that in the 1930s and 1940s a wide variety of foods and beverages as well as personal care products were fortified with vitamin D.19 They included not only milk and other dairy products but also soda pop, beer, hot dogs, custard and even soap and shaving cream (Figs. 9A-D).19
Figure 9. (A) Seal denoting that this product was fortified with vitamin D. (B) Bottle of oil denoting that it contained irradiated ergosterol. (C) Beer can denoting that it was fortified with vitamin D. (D) Advertisement denoting that Bird’s custard contained vitamin D. Holick, copyright 2013. Reproduced with permission.
However in the early 1950s an outbreak of hypercalcemia in infants who had elfin faces, heart problems, and mental retardation led to an investigation by the Royal College of Physicians. The experts concluded that this was most likely due to vitamin D intoxication since a similar presentation had been observed in neonatal rodents born of mothers who were fed high doses of vitamin D.20-22 Legislation quickly followed banning the fortification of any food or personal use products with vitamin D in Great Britain.8,17,22 This ban quickly spread across Europe and for the most part remains in effect today with the exception of a few foods including margarine and some cereals being fortified with vitamin D.8,19-22 It is however likely that these children had Williams syndrome which is associated with elfin faces, mental retardation, heart problems, and hypercalcemia due to a hypersensitivity to vitamin D.23 Sweden and Finland now permit milk to be fortified with vitamin D.22,24 It is worth noting that milk has been fortified with 100 IU vitamin D/8 oz for the past 80+ years without any reports of toxicity in infants. In the past 10 y juice products including orange juice have been fortified in the United States with 100 IU vitamin D/8 oz without any reports of toxicity.22
Photochemistry of Provitamin D3
During exposure to sunlight solar radiation with wavelengths of 290–315 nm penetrate into the skin and are absorbed by proteins, DNA and RNA as well as 7-dehydrocholesterol.1,2 Most of this UVB radiation is absorbed in the epidermis and as a result when exposed to sunlight most of the vitamin D3 that is produced in the skin is made in the living cells in the epidermis. This is the reason why after exposure to sunlight vitamin D3 remains in the skin even when the skin is washed with soap and water immediately after the exposure to sunlight.
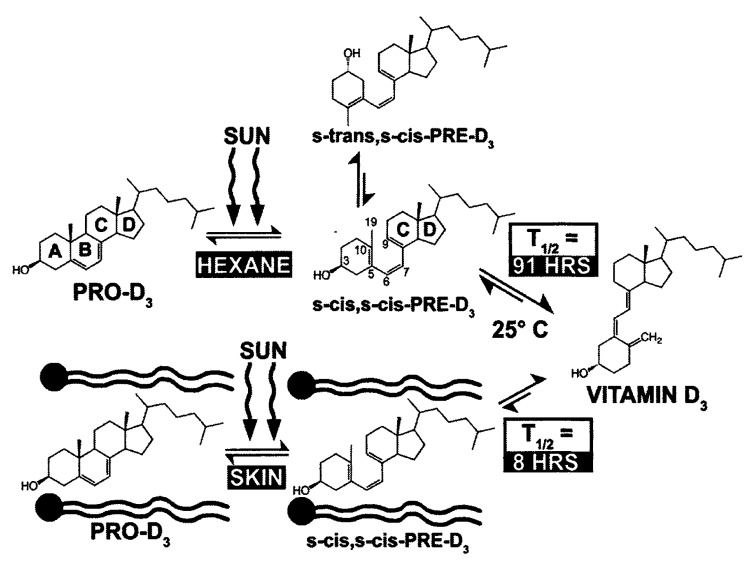
When epidermal 7-dehydrocholesterol absorbs solar UVB radiation with energies of 290–315 nm (Fig. 10), it causes an activation of the double bonds causing them to rearrange and open up the B ring to form the seco-steroid (split steroid) previtamin D3 (Fig. 11).25
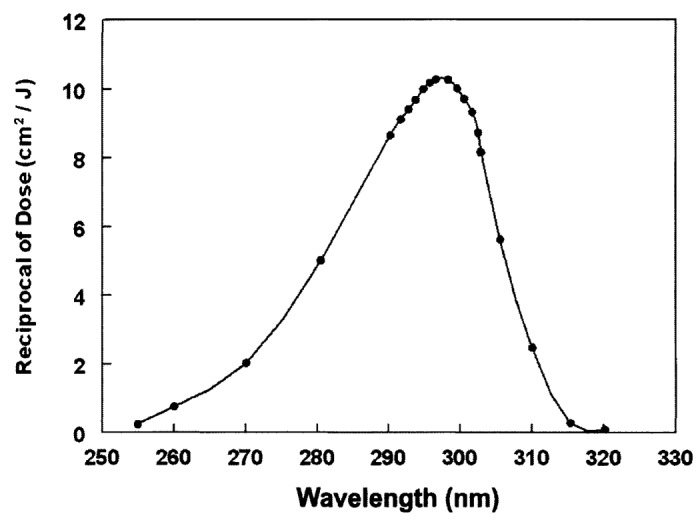
Figure 10. Action spectrum for the conversion of 7-dehydrocholesterol to previtamin D3 in human skin. Holick, copyright 2007. Reproduced with permission.
Figure 11. Photolysis of provitamin D3 (pro-D3. 7-dehydrocholesterol) into previtamin D3 (pre-D3) and its thermal isomerization to vitamin D3 in hexane and in lizard skin at 25°C. In hexane pro-D3 is photolyzed to s-cis,s-cis-pre-D3. Once formed, this energetically unstable conformation undergoes a conformational change to the s-trans,s-cis-pre-D3. Only the s-cis,s-cis-pre-D3 can undergo thermal isomerization to vitamin D3. The s-cis,s-cis conformer of pre-D3 is stabilized in the phospholipid bilayer by hydrophilic interactions between the 3β-hydroxl group and the polar head of the lipids, as well as by the van der Waals interactions between the steroid ring and side-chain structure and the hydrophobic tail of the lipids. These interactions significantly decrease the conversion of the s-cis,s-cis conformer to the s-trans,s-cis conformer, thereby facilitating the thermal isomerization of s-cis,s-cis-pre-D3 to vitamin D3. Holick, copyright 1995. Reproduced with permission.
Previtamin D3 when made in a test tube exists in two confomeric forms, a 5,6-sec-cis-s-cis (czc) and a 5,6-sec-trans-s-cis (czt) form. The czt conformer is the most thermodynamically stable form and thus most of the previtamin D3 when produced in a test tube exists as this conformer. This conformer however being stable cannot isomerize to vitamin D3. However the less thermodynamically stable conformer czc does undergo a 1–7 antarafacial sigma shift of a hydrogen from C-19 to C-9 causing rearrangement of the double bonds to form vitamin D3. As a result it takes several days in a test tube at room temperature and even at body temperature for the czt conformer to isomerize to the czc conformer which then is converted to vitamin D3 (Fig. 12).6,7,26,27
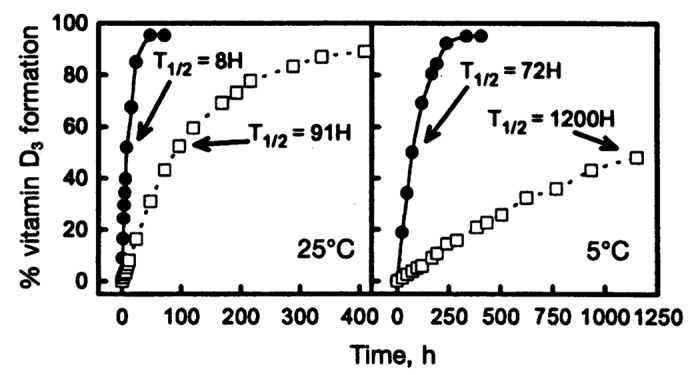
Figure 12. Thermal isomerization of previtamin D3 to vitamin D3 as a function of time in lizard skin (●) and in hexane (☐) at 25°C (left) and 5°C (right). Each point represents the mean value from three separate analyses. Holick, copyright 1995. Reproduced with permission.
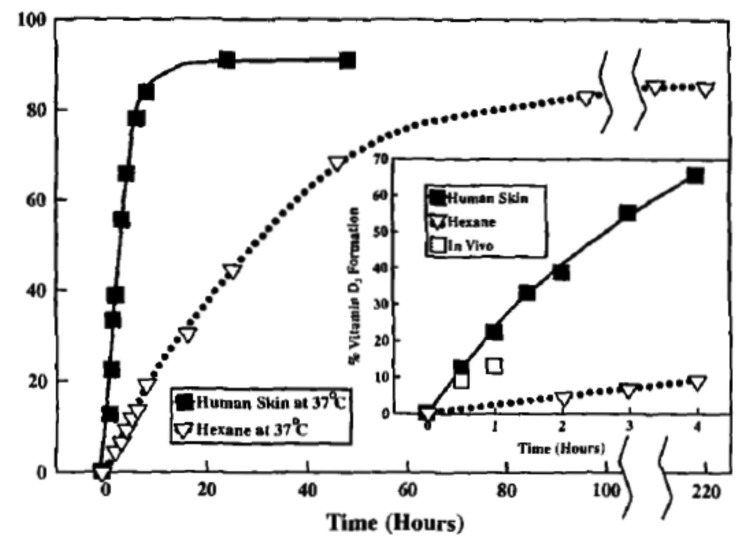
From a physiologic perspective it made little sense for it to take several days for previtamin D3 to convert to vitamin D3 in the skin as it does in a test tube. Furthermore this was a significant problem for poikilothermic (cold blooded) vertebrates since a lower outside temperature would make it take a much longer time for previtamin D3 that was produced in the skin to convert to vitamin D3. Studies of lizard skin exposed to simulated sunlight revealed that the conversion of previtamin D3 to vitamin D3 was 10 times faster when compared with previtamin D3 in a isotropic organic solution (Fig. 12).6 A similar observation was made in human skin (Fig. 13).7
Figure 13. Thermoconversion of pre-D3 to vitamin D3 as a function of time in human skin and in n-hexane at 37°C. The inset depicts the thermoconversion of pre-D3 to vitamin D3 in human skin in vivo (☐) and compares them with those in n-hexane (▽) and in human skin in vitro (ν) at 37°C. Reproduced with permission from.7
This suggested that the skin had some property that accelerated the conversion of previtamin D3 to vitamin D3. One possible explanation was that an enzyme existed that catalytically converted previtamin D3 to vitamin D3. Skin homogenates incubated with previtamin D3 did not result in an enhancement in its conversion to vitamin D3. Thus another explanation needed to be found. It was hypothesized and finally proven with several studies that 7-dehydrocholesterol in the skin cell principally existed in the plasma membrane and was incorporated within the fatty acid hydrocarbon side chain and polar head group of the triglycerides (Figs. 11 and 14). The rigid planar structure of 7-dehydrocholesterol sandwiched in between the triglyceride fatty acid hydrocarbon tails could only be transformed into the planar czc conformer of previtamin D3 upon exposure to solar UVB radiation (Fig. 11).6,26-28

Figure 14. Proposed theoretical structural model for the localization of the cZc-previtamin D3 in the phospholipids of a membrane. Based on the amphipathic nature and conformational mobility of previtamin D3, we proposed the following model to show the spatial relationship between previtamin D3 and phospholipids. We postulated that in the membrane, the cholesterol like cZc-previtamin D3 is aligned parallel to its neighboring phospholipids, with its polar 3β-hydroxy interacting with the polar head groups of the phospholipids through hydrogen bonding, and the hydrophobic rings and side chain interacting with the nonpolar acyl chains of the lipids through hydrophobic and van der Waals interactions. A, phosphatidylcholine; B, cZc-previtamin D3. Reproduced with permission from.27
Once formed, this unstable conformer rapidly converted to vitamin D3.6,7,26-28 To confirm this hypothesis studies were done in liposomes that mimicked the plasma membrane and it was demonstrated that adding double bonds to the triglyceride’s side chain or shortening or lengthening of the side chain resulted in a decrease in the kinetics for the conversion of previtamin D3 to vitamin D3 (Fig. 15).27
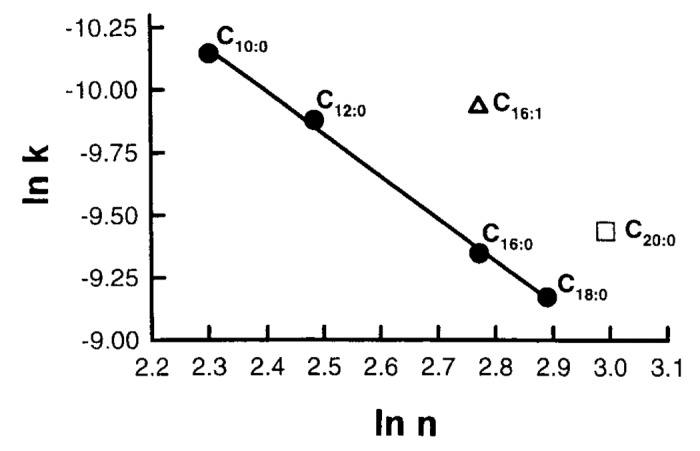
Figure 15. Effects of phospholipid carbon chain length and saturation on the rate of pre-D3 to vitamin D3 isomerization in liposomes. k, rate constant; n, carbon number of phospholipid chain. Reproduced with permission from.27
Because vitamin D3 is thermodynamically more stable and also more flexible it is ejected out of the plasma membrane into the extracellular space and diffuses into the capillary bed in the dermis where it is bound to the vitamin D binding protein (DBP) for transport to the liver.2,5,6,22,29,30
There has been a lot of discussion as to whether ingesting vitamin D from the diet or from a supplement is the same as producing vitamin D3 in the skin. Because it takes approximately ~8 h for previtamin D3 in the skin to fully convert to vitamin D326,27 and it takes additional time for the vitamin D3 to enter the dermal capillary bed this is at least 2 of the explanations for why it was observed that vitamin D3 produced in the skin last 2–3 times longer in the circulation when compared with ingesting it orally (Fig. 16).29 Furthermore when vitamin D3 is produced in the skin 100% of it is potentially bound to the vitamin D binding protein (Fig. 17). When vitamin D3 is ingested from the diet or supplement it gets incorporated into chylomicrons which are transported into the lymphatic system and then into the venous system were approximately 60% of the vitamin D3 is bound to the vitamin D binding protein and 40% is rapidly cleared in the lipoprotein bound fraction.29
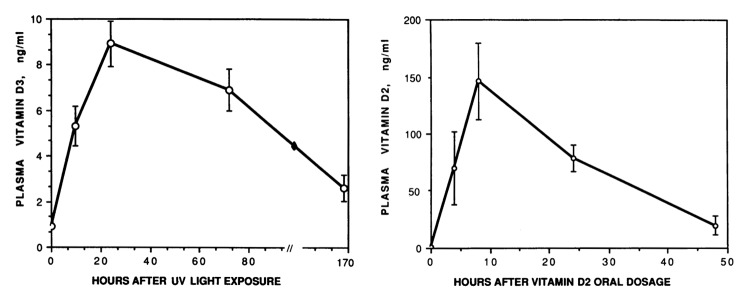
Figure 16. Plasma vitamin D3 or vitamin D2 after UV light exposure or vitamin D2 oral dosage. Reproduced with permission from.29
Figure 17. A schematic representation of the photochemical and thermal events that result in the synthesis of vitamin D3 in the skin, and the photodegradation of previtamin D3 and vitamin D3 to biologically inert photoproducts. 7-dehydrocholesterol (7-DHC) in the skin is converted to previtamin D3 by the action of solar UV B radiation. Once formed, previtamin D3 is transformed into vitamin D3 by a heat-dependent (ΔH) process. Vitamin D3 exits the skin into the dermal capillary blood system and is bound to a specific vitamin D-binding protein (DBP). When previtamin D3 and vitamin D3 are exposed to solar UV B radiation, they are converted to a variety of photoproducts that have little or no activity on calcium metabolism. Holick, copyright 1995. Reproduced with permission.
Sun Controlled Cutaneous Production of Vitamin D3
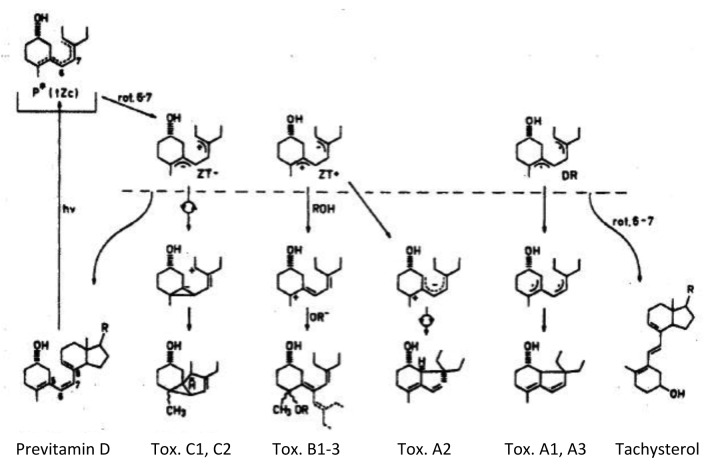
During exposure to sunlight after previtamin D3 is produced it will absorb solar UVB radiation and isomerize into two major photoproducts, lumisterol3 and tachysterol3 (Fig. 17).31,32
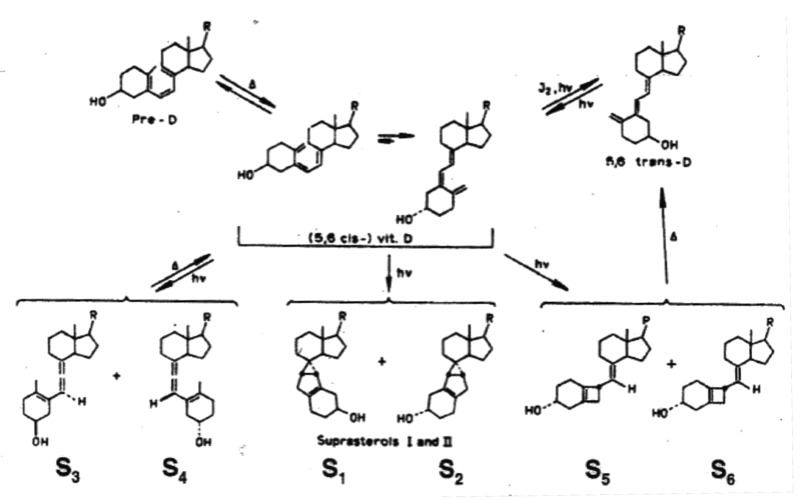
Neither of these two photoproducts has any effect on calcium metabolism.32 Thus when the skin is exposed to sunlight it can only convert approximately 15% of 7-dehydrocholesterol to previtamin D3 (Fig. 18).32 Any further exposure will result in a photoequilibrium whereby previtamin D3 is converted into lumisterol3 and tachysterol3 as well as revert back to 7-dehydrocholesterol (Fig. 17). In addition when vitamin D3 is made from previtamin D3 in the skin if it is exposed to solar UVB radiation it will absorb UVB radiation and be converted into several suprasterols and 5,6-trans-vitamin D3 (Figs. 17 and 19). In addition previtamin D3 can also be converted to several toxisterols (Fig. 20).33-36 Therefore no matter how much sun a human is exposed to vitamin D intoxication will not occur because any excess previtamin D3 and vitamin D3 is photodegraded into products that have no calcemic activity.31,32
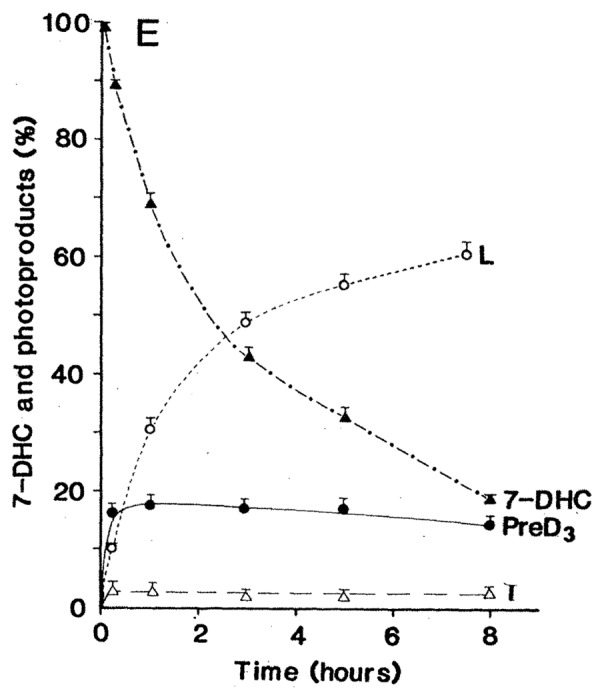
Figure 18. An analysis of the photolysis of 7-dehydrocholesterol (7-DHC) in the basal-cell layer and the appearance of the photoproducts previtamin D3 (Pre-D3), lumisterol3 (L), and tachysterol3 (T) with increasing time of exposure to equatorial simulated solar UV radiation. Bars above data points show the standard error of the mean of three determinations. Holick, copyright 1981. Reproduced with permission.
Figure 19. When vitamin D3 is irradiated, it is converted to 5,6-trans-vitamin D3 and at least 6 photoproducts known as suprasterols. Holick, copyright 2013. Reproduced with permission.
Figure 20. Once previtamin D3 is formed, it has the ability to rotate around the 6–7 bond. Relaxation via rotation about the 6–7 bond followed by UV irradiation can give rise to a wide variety of toxisterols and tachysterol. Holick, copyright 2013. Reproduced with permission.
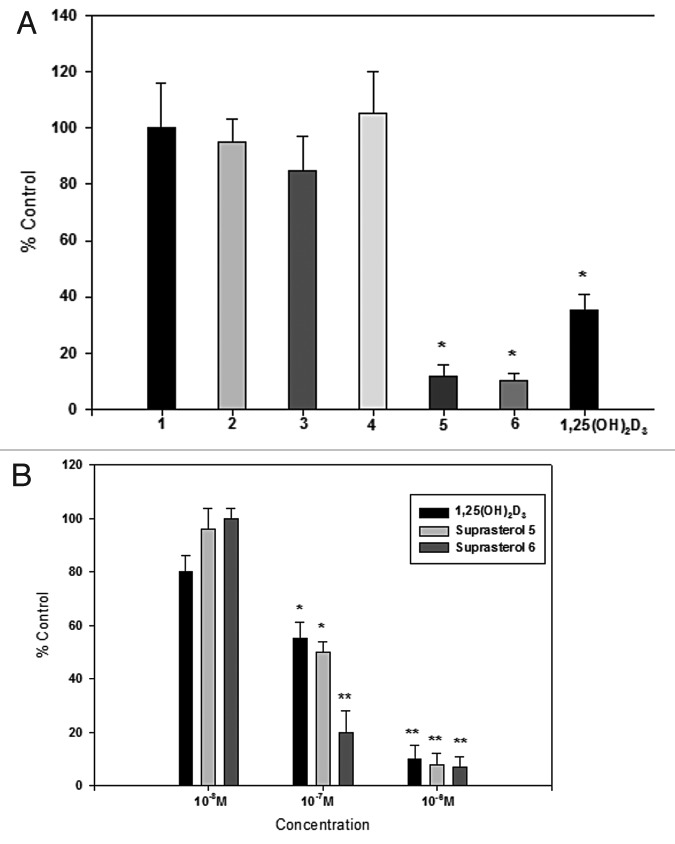
This however does not mean that these myriad of photoproducts don't have other biologic effects such as regulating epidermal cell growth and reducing risk of skin cancer. One product, lumisterol3, if converted to 1,25-dihydroxylumisterol3 may have anti-tumor effects in the skin.37 Some of the suprasterols also have antiproliferative activity in cultured human keratinocytes (Fig. 21). Therefore sensible sun exposure to produce previtamin D3, vitamin D3 and its photoproducts may have some additional benefits above and beyond simply taking a vitamin D3 supplement or ingesting vitamin D3 from dietary sources.
Figure 21. (A) Proliferation of human keratinocytes after incubation with different suprasterols compared with negative control (100%). Suprasterols 5 and 6 show a strong antiproliferative activity as well as the positive control 1,25(OH)2D3. (B) Dose dependent antiproliferative activity of suprasterols 5 and 6 compared with 1,25(OH)2D3 in keratinocytes. Mean ± SEM *p < 0.01, **p < 0.001 compared with control 100%. Holick, copyright 2013. Reproduced with permission.
Factors that Influence Cutaneous Vitamin D3 Synthesis
Zenith Angle
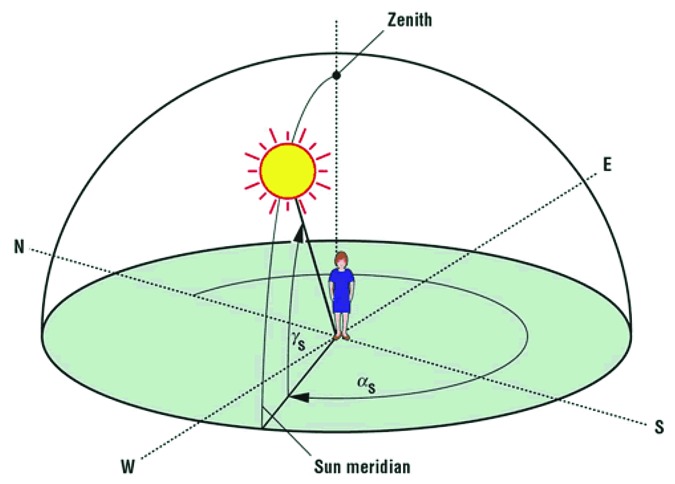
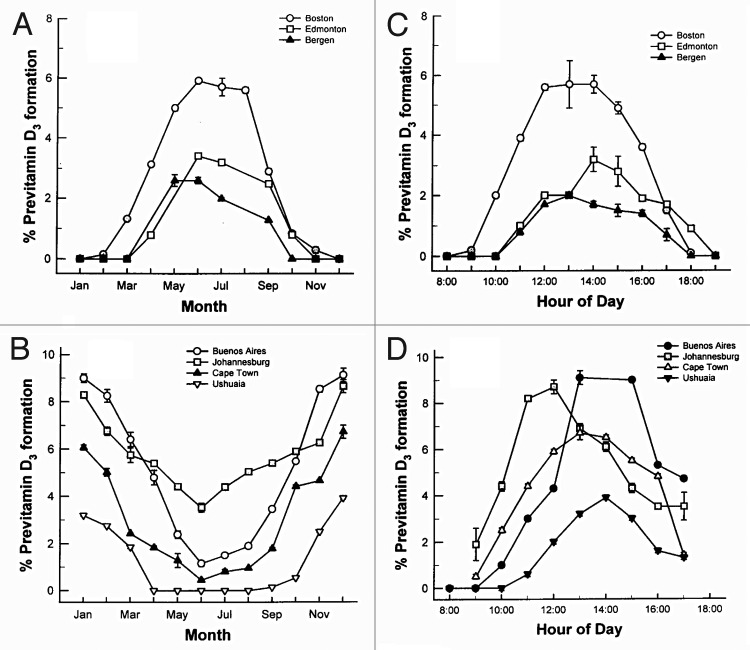
Only about one percent of solar UVB radiation ever reaches the earth’s surface even in the summer at noon time.38 The reason is that all of the UV C (200–280 nm) and all of the UVB radiation up to approximately 290 nm is efficiently absorbed by the stratospheric ozone layer.38,39 In addition the ozone layer absorbs approximately 99% of the UVB radiation with wavelengths 291–320 nm. Therefore increasing the path length by which solar UVB has to travel through the ozone layer will result in a decrease in the number of UVB photons that reach the earth's surface (Fig. 22).40 This is the explanation for why during the winter when living above and below approximately 33° latitude very little if any vitamin D3 can be produced in the skin from sun exposure. People who live farther North and South often cannot make any vitamin D3 in their skin for up to 6 mo of the year.41 For example in Boston at 42° North essentially no vitamin D3 can be produced in the skin from November through February. Inhabitants living in Edmonton Canada at 52° North, Bergen Norway at 60° North, or Ushuaia Argentina at 55° South are unable to produce any significant vitamin D3 for about 6 mo of the year (Figs. 23 and 24).2,39,41
Figure 22. The solar zenith angle is the angle made by the sun’s light with respect to the vertical (the sun being directly overhead). This angle is increased at higher latitudes, early morning and late afternoon when the sun is not directly overhead, and during the winter months. As the solar zenith angle increases, the amount of UVB radiation reaching the earth’s surface is reduced. Therefore, at higher latitudes, greater distance from the equator, more of the UVB radiation is absorbed by the ozone layer thereby reducing or eliminating the cutaneous production of vitamin D3. Holick, copyright 2006. Reproduced with permission.
Figure 23. Influence of season, time of day, and latitude on the synthesis of previtamin D3 in Northern (A and C) and southern hemispheres (B and D). The hour indicated in C and D is the end of the 1 h exposure time. Holick, copyright 1998. Reproduced with permission.
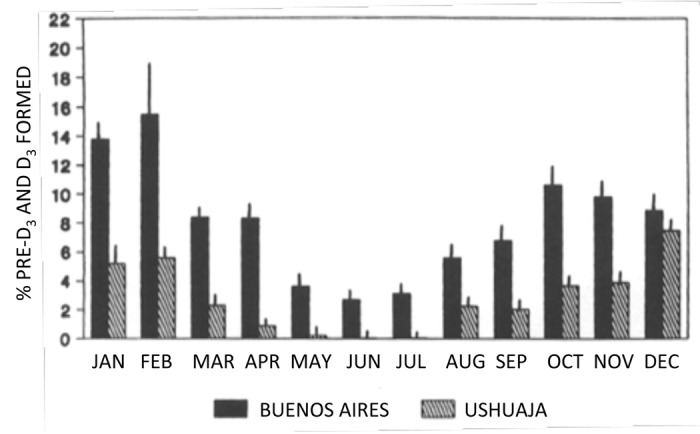
Figure 24. Photoproduction of previtamin D3 and vitamin D3 from 7-DHC throughout the year in Ushuaia, Argentinia (slashed bars, 55 degrees South) and Buenos Aires (closed bars, 34 degrees South). Values are percentages of initial 7-DHC. Each bar represents the mean ± SEM of three determinations of the sample ampules. Reproduced with permission from Ladizesky.41
People who live in the far Northern and Southern hemispheres had apparently appreciated this fact and were able to satisfy their vitamin D requirement by eating vitamin D rich foods including oily fish, seal blubber, polar bear liver and whale blubber and liver all of which contain large amounts of vitamin D3.42,43
In the early morning and late afternoon the zenith angle of the sun is also more oblique similar to winter sunlight and as a result very little if any vitamin D3 can be produced in the skin before 10 a.m. and after 3 p.m. even in the summer time (Figs. 23 and 25).44
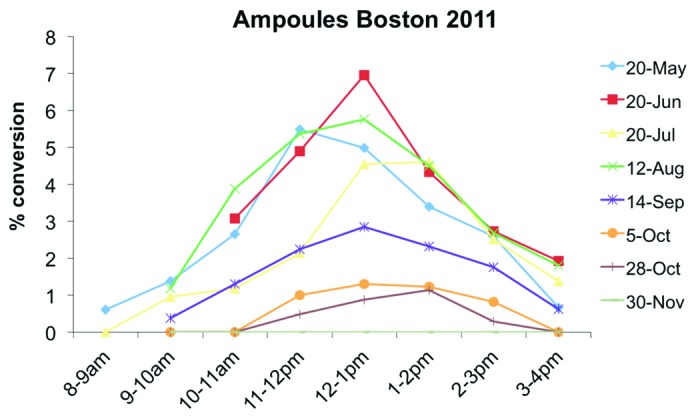
Figure 25. Conversion rate of 7-dehydrocholesterol (7-DHC) to vitamin D depending on time of the day and season in Boston (42° North). The measurements were conducted after exposing ampoules filled with 7-DHC to sunlight. Holick, copyright 2013, reproduced with permission.
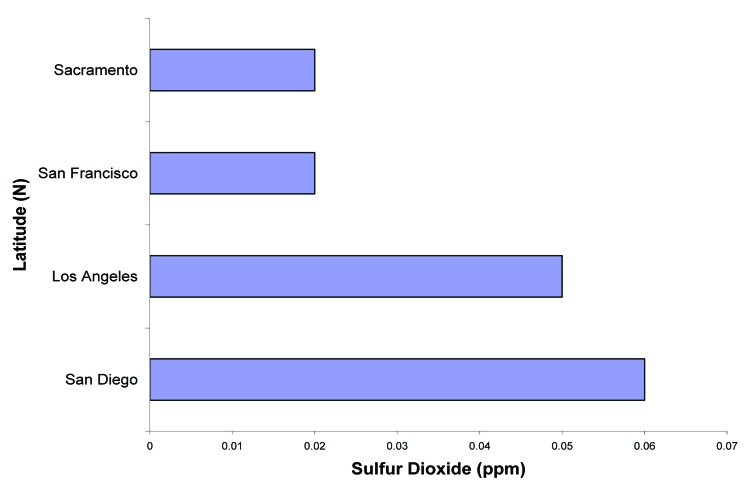
Air pollution including nitrous oxide and ozone is common in many large cities including Los Angeles and San Diego (Fig. 26) and will absorb solar UVB radiation and therefore reduce the effectiveness of sun exposure in producing vitamin D3 in the skin (Fig. 27).40,45 The amount of UVB radiation available for cutaneous vitamin D3 production is markedly reduced by the increase of sulfur dioxide in San Diego and Los Angeles, offsetting the fact that both cities are at lower latitudes.45
Figure 26. The amount of sulfur dioxide (ppm) measured over a one hour period in San Diego, Los Angeles, San Francisco, and Sacramento on the same day. Reproduced with permission from.45
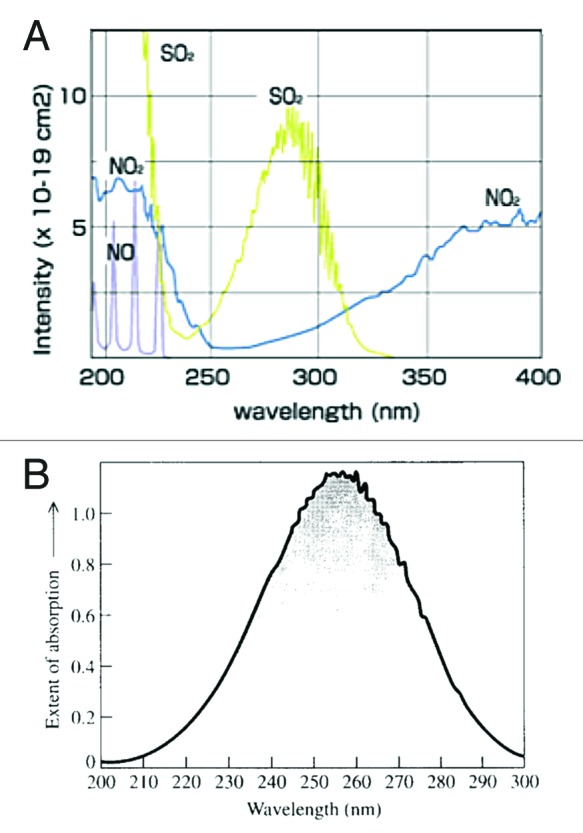
Figure 27. (A) Absorption spectra for NO, NO2, and SO2. The absorption spectra show that SO2 and NO2 absorb UVB radiation (280–315 nm) required for cutaneous production of vitamin D. (B) Absorption spectrum of ozone which also absorbs UVB radiation. Reproduced with permission from.45
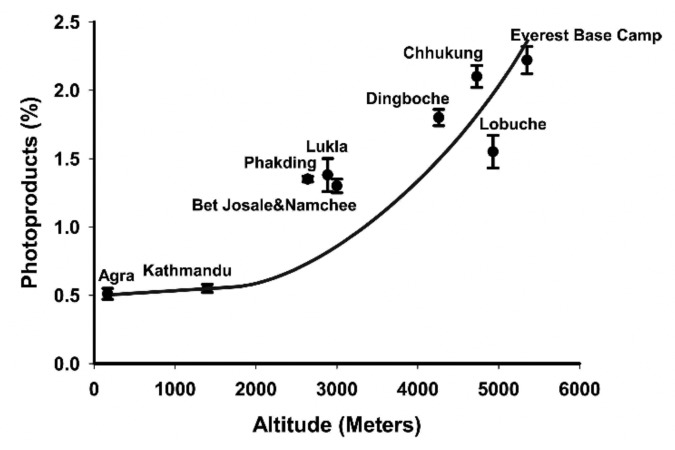
Altitude can also have a dramatic influence on the amount of solar UVB that reaches the earth’s surface because the higher the altitude the shorter the path length that UVB has to travel through and thus the skin can produce more vitamin D3. This was dramatically demonstrated in Agra (169 M altitude), Katmandu (1400 M), and Mount Everest base camp (5300 M), India (27° North). An analysis of sun-induced vitamin D3 synthesis in vitro was conducted at higher altitudes at the same latitude during the same month. In November in Agra very little previtamin D3 was produced during exposure to the sun. It was observed that there was a direct correlation with increased previtamin D3 production with increased altitude. At Mt Everest base camp (5300 min) there was almost a 5-fold increase in previtamin D3 production compared with what was observed in Agra (Fig. 28).46 Since glass absorbs all UVB radiation, exposure of the skin to sunlight that passes through glass, plexiglass, and plastic will not result in any production of vitamin D3 in the skin (Fig. 29).31
Figure 28. Ampoules containing 7-dehydrocholesterol in ethanol were exposed for 1 h between 11:30 a.m. and 12:30 p.m. at 27° North in India at various altitudes. The conversion of 7-dehydrocholesterol to previtamin D3 and its photoproducts was determined by HPLC. Reproduced with permission from.46
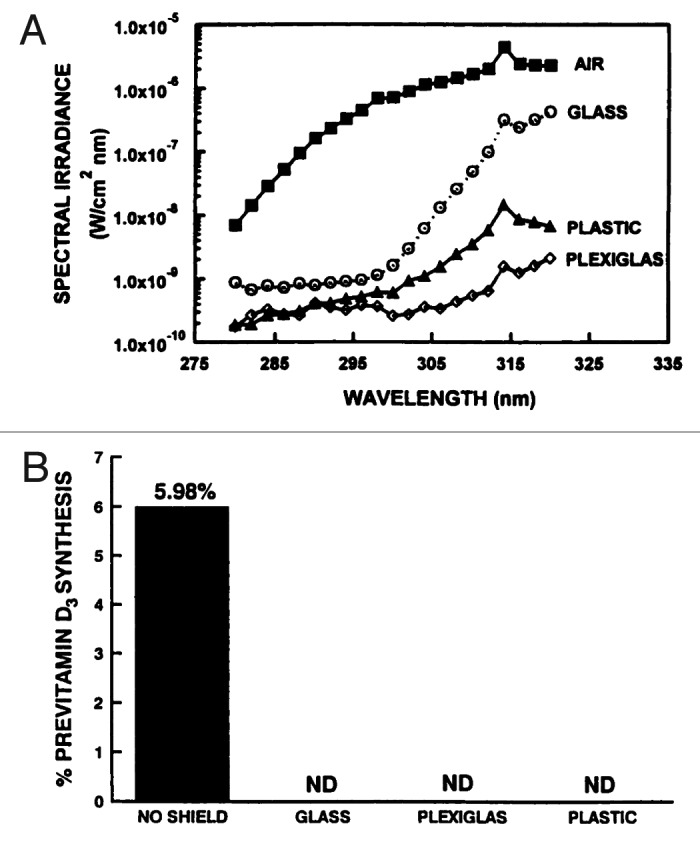
Figure 29. (A) Transmission of UV radiation through air, glass, plastic, and Plexiglas (Dupont Chemical Company, Memphis TN). Holick, copyright 2003. (B) Prevention of previtamin D3 formation as a result of glass, plastic, or plexiglass (Dupont Chemical Company, Memphis, TN) placed between the simulated-sunlight source and the provitamin D3 (7-DHC). Reproduced with permission from.31
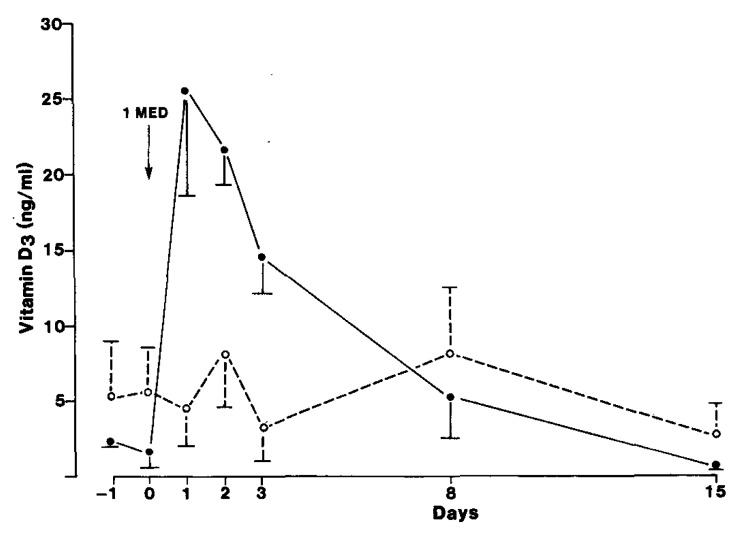
Sunscreens were designed to absorb solar UVB radiation.47 A sunscreen with a sun protection factor (SPF) of 30 absorbs approximately 95–98% of solar UVB radiation. Therefore the topical application of a sunscreen with an SPF of 30 reduces the capacity of the skin to produce vitamin D3 by the same amount i.e., 95–98%.22 This was confirmed with the report that the application of sunscreen with a SPF of only 8 dramatically reduced the blood level of vitamin D3 after exposure to simulated sunlight in a tanning bed (Fig. 30).47,48 Farmers in the Midwest who had a history of non-melanoma skin cancer and who wore a sunscreen all the time before going outdoors for more than a year demonstrated that at the end of the summer their blood levels were significantly lower (most were vitamin D deficient) than the levels of the control group (Fig. 31).48
Figure 30. Mean (± SEM) serum vitamin D3 concentrations in eight normal subjects. Four subjects (o) applied PABA (para-aminobenzoic acid) with a SPF of 8 and four applied vehicle (●) to the entire skin before exposure to UVB. On day 0, all subjects underwent total body exposure to 1 MED (minimal erythema dose) UVR (UV radiation). To convert nanograms of vitamin D per mL to nanomoles per L, multiply by 2.599. Reproduced with permission from.47
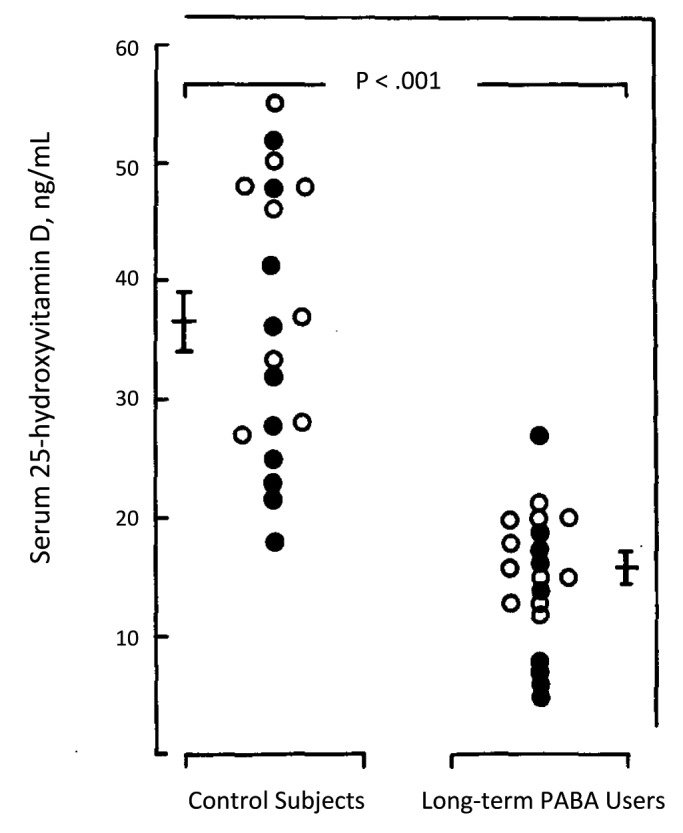
Figure 31. Serum concentration of 25-hydroxyvitamin D in longterm sunscreen users and in age- and sex-matched controls from same geographical area. Blood samples were obtained simultaneously from patients and controls. Mean serum 25-hydroxyvitamin D level was significantly lower in long-term sunscreen users (p < 0.001). Two long-term sunscreen users had absolute vitamin D deficiency, 25-hydroxyvitamin D level below 20 nmol/L. PABA indicates p-aminobenzoic acid; open circles, subjects from Philadelphia; closed circles, subjects from Springfield, III. Reproduced with permission from.48
Skin Pigment
Humans evolved at the equator. They were constantly exposed to sunlight and developed an efficient natural sunscreen melanin,49 which has an absorption spectrum of 290–700 nm and thus can effectively absorb solar UVB radiation (Fig. 32).45 However even though Africans have extremely dark heavily pigmented skin a small amount of UVB radiation is able to penetrate into the epidermis to produce vitamin D3. This was demonstrated when adult whites (skin type 2) and blacks (skin type 5) were exposed to the same amount of UVB radiation in a tanning bed. Whereas the white adults raised their blood levels of vitamin D3 more than 30 fold the black adults demonstrated no significant increase in their blood levels of vitamin D3. However when the black adults were exposed to 5 times more UVB radiation, they increased their blood level by about 15-fold (Fig. 33).50 This was confirmed when surgically obtained white and black skin was exposed to sunlight in Boston in summer. After 30 min approximately 3% of cutaneous 7-dehydrocholesterol was converted to previtamin D3 in the white skin sample whereas only about 0.3% of 7-dehydrocholesterol was converted to previtamin D3 in the black skin (Fig. 34).51 These findings could explain the positive association between skin lightness and 25-hydroxyvitamin D [25(OH)D] levels as found by Armas et al.52 (Fig. 35). The associations between skin lightness, UVB dose and 25(OH)D are documented in Figure 36.
Figure 32. Absorption spectrum of melanin. Reproduced with permission from.45
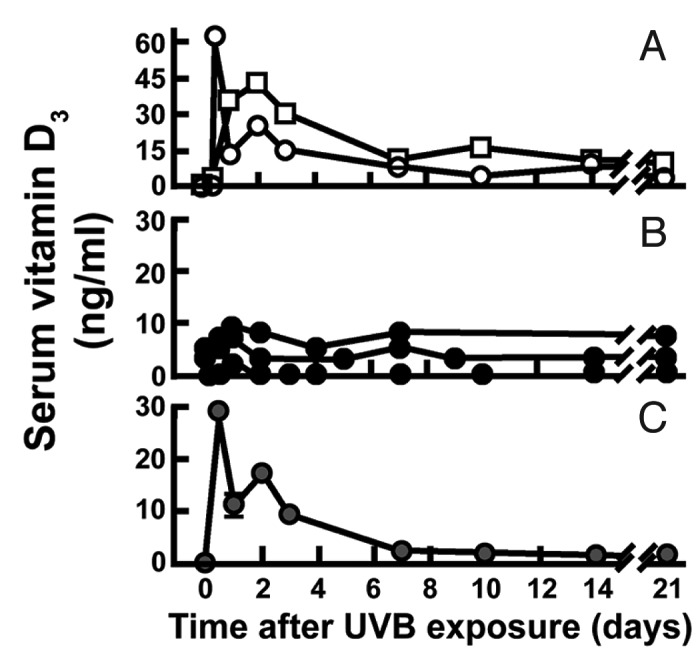
Figure 33. In two lightly pigmented Caucasian (A) and three heavily pigmented Afroamerican subjects (B) after total body exposure to 0.054 J/cm2 of UVR. (C) Serial change in circulating vitamin D after re-exposure of on Afroamerican subject (● in panel B) to a 0.32 J/cm2 dose of UVR. Reproduced with permission from.50
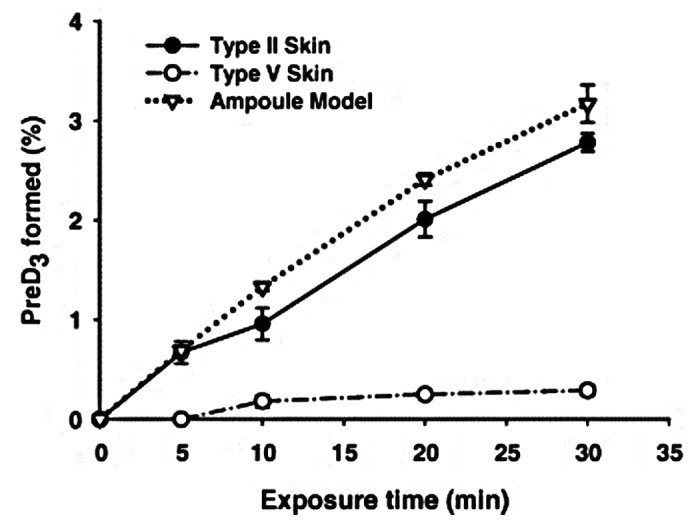
Figure 34. The conversion of 7-dehydrocholesterol to previtamin D3 in an ampoule model, Type II and Type V skin after exposing to noon sunlight in June at Boston (42°N), Massachusetts. The data represent the means ± SEM of duplicate determinations. Reproduced with permission from.51
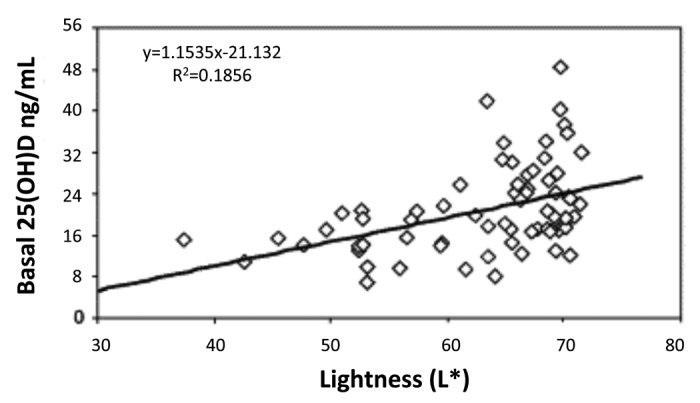
Figure 35. Scatter plot of baseline serum 25-hydroxyvitamin D (25-OH-D) on skin lightness (L∗) score for unexposed skin, showing significant positive correlation of serum 25-OH-D and L∗ (r2 = 0.1856). Heaney, copyright 2006. Reproduced with permission.
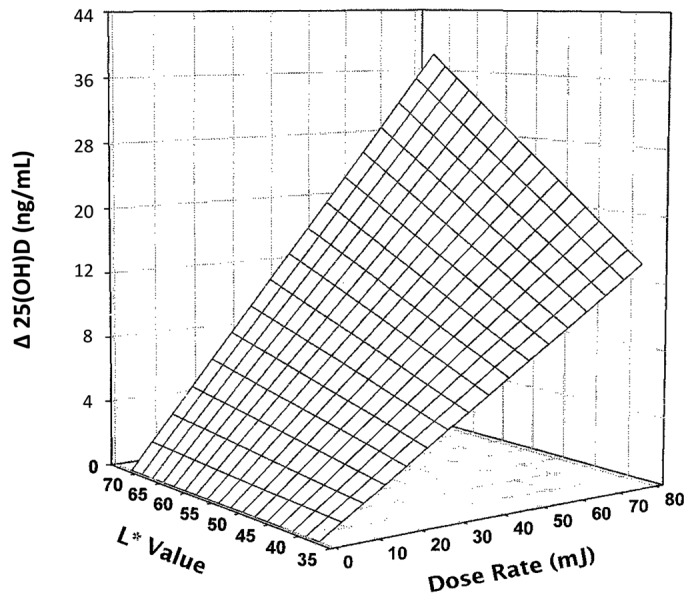
Figure 36. Three-dimensional scatter plot of 4-week serum 25-hydroxyvitamin D response change above baseline expressed as function both of basic skin lightness (L∗) and UV-B dose rate. Surface is a hyperboloid, plotting equation and was fitted to data by least squares regression methods. Heaney, copyright 2006. Reproduced with permission.
Skin pigmentation or the lack thereof was important in the evolution of humans as they migrated North and South of the equator. Africans such as the Maasai (Fig. 37) living outdoors exposed to sunlight daily throughout the year have robust circulating concentrations of the major circulating form of vitamin D, 25(OH)D, in the range of 46 ng/mL.53
Figure 37. Maasi men demonstrate their muscle strength who have been reported to have 25(OH)D ~46 ng/ml. Holick, copyright 2013, reproduced with permission.
Although there have been several explanations for why skin pigment devolved as humans migrated North and South of the equator one of the most likely explanations is as humans migrated farther North and South of the equator the zenith angle of the sun increased resulting in a decrease in the amount of solar UVB radiation reaching the earth thereby reducing vitamin D3 synthesis. A decrease in the amount of skin pigment resulted in a decrease in the sun screening protection permitting more of the UVB radiation to reach the epidermal cells. This provided an evolutionary advantage by being more efficient in producing vitamin D3.49 It had long been speculated that our Neanderthal ancestors were heavily pigmented hairy creatures. This however did not make a lot of sense since heavy pigmentation and excessive hair would markedly reduce cutaneous production of vitamin D3 which was essential for maximizing skeletal health throughout life thereby reducing risk of life-threatening fractures. However, more important is the fact that vitamin D deficiency in utero and during the first few years of life would have caused infantile rickets resulting in a flat deformed pelvis with a small pelvic outlet. Furthermore vitamin D is important for muscle function which is also crucial for birthing.22,54 These conditions caused by vitamin D deficiency would have made it difficult for females to give birth. Therefore in order to survive and procreate skin pigmentation had to markedly decrease in order to permit more UVB photons to enter the skin to produce sufficient amounts of previtamin D3.54,55 Recent evidence has suggested that Neanderthals had a mutation of their melanocyte stimulating hormone receptor resulting in them being redheaded and having Celtic-like fair skin.56,57 This is the likely explanation for why people in Northern Europe have skin types 1 and 2.
Aging
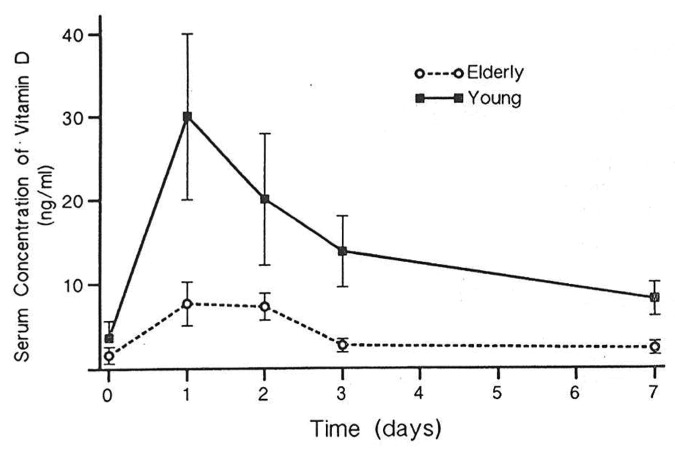
It was observed that 7-dehydrocholesterol concentrations in human epidermis were inversely related to age (Fig. 38).58 The effect of aging on the cutaneous production of vitamin D3 was demonstrated in a study that exposed healthy young adults and older adults to the same amount of UVB radiation in a tanning bed. The increase in the blood level of vitamin D3 in six young adults aged 20–30 was at least three-fold higher compared with the six older adults aged 62–80 demonstrating that aging significantly decreased the capacity of the skin to produce vitamin D3 (Fig. 39).59 With this marked age-related decrease in the cutaneous production of vitamin D3 could the elderly benefit from being exposed to sunlight or UVB radiation? The skin has a large capacity to produce vitamin D3. Exposure of a young adult in a bathing suit to one minimal erythemal dose (MED) of UV radiation in a tanning bed was equivalent to ingesting approximately 20,000 IUs of vitamin D2 (Fig. 40).25 When a healthy 75 y old male in a bathing suit was exposed to UVB radiation in a tanning bed three times a week for 7 weeks he was able to raise and maintain his blood levels of 25(OH)D into the healthy normal range of ~50 ng/ml (Fig. 41C). The percent increase in circulating 25(OH)D concentrations was similar to what was observed in healthy young adults (Fig. 41B). Ampoules containing 7-DHC that were also irradiated served to demonstrate the efficacy of the tanning bed in producing previtamin D3 (Fig. 41A).5 A study in elderly in a nursing home that had an activity room with an UVB emitting lamps on the ceiling (Fig. 42) reported that this was effective in raising and maintaining 25(OH)D levels in these residents (Fig. 43).60
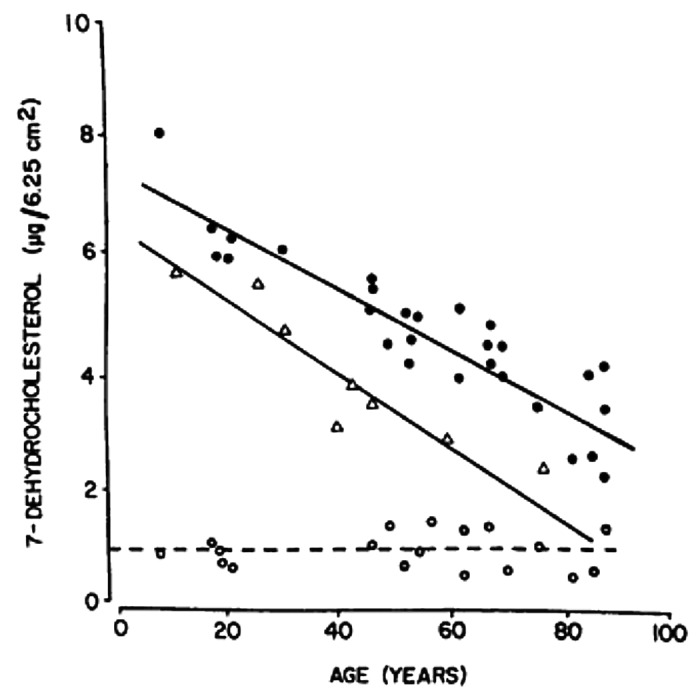
Figure 38. Concentrations of 7-dehydrocholesterol (provitamin D3) per unit area of human epidermis (●), stratum basale (∆) and dermis (○) obtained from surgical speciments from donors of various ages. A linear regression analysis revealed slopes of -0.05, -0.06, and -0.0005 for the epidermis (r = -0.89), stratum basale (r = -0.92), and dermis (r = -0.04), respectively. The slopes of the epidermis and stratum basale are significantly different from the slope of the dermis (p < 0.001). Reproduced with permission from.58
Figure 39. Circulating concentrations of vitamin D in healthy young and elderly volunteers exposed to UV radiation. Reproduced with permission from.59
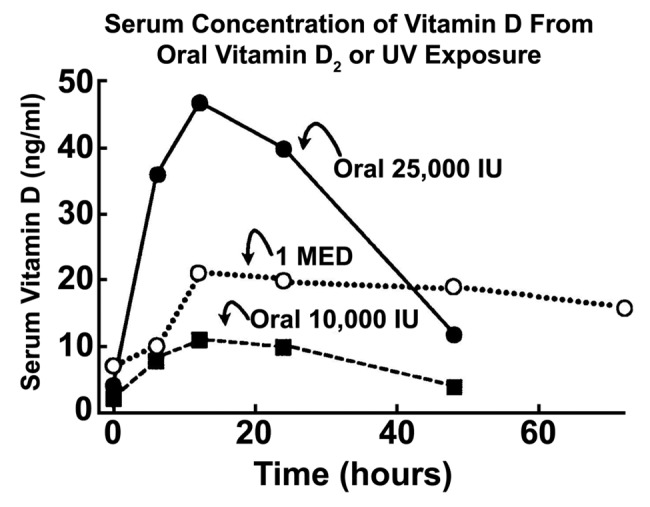
Figure 40. Comparison of serum vitamin D3 levels after a whole-body exposure (in a bathing suit; bikini for women) to 1 MED (minimal erythemal dose) of simulated sunlight compared with a single oral dose of either 10,000 or 25,000 IU of vitamin D2. Holick, copyright 2004. Reproduced with permission from.25
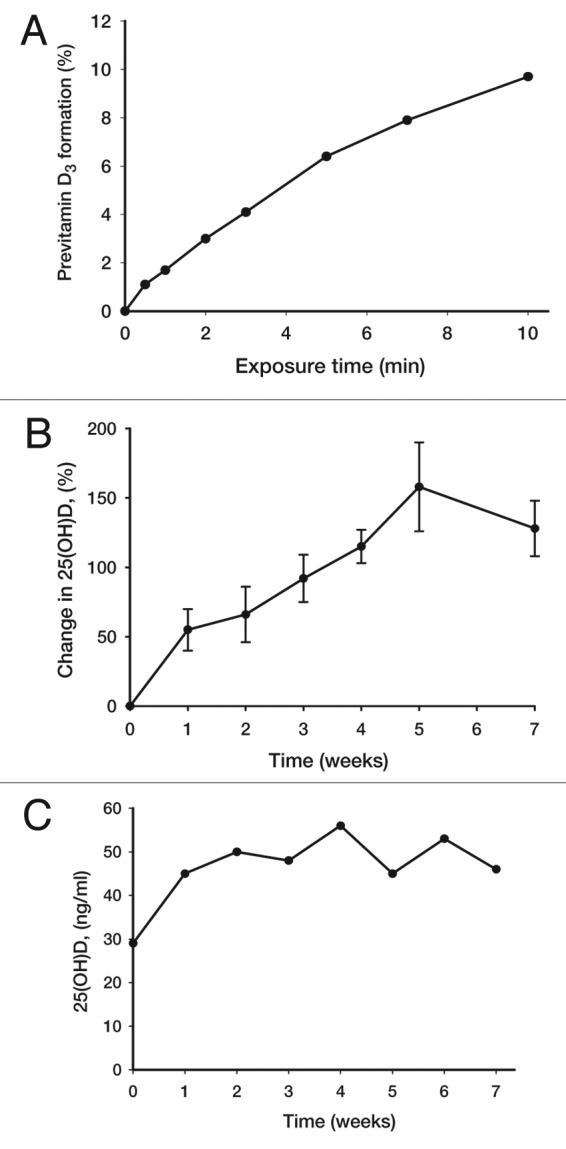
Figure 41. Production of previtamin D3 and serum level of 25(OH)D after the exposure of 7-DHC solution in ampoules and human volunteers to a tanning bed lamp. (A) Ampoules containing 7-DHC were placed and exposed to a tanning bed lamp. At various times, an ampoule was removed and the conversion of 7-DHC to previtamin D3 was measured by HPLC. (B) Healthy young adults were exposed to 0.75 MED in a tanning bed three times a week for 7 weeks. Circulating concentrations of 25(OH)D were determined at baseline and once a week thereafter. (C) A healthy 76-y-old man was exposed to tanning bed radiation equivalent to 0.75 MED three times a week for 7 weeks. His circulating concentrations of 25(OH)D were obtained at weekly intervals. Holick copyright 2007, reproduced with permission from.30
Figure 42. The UVB lamps and residents in a day room of a nursing home. Reproduced with permission from.60
Figure 43. Mean (± 1 sd) 25(OH) vitamin D values pre-irradiation, 12–24 weeks and 56–72 weeks after irradiation in 7 subjects with abnormal baseline values (< 25 nmol/l). Reproduced with permission from.60
Influence of Latitude and Season on Vitamin D Status
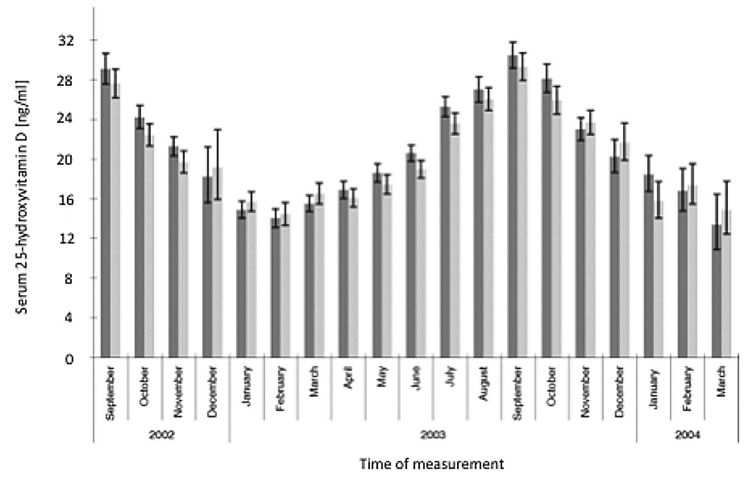
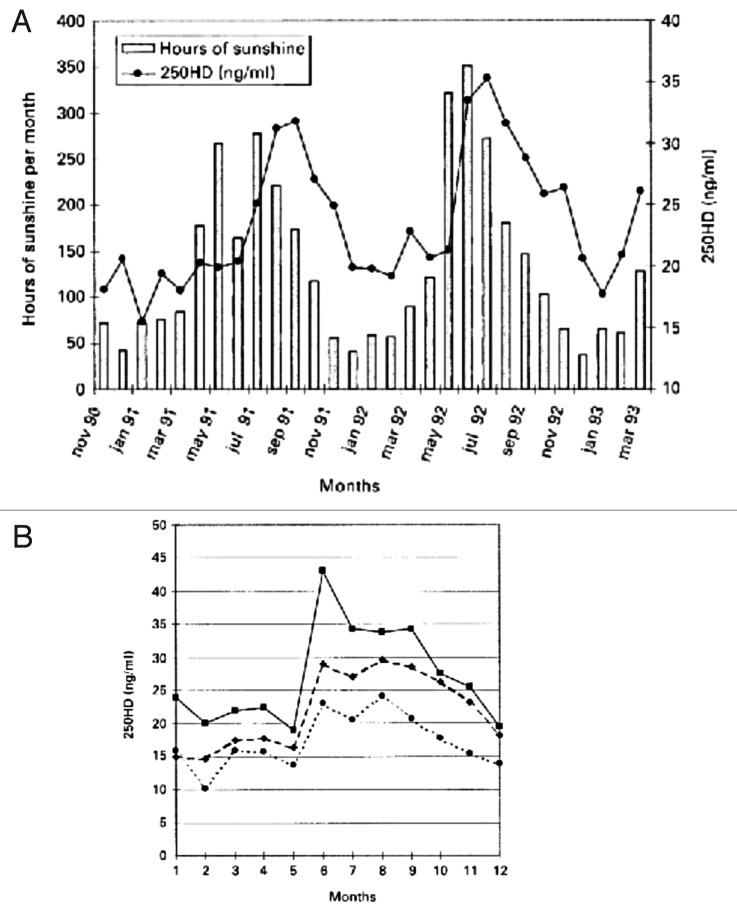
It is well documented that seasonal differences in cutaneous vitamin D3 production has a dramatic influence on both children’s and adults’ vitamin D status (D represents vitamin D2 or vitamin D3).22 A study of 7437 Caucasian men and women from the 1958 British birth cohort at age 45 y revealed that the peak blood levels for 25(OH)D were observed in September (~30 ng/mL) and the nadir was observed in February (~14 ng/mL) (Fig. 44).61 A similar observation was made in postmenopausal women in Denmark. Those who had regular sun exposure achieved a blood level of 25(OH)D of ~45 ng/mL compared with women who avoided direct sun exposure had a 25(OH)D of ~23 ng/mL. This was also supported by the fact that hours of sun exposure was directly related to circulating concentrations of 25(OH)D (Fig. 45).62
Figure 44. Geometric mean (95% CI) monthly variation in serum 25-hydroxyvitamin D [25(OH)D] concentrations in men (■; n = 3725) and women (□; n = 3712) in the 1958 British birth cohort at age 45 y. The interaction between sex and month was significant [p = 0.02, linear regression analyses on log 25(OH)D]. n per sex and month ranged from 17 to 340: 98 in December 2003 for women and < 100 for both sexes in December 2002 (n = 40 M, 37 F), January 2004 (n = 95 M, 75 F), February 2004 (n = 58 M, 70 F), and March 2004 (n = 22 M, 17 F). Reproduced with permission from.61
Figure 45. (A) Seasonal fluctuation of serum 25(OH)D in healthy perimenopausal Danish women and relationship between hours of sunshine and serum 25(OH)D. (B) Seasonal fluctuation of serum 25(OH)D according to frequency of sun exposure. ■, regular sun exposure; ◆, occasional sun exposure; ●, avoiding direct sun exposure. Reproduced with permission from.62
Latitude also has a dramatic influence on the cutaneous production of vitamin D3 and therefore on a person’s vitamin D status (Fig. 46).39,63 Mean circulating 25(OH)D in children, adolescents and adults at various latitudes revealed that there was a significant inverse relationship with the highest levels for those living near the equator with blood levels of 25(OH)D ~40 ng/mL compared with those living far North and South of the equator with blood levels of 25(OH)D ~15 ng/mL (Fig. 46).64

Figure 46. Mean circulating 25-hydroxyvitamin D levels in children, adolescents, and adults according to geographic latitude. Reproduced with permission from.64
However another study reported that those living at the highest latitudes in Europe at higher circulating concentrations of 25(OH)D (Fig. 47).65
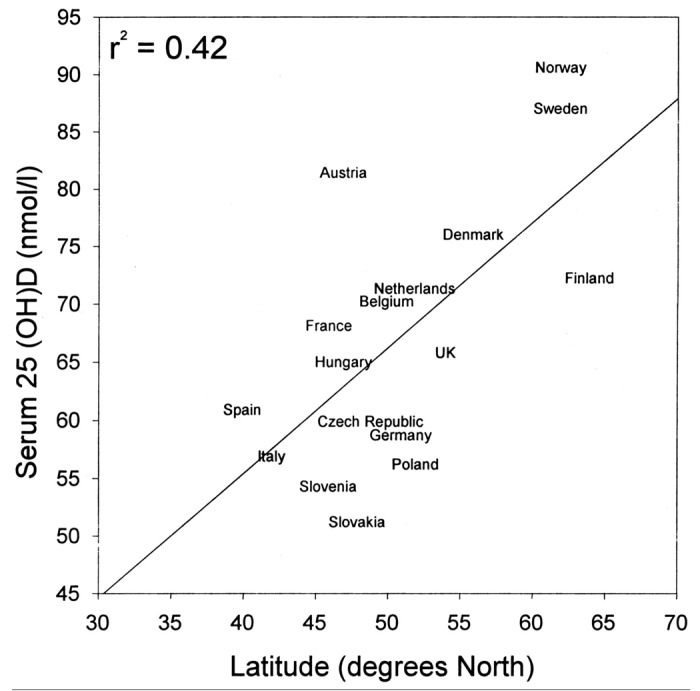
Figure 47. Relationship between the serum 25OHD concentration and northern latitude in Europe. The relationship was very significant (p < 0.001). Reproduced with permission from.65
The likely explanation is that some people living far North and South of the equator who could not make any significant amount of vitamin D3 in their skin for more than half of the year (Fig. 24) adapted by eating foods rich in vitamin D including oily fish22,42,43 while others did not.39,44,66
Sunlight and Non-Calcemic Health Benefits
Historical perspective
People have a feeling of wellbeing when exposed to sunlight. This may be due to the fact that keratinocytes produce β-endorphin when exposed to UV radiation.67 In the early 1900s Finsen (Fig. 48) observed that exposure to sunlight (Fig. 49) dramatically improved cutaneous skin lesions caused by a tuberculosis infection (lupus vulgaris) and received the Nobel Prize in 1903 for his enlightening observations. This led to the use of solariums as a way to treat patients with tuberculosis and gave rise to the use of heliotherapy to improve health.19,68,69 Heliotherapy was used to treat a wide variety of chronic illnesses in the early 1900s and it is still practiced throughout the world and especially in Northern Europe.19,70-72 The rise in the use of pharmaceuticals to treat acute and chronic diseases led to the demise of heliotherapy especially in United States.19,68,69,73

Figure 48. Niels Ryberg Finsen, * December 15, 1860, Thorshavn, Faroe Islands; + 24 September 1904; Nobel Prize was awarded “in recognition of his contribution to the treatment of diseases, especially lupus vulgaris, with concentrated light radiation, whereby he has opened a new avenue for medical science.” Reproduced with permission from.69

Figure 49. (A) 1901 Illustration from Scientific American showing phototherapy with the Finsen carbon-arc UV lamp Reproduced with permission from.21 (B) Sunbathing individuals at sanatorium Leysin in Switzerland. Reproduced with permission from.222
Sunlight and Vitamin D: The Cancer Connection
One of the first association studies relating sun exposure with reduced risk for cancer was reported in 1916 by Hoffman,74 who found that living at a higher latitude was associated with an increased risk for mortality from cancer. He compared cancer mortality between 1908 and 1912 and observed that cancer mortality increased with increasing distance from the equator (Fig. 50). In 1937 Peller and Stephenson75 analyzed the incidence of cancer in navy personnel in the United States Navy who were documented to have increased exposure to solar UV radiation with age matched controls and reported that the rate of skin cancer was eight times higher in the navy personnel while the total number of deaths from other cancers was 60% less than the civilian population.75
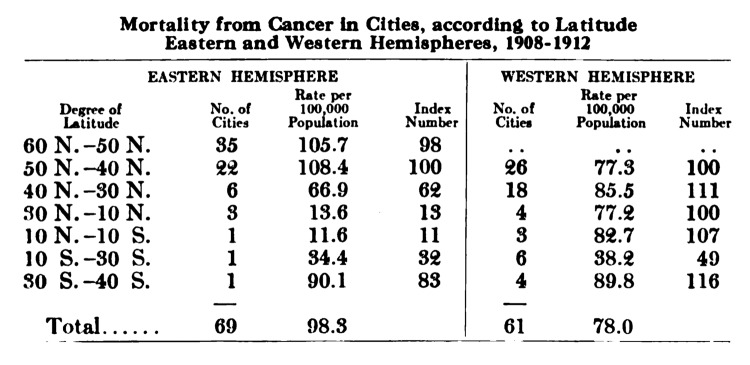
Figure 50. Mortality from cancer in cities according to latitude, 1908–1912. Reproduced with permission from.74
Four years later, Apperly76 compared total cancer mortality in the populations studied with the percentage of Americans and Canadians in the same population who were engaged in agriculture. He concluded that cancer mortality was highest in farmers living in the Northeast compared with those living in the South (Fig. 51).76 He also reported that farmers living in the South exposed to more sunlight were at a higher risk for nonmelanoma skin cancer which he noted was easy to detect and easy to treat. He concluded that the fact that these Southern farmers had nonmelanoma skin cancer resulted in them developing an immunity to the skin cancer which also resulted in an immunity to all cancers including those with high mortality rate.76
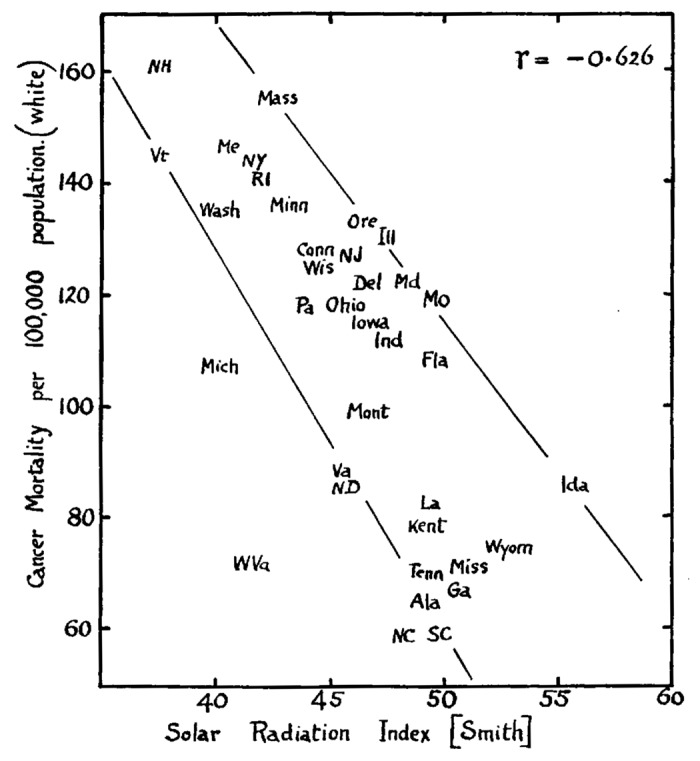
Figure 51. Showing the relation of total cancer mortality rates to Smith's Solar Radiation Index in the American states, (white population only). Reproduced with permission from.76
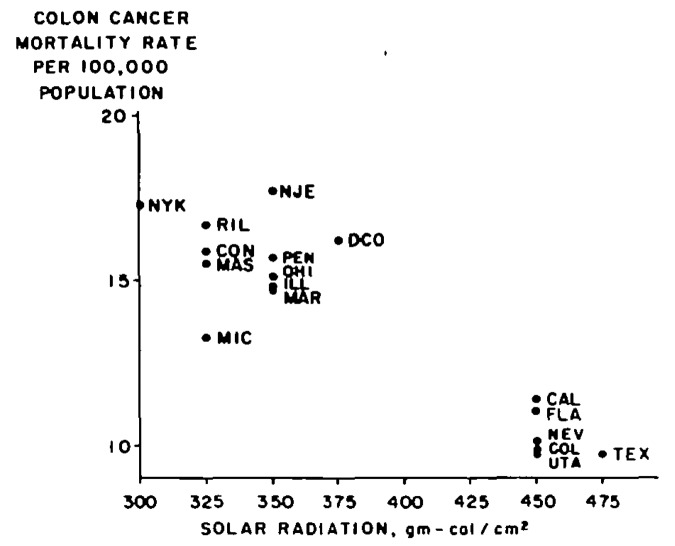
These observations essentially went unnoticed and the curious relationship of increased sun exposure and living at a lower latitude reducing risk of cancer mortality was buried in the literature. Forty years would pass before Garland et al.77 reported that there was a strong significant negative correlation with colon cancer mortality and mean daily solar radiation in the United States (Fig. 52).
Figure 52. Annual mean daily solar radiation (gm-cal/cm1) and annual age-adjusted colon cancer death rates per 100 000 population, white males, 17 metropolitan states. United States, 1959–61. Reproduced with permission from.77
They followed up these findings with an eight-year prospective case-control study of adults living in Washington County and reported that the risk of getting colon cancer decreased three-fold in people with a serum 25(OH)D > 20 ng/ml. These results together suggested that living at higher latitudes meant less exposure to vitamin D producing sunlight and therefore the connection with the first association with latitude and cancer mortality could be linked to an inverse relationship with cancer mortality and vitamin D status.78 Even in California where there is a large difference in latitude there was a positive association with colorectal cancer prevalence with latitude (Fig. 53).45
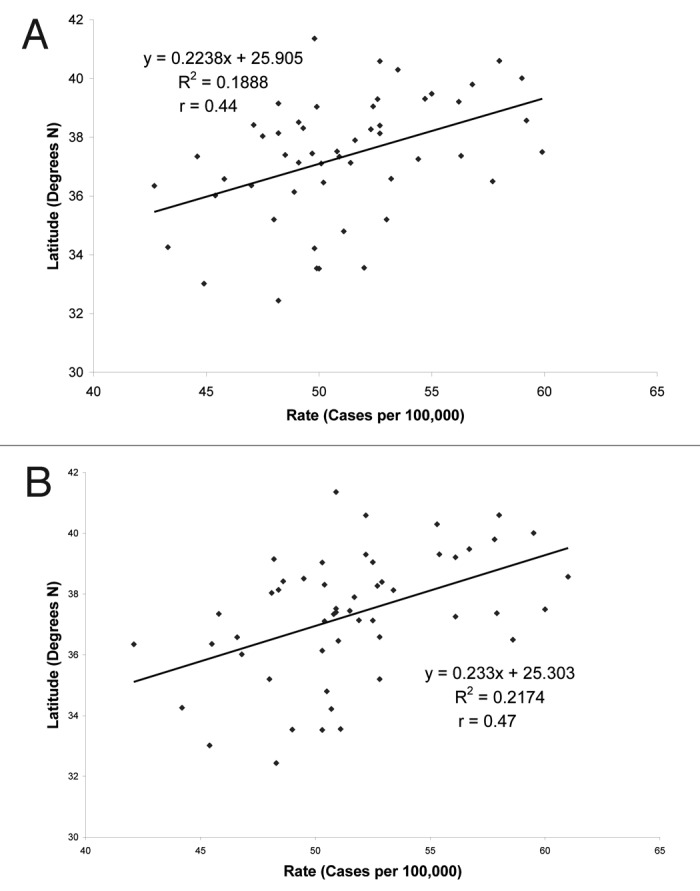
Figure 53. (A) Latitude vs. number of individuals diagnosed with colon cancer in California, independent of race. (B) Latitude vs. the number of Caucasian individuals diagnosed with colon cancer in the state of California. Reproduced with permission from.45
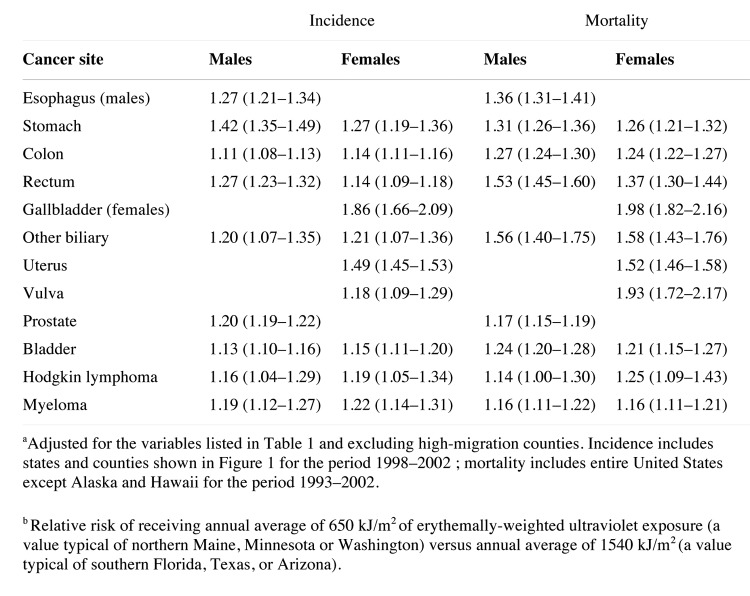
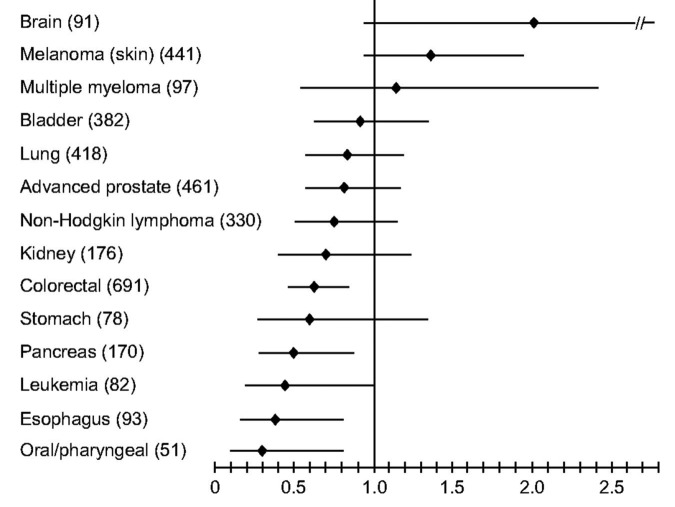
A multitude of epidemiologic studies followed these initial observations not only in the United States and Canada but worldwide. Grant79 reported a dramatic inverse relationship between premature mortality due to cancer with UV exposure in both men and women (Fig. 54). In the United States, inverse associations with exposure to solar UVB radiation and cancer risk and mortality were reported for ovarian80 and breast cancer,81 cancers of the digestive system79,82 and prostate cancer.83,84 A meta-analysis of studies reporting cancer incidence rates for more than 100 countries including Australia, China, Japan, Spain among others revealed an inverse relationship with solar UVB exposure for 15 types of cancer including bladder, breast, cervical, colon, endometrial, esophageal, gastric, lung, ovarian, pancreatic, rectal, renal and vulvar cancer as well as Hodgkin's and non-Hodgkin's lymphoma.85 Boscoe and Schymura86 found that the relative risk of cancer incidence and mortality for numerous malignancies was strongly related to solar UVB exposure (Fig. 55). Giovannucci et al.87 conducted a prospective study in men relating predictors of vitamin D status and cancer incidence and also found an inverse association (Fig. 56). Luscombe et al.88 reported men who worked outdoors had a 3-y hiatus before developing prostate cancer compared with indoor workers (Fig. 57). It was also reported that adults who developed lymphoma had a decreased risk for mortality if they had more sun exposure as a teenager.89 Knight et al.90 asked women in Canada who had breast cancer how much sun exposure they had during their teenage and young adult life and compared this sun exposure to women matched for age, ethnicity and place of residence. She concluded that women who had the most sun exposure from ages 10 to 19 y reduced their risk of developing breast cancer by more than 60% when comparing the highest quartile of outdoor activities with the lowest. Also women over 40 y who had the most sun exposure lost the benefit since their risk was no different than those who had the least sun exposure.

Figure 54. (A) Premature mortality due to cancer with insufficient UVB in white males, US, 1970–1994, vs. July 1992 DNA-weighed UVB radiation. (B) Premature mortality due to cancer, white females, vs. TOMS July 1992 DNA-weighed UVB. Reproduced with permission from.79
Figure 55. Relative risk of cancer incidence and mortality related to solar UV-B exposure, northern vs. southern United States boundary, non-Hispanic whites (95% CI in parentheses): Cancer sites with strongest evidence of an inverse association with solar UV-B exposure 86.
Figure 56. Multivariable relative risks and 95% confidence intervals for an increment of 25 nmol/L in predicted plasma 25-hydroxy-vitamin D level for individual cancers in the Health Professionals Follow-up Study (1986–2000). Number in parentheses = number of cases. Covariables included in the Cox proportional hazards model: age, height, smoking history, and intakes of total calories, alcohol, red meat, calcium, retinol, and total fruits and vegetables. Reproduced with permission from.87
Figure 57. Kaplan-Meier plot showing association of UVR exposure and age at diagnosis with prostate cancer. Reproduced with permission from.88
The observational and epidemiologic studies relating increased latitude with increased risk for cancers suggest a possible role of sun induced vitamin D3 synthesis as the beneficial factor responsible for these observations.87 It is known that exposure to sunlight also has other physiologic effects on the skin including altering the immune system,91-93 increasing production of β endorphin67 and nitric oxide.94 There are however a variety of studies including interventional studies and association studies supporting the notion that improvement in vitamin D status reduced risk of many deadly cancers.73,87,95-104 Woo et al.105 reported that more than 50% of men with completed local treatment of prostate cancer and rising PSA levels in the absence of symptoms had a decrease in their PSA serum levels when commencing the supplementation of 2000 IUs vitamin D3 per day had a statistically significant decrease in the rate of PSA rise (Fig. 58).
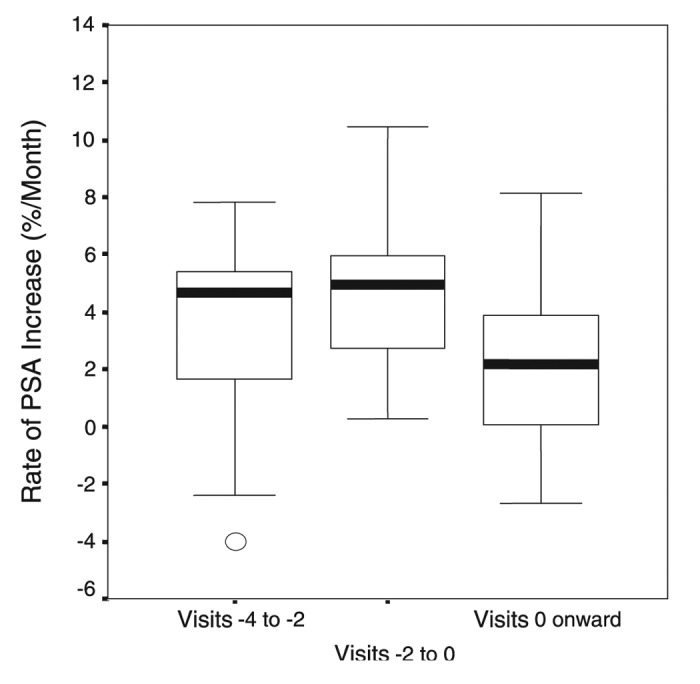
Figure 58. Effect of cholecalciferol on rate of rise of PSA. Median and quartiles of rate of PSA increase prior to starting cholecalciferol (visits –4 to –2 and visits –2 to 0) and after starting cholecalciferol (visits 0 onward). Reproduced with permission from.105
Lappe et al.101 reported a more than 60% reduction in the development of all cancers in a small study of postmenopausal women who received calcium supplementation (1500 mg) along with 1100 IUs of vitamin D3 daily compared with women who received placebo (Fig. 59). The Women's Health Initiative (WHI) initially reported that women who took 1000 mg of calcium and 400 IUs of vitamin D3 daily for up to 8 y had no reduced risk for colorectal cancer.106 However the women in this study who had a baseline 25(OH)D < 12 ng/mL had a 253% increased risk for developing colorectal cancer compared with women who had a baseline 25(OH)D of at least 23 ng/mL. Further scrutiny of the data from the WHI revealed that only 60% of the women admitted taking their calcium and vitamin D supplement at least 80% of the time. However those women not on personal calcium and or vitamin D supplementation but who took 400 IUs of vitamin D3 daily along with calcium supplementation as part of the WHI study for 8 y had a 14–20% reduced risk for developing breast cancer and a 17% reduced risk for developing colorectal cancer.107 The importance of vitamin D in reducing risk of colorectal cancer has also been supported by the observation that the vitamin D receptor polymorphisms were associated with colorectal cancer108 and a quantitative meta-analysis on the optimal status for colorectal cancer prevention showed that a 25(OH)D level of 34 ng/ml was associated with a 50% reduced risk of developing colorectal cancer.109 Another study showed that the 25(OH)D level and the risk for developing colorectal adenoma were inversely correlated and that the association was modified by the Taql polymorphism of the VDR.110 The United States Preventative Services Task Force evaluated vitamin D supplementation and risk for colorectal cancer and concluded that for every 4 ng/ml increase in circulating concentrations of 25(OH)D was associated with a 6% (95% Cl 3–9%) reduced risk for colorectal cancer.111
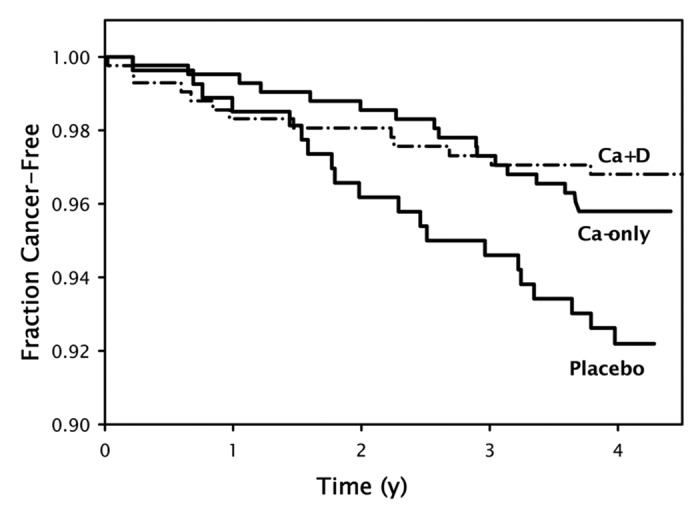
Figure 59. Kaplan-Meier survival curves (i.e., free of cancer) for the 3 treatment groups randomly assigned in the entire cohort of 1179 women. Sample sizes are 288 for the placebo group, 445 for the calcium-only (Ca-only) group, and 446 for the calcium plus vitamin D (Ca + D) group. The survival at the end of study for the Ca + D group is significantly higher than that for placebo, by logistic regression. Copyright Robert P. Heaney, 2006. Reproduced with permission.
Sunlight and Vitamin D: Innate Immune Health
Cod liver oil was used in the mid-1800s to treat tuberculosis.73,112,113 In the early 1900s heliotherapy was promoted for treating both skin and pulmonary tuberculosis.69,114 It was also recognized that young children with rickets had a much higher risk of developing pneumonia and upper respiratory tract infections and were more likely to die of them.8,14,115,116 Therefore sun exposure and vitamin D were used in the early 1900s to treat and prevent tuberculosis113,114,117 and upper respiratory tract infections.72
Hope-Simpson118 had speculated that there was a seasonal stimulus responsible for reducing infectious diseases during the summer. It is known for example that influenza infection is most prevalent in the winter months at latitudes North and South of the equator but is sporadic throughout the year in children and adults who live near the equator (Fig. 60). It has been hypothesized that the seasonal variation could be due to a seasonal variation in circulating levels of 25(OH)D.119 Several observational and intervention studies have helped to support this hypothesis. Healthy adults living in New England who had 25(OH)D blood levels of ~38 ng/mL approximately halved their risk of developing acute viral respiratory tract infections.120 School children in Japan who received 1200 IUs of vitamin D3 daily for 4 mo during the winter reduced their risk of developing influenza infection by 42%.121 A study of 156 neonates revealed that the risk for acquiring respiratory syncytial virus (RSV), a pathogen causing severe lower respiratory tract infection, was 6-fold higher in infants who had a blood level of 25(OH)D < 20 ng/mL compared with infants who had a blood level of 25(OH)D > 30 ng/mL.122
Figure 60. The seasonal and latitudinal distribution of outbreaks of type A influenza in the world, 1964–1975, summarized from the Weekly Epidemiological Record of the World Health Organization into major zones. The diagrams show for each calendar month the percentage of each zone's total outbreaks. In both north and south temperate zones the epidemics are distributed around the local midwinter, whereas the tropical zones show a transition, each approximating toward the distribution of its own temperate zone. The curve indicates the ‘midsummer’ path taken annually by vertical solar radiation. The ‘epidemic path’ seems to parallel it, but to lag 6 mo behind it. Reproduced with permission from.118
Macrophages play an important role in fighting infectious diseases by ingesting and then destroying them.123 When a macrophage ingests an infectious agent like tuberculosis toll-like receptors are activated to initiate an innate immune response.124 One of the first responses is signal transduction to the nucleus to increase the expression of the VDR and the 25-hydroxyvitaminD-1-hydroxylase (CYP27B1). This results in the conversion of 25(OH)D to 1,25(OH)2D. 1,25(OH)2D interacts with the VDR and increases the expression of cathelicidin125 which is a member of the defensin proteins and rapidly permealizes susceptible infectious agents resulting in their destruction (Fig. 61). This is one of the mechanisms believed to be responsible for vitamin D reducing risk of infectious diseases. Liu et al.125 also reported that the extent of antimicrobial activity of a monocyte exposed to Mycobacterium tuberculosis depended on the 25(OH)D levels of the medium in which the monocytes were cultured. Monocytes cultured in serum of African-American individuals who were vitamin D deficient (mean ~8 ng/mL) mounted an ineffective cathelicidin mRNA response upon exposure to M. tuberculosis, however the supplementation of the sera with 25(OH)D (mean ~30 ng/mL) restored the toll-like receptor mediated induction of cathelicidin mRNA. This was substantiated by Adams et al.126 who not only showed, that the expression of cathelicidin by monocytes exposed to M. tuberculosis lipopeptides was significantly enhanced by addition of exogenous 25(OH)D to the vitamin D deficient serum but that serum from vitamin D-supplemented subjects had the same effect. This data added support for the importance of maintaining a serum 25(OH)D > 30 ng/mL to generate an effective cathelicidin response following activation of monocytes/macrophages.
Figure 61. Metabolism of 25-hydroxyvitamin D [25(OH)D] to 1,25-dihydroxyvitamin D 1,25(OH)2D for non-skeletal functions. When a monocyte/macrophage is stimulated through its toll-like receptor 2/1 (TLR2/1) by an infective agent such as Mycobacterium tuberculosis (TB), or its lipopolysaccharide (LPS) the signal upregulates the expression of vitamin D receptor (VDR) and the 25-hydroxyvitamin D-1-hydroxylase (1-OHase). 25(OH)D levels > 30 ng/mL provides adequate substrate for the 1-OHase to convert it to 1,25(OH)2D. 1,25(OH)2D returns to the nucleus where it increases the expression of cathelicidin which is a peptide capable of promoting innate immunity and inducing the destruction of infective agents such as TB. It is also likely that the 1,25(OH)2D produced in the monocytes/macrophage is released to act locally on activated T (AT) and activated B (AB) lymphocytes which regulate cytokine and immunoglobulin synthesis respectively. When 25(OH)D levels are ~30 ng/mL, it reduces risk of many common cancers.22-32 It is believed that the local production of 1,25(OH)2D in the breast, colon, prostate, and other cells regulates a variety of genes that control proliferation. Once 1,25(OH)2D completes the task of maintaining normal cellular proliferation and differentiation, it induces the 25-hydroxyvitamin D-24-hydroxylase (24-OHase). The 24-OHase enhances the metabolism of 1,25(OH)2D to calcitroic acid which is biologically inert. Thus, the local production of 1,25(OH)2D does not enter the circulation and has no influence on calcium metabolism. The parathyroid glands have 1-OHase activity and the local production of 1,25(OH)2D inhibits the expression and synthesis of PTH. The production of 1,25(OH)2D in the kidney enters the circulation and is able to downregulate renin production in the kidney and to stimulate insulin secretion in the β-islet cells of the pancreas. Holick, copyright 2007. Reproduced with permission.
It has also been suggested based on studies in mice and in vitro that the local keratinocyte production of 1,25(OH)2D3 from 25(OH)D3 in the skin and oral pharynx enhanced the production of cathelicidin supporting the concept that maintaining serum 25(OH)D above 30 ng/ml may also be important in fighting infections in both the skin and oropharynx.127-130 This may also help explain the observation that the risk for periodontal disease is higher in adults who have the lowest circulating concentrations of 25(OH)D.131 Calcium and vitamin D supplementation was associated with a lower risk of tooth loss in elderly men and women1 respectively and with better periodontal health.132,133
Sunlight and Vitamin D: Autoimmunity Protection
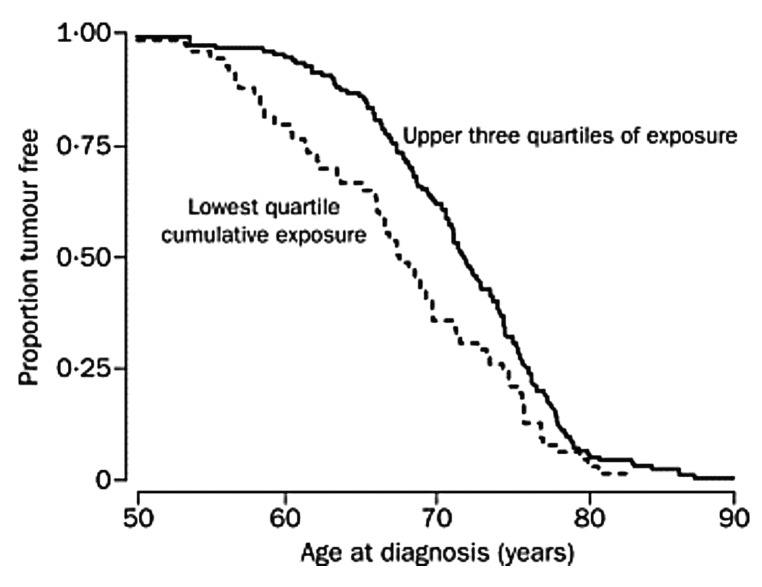
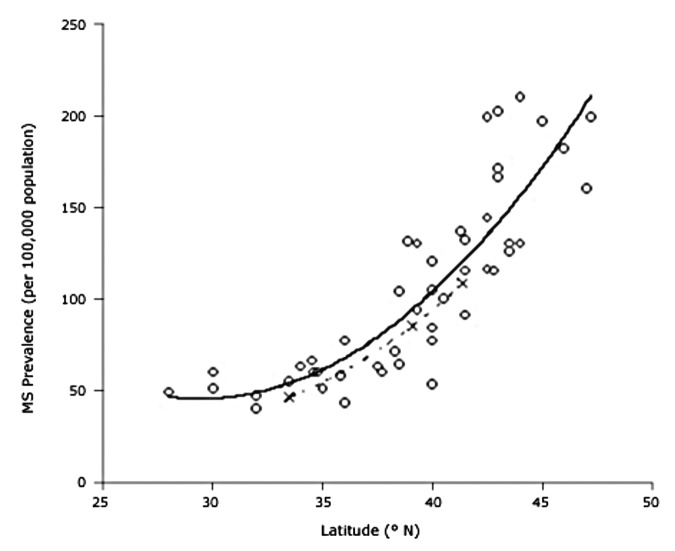
There are a variety of association studies demonstrating that being born or living near the equator reduces risk of several autoimmune diseases.73,134-139 Being born and living for the first 10 y at a latitude of ~40° North compared with ~33° North increases a person's risk of developing multiple sclerosis by 100% (Fig. 62).134,140,141 Munger et al.142 made the observation that high circulating levels of 25(OH)D were associated with a lower risk of multiple sclerosis and that women who had an intake of vitamin D of ≥ 400 IU vitamin D per day reduced their risk of developing multiple sclerosis by more than 40%.143
Figure 62. Prevalence of multiple sclerosis (MS) by latitude in the United States according to data from Noonan et al.140 ( × ) and Wallin et al.141 (o). The dashed line is a quadratic fit to the data from Noonan et al.,140 and the solid line is a fit to the data from Wallin et al.141 Reproduced with permission from.134
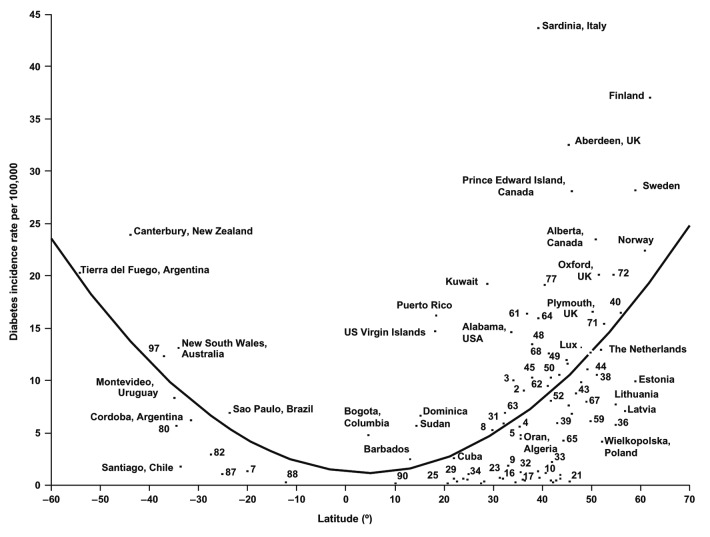
A plot of the incidence of type 1 diabetes vs. latitude demonstrated an impressive U-shaped curve. Children younger than 14 y during 1990–1994 in 51 regions worldwide demonstrated a 10–15 fold increase in risk for developing type 1 diabetes if they were born in far Northern and Southern latitudes (Fig. 63).144
Figure 63. Age-standardized incidence rates of type 1 diabetes per 100,000 boys < 14 y of age, by latitude, in 51 regions worldwide, 2002. Data points are shown by dots; names shown adjacent to the dots denote location, where space allows. Where space was limited, numerical codes (below) designate location. Source: data from WHO DiaMond [3]. Lux., Luxembourg. Numerical codes for areas: 2. Beja, Tunisia; 3. Gafsa, Tunisia; 4. Kairoan, Tunisia; 5. Monastir, Tunisia; 7. Mauritius; 8. Wuhan, China; 9. Sichuan, China; 10. Huhehot, China; 16. Nanjing, China; 17. Jinan, China; 21. Harbin, China; 23. Changsha, China; 25. Hainan, China; 29. Hong Kong, China; 31. Israel; 32. Chiba, Japan; 33. Hokkaido, Japan; 34. Okinawa, Japan; 36. Novosibirsk, Russia; 38. Antwerp, Belgium; 39. Varna, Bulgaria; 40. Denmark; 43. France; 44. Baden, Germany; 45. Attica, Greece; 48. Sicily, Italy; 49. Pavia, Italy; 50. Marche, Italy; 52. Lazio, Italy; 59. Krakow, Poland; 61. Algarve, Portugal; 62. Coimbra, Portugal; 63. Madeira Island, Portugal; 64. Portalegre, Portugal; 65. Bucharest, Romania; 67. Slovakia; 68. Catalonia, Spain; 71. Leicestershire, UK; 72. Northern Ireland, UK; 77. Allegheny, PA, USA; 80. Avellaneda, Argentina; 82. Corrientes, Argentina; 87. Paraguay; 88. Lima, Peru; 90. Caracas, Venezuela; 97. Auckland, New Zealand. Data points not labeled because of space constraints (latitude in degrees, rate per 100,000): 11. Dalian, China (39, 1.1); 12. Guilin, China (24, 0.6); 13. Beijing, China (40, 0.7); 14. Shanghai, China (32. 0.7); 15. Chang Chun, China (44, 0.6); 18. Jilin, China (43, 0.4); 19. Shenyang, China (42, 0.4); 20. Lanzhou, China (36, 0.5); 22. Nanning, China (23, 0.3); 24. Zhengzhou, China (35, 0.2); 26. Tie Ling, China (42, 0.2); 27. Zunyi, China (28, 0.1); 28. Wulumuqi, China (44, 0.9); 35. Karachi, Pakistan (25, 0.5); 37. Austria (48, 9.8); 46. Hungary (47, 8.7); 51. Turin, Italy (45, 11.9); 53. Lombardia, Italy (46, 7.6); 66. Slovenia (46, 6.8); 79. Chicago, IL, USA (42, 10.2). R2 = 0.25, p < 0.001. Reproduced with permission from.137
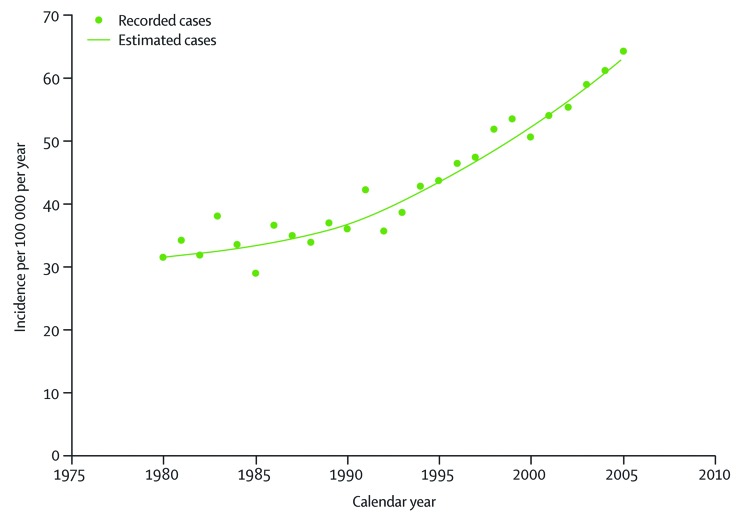
It was also reported that spring births were associated with increased likelihood of developing type 1 diabetes. These findings indicate a potential role of vitamin D in the pathogenesis of type 1 diabetes mellitus. This hypothesis is supported by an observational study that children in Finland who received 2000 IUs of vitamin D daily during their first year of life in the 1960s reduced their risk of developing type 1 diabetes 31 y later by 88%.145 Because of concern about vitamin D toxicity the amount of vitamin D recommended for infants in Finland was reduced first to 1000 IUs daily and then to 400 IUs daily. Interestingly as a result of this decrease in vitamin D intake there is an impressive increase in the incidence of type 1 diabetes occurring in Finland over the past 3 decades (Fig. 64).145
Figure 64. Incidence rate of type 1 diabetes diagnosed at or before 14 y of age in Finland. Reproduced with permission from.271
A pronounced North-South gradient has also been reported for inflammatory bowel disease, in particular Crohn's disease (Fig. 65).138 A complete data set including demographic data and lifestyle factors based on the two prospective Nurses’ Health Studies (NHSs) and comprising almost 240,000 nurses, also showed that women from lower latitudes had a consistently lower risk of developing ulcerative colitis and Crohn’s disease compared with women living in higher latitudes.139 These observations were supported by a prospective cohort study of 72,719 women enrolled in the Nurses' Health Study showed that a higher predicted vitamin D status was associated with a reduced risk of Crohn's disease.146
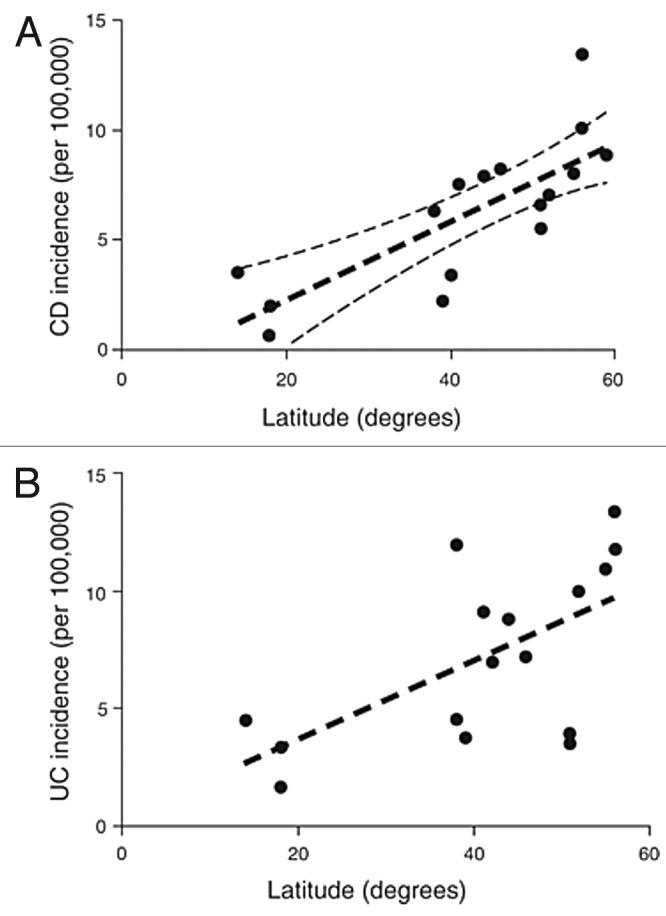
Figure 65. Variation in inflammatory bowel disease incidence rates with degrees latitude from the equator. (a) Variation in Crohn's disease incidence rates. R2 = 0.62, p = 0.0002. (b) Variation in ulcerative colitis incidence rates. R2 = 0.38, p = 0.011. Reproduced with permission from.138
A case-control study investigating the association between latitude and rheumatoid arthritis using data from the Nurses’ Health Study suggested that women living in higher latitudes were at greater risk for rheumatoid arthritis (Fig. 66).147 These latitude-dependent differences in the prevalence of rheumatoid arthritis could be explained by differences in the vitamin D status. Merlino et al.148 showed in a study in Iowa that women with the highest intake of vitamin D reduced their risk of developing rheumatoid arthritis by more than 30%.73 However, other investigators did not find such an association when reviewing the data from this study.149
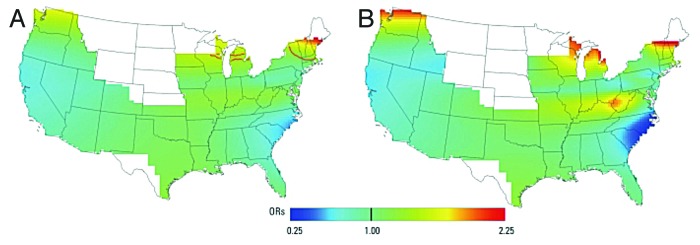
Figure 66. This figure illustrates the geographic variation in rheumatoid arthritis risk and shows a clear North-South gradient. Odds ratios are relative to the whole study area. (A) Adjusted, optimal span of 0.55 (global p = 0.034); contour lines denote areas of significantly increased (red) and decreased (blue) risk at the 0.05 level. (B) Adjusted, span of 0.20. Small span size results in more spatial variation in risk. Results for addresses at time of censoring. Reproduced with permission from.147
Although the exact mechanisms by which vitamin D may reduce risk for autoimmune diseases are not fully understood we do know that vitamin D plays an important role in cellular immunity.135 Inactivated T- and B-lymphocytes are unable to respond to 1,25(OH)2D because they lack a VDR. However when they become activated they express a VDR and are now responsive to the immunomodulatory activity of 1,25(OH)2D (Fig. 61).22,63,150
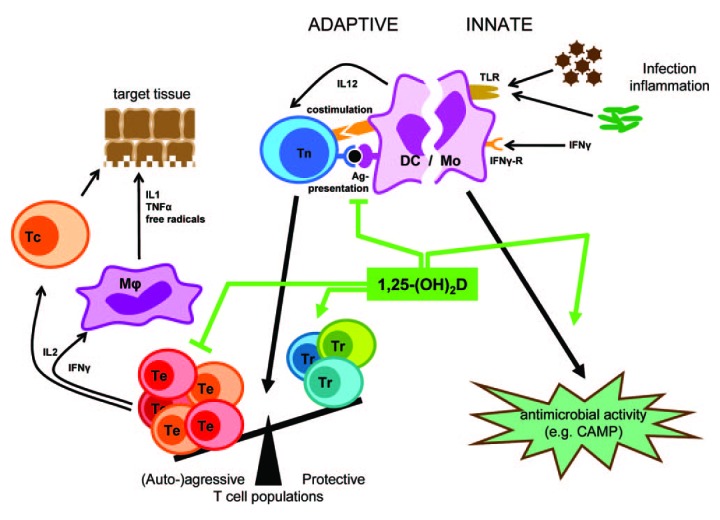
In B-cells 1,25(OH)2D downregulates immunoglobulin synthesis151 and B-cell memory. Thus by doing so it may reduce production of autoantibodies responsible for causing autoimmune diseases.152 1,25(OH)2D also has a multitude of functions in activated T cells.153-158 This hormone decreases T cell proliferation154 as well as the number of Th1-Th17 lymphocytes while increasing T-regulatory lymphocytes155 by increasing the production of Th2-Th3 lymphocytes.156 1,25(OH)2D also directly influences the expression and synthesis of several immunomodulatory cytokines. Bouillon et al.151 summarized that 1,25(OH)2D downregulates pro-inflammatory cytokines and interleukins (IL) such as IL-2, IL-4, IL-8, IL-12, tumor necrosis factor-α, and interferon-γ and upregulates anti-inflammatory interleukins such as IL-10 (Fig. 67).
Figure 67. Overview of the pharmacological effects of 1,25(OH)2D in the immune system. 1,25(OH)2D inhibits the surface expression of MHC II-complexed antigen and of costimulatory molecules, as well as the production of the cytokine IL-12 in antgen presenting cells (such as dendritic cells), thereby shifting the polarization of T cells from an (auto-)aggressive effector (Te) toward a protective or regulatory (Tr) phenotype. 1,25(OH)2D exerts its immunomodulatory effects also directly on the level of T cells. Together, these immunomodulatory effects of 1,25(OH)2D onto players of the adaptive immune system can lead to the protection of target tissues in autoimmune diseases and transplantation. In the innate immune system on the other hand, 1,25(OH)2D strengthens the antimicrobial function of monocytes and macrophages, for example through enhanced expression of the CAMP, eventually leading to better clearance of pathogenic microorganisms. Reproduced with permission from.151
Sunlight and Vitamin D: Cardiovascular Health
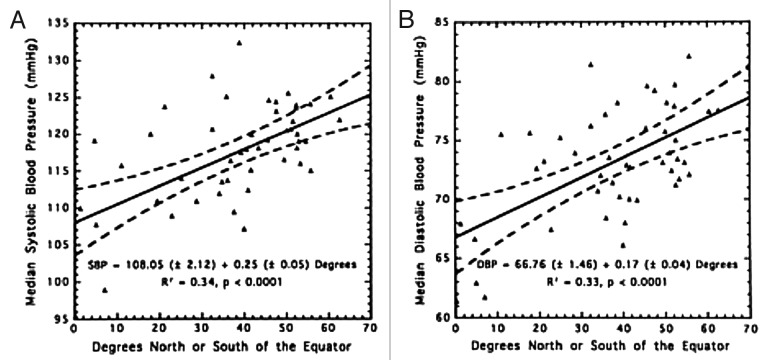
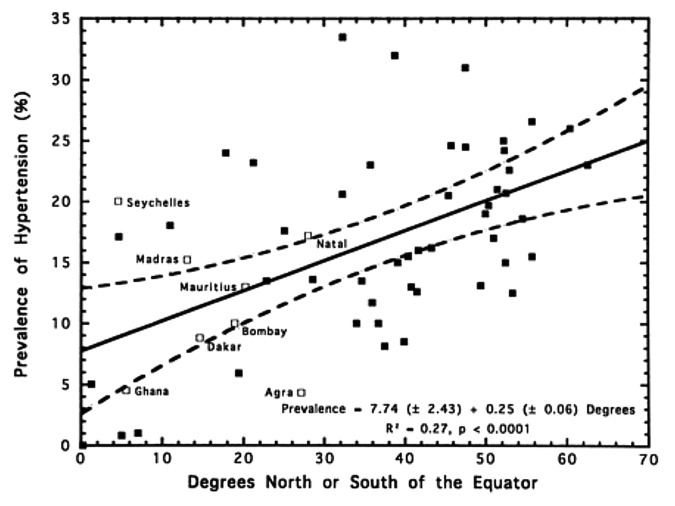
In 1997 Rostand et al.159 reported that there was an inverse association with latitude and blood pressure (Fig. 68) and the prevalence of hypertension (Fig. 69). This was followed by the observation of Krause et al.160 who reported that exposure to UVB radiation in a clinical setting not only improved circulating concentrations of 25(OH)D by more than 160% but also significantly reduced both systolic and diastolic blood pressure in patients with hypertension. A control group was exposed to the same UV lamps that were covered by an acrylic shield absorbing all UVB radiation and thus was exposed to UVA radiation only. The control group’s subjects demonstrated no significant change in their circulating concentrations of 25(OH)D as well as no change in their hypertension (Fig. 70). These data suggested that vitamin D may somehow be involved in cardiovascular health. One of the first insights as to how vitamin D could influence cardiovascular health was the observation that 1,25(OH)2D3 suppressed the production of renin.161 This observation was also supported by the report that VDR knockout mice have a dysregulation of the renin-angiotensin-aldosterone axis.162
Figure 68. (A) Relationship of mean systolic blood pressure (SBP) and distance north or south of the equator. Symbols represent north or latitudes of INTERSALT Centers. (B) Relationship of mean diastolic blood pressure (DBP) to distance north or south of the equator. For both figures, broken lines represent 95% confidence limits. Reproduced with permission from.159
Figure 69. Relationship of prevalence of hypertension to distance north or south of the equator. Labeled open boxes represent non-INTERSALT centers; solid boxes are INTERSALT centers. Broken lines represent 95% confidence limits. Regression line and confidence limits are derived from INTERSALT centers only. Reproduced with permission from.159
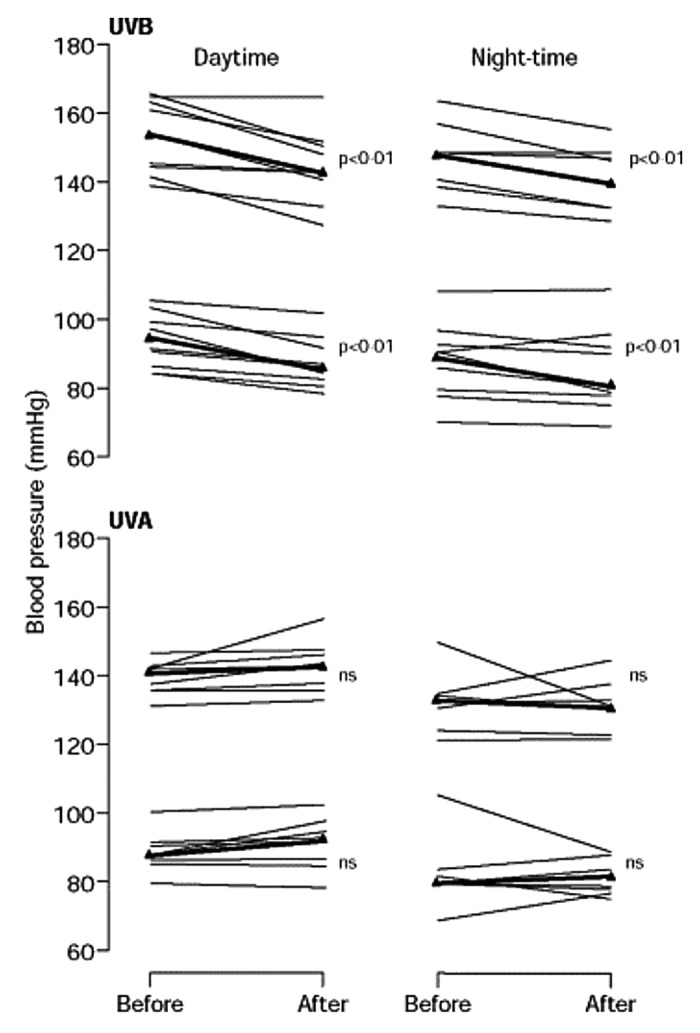
Figure 70. Effect of UV irradiation on ambulatory daytime and night-time blood pressures was non-significant. Thick line represents the mean. Reproduced with permission from.160
There have been a multitude of association studies suggesting that vitamin D deficiency not only increases risk for a myocardial infarction by as much as 50% but also was associated with more than one 100% increased risk of mortality from the heart attack.73,106,163-168 In the US an estimated 50 million teenagers are vitamin D deficient or insufficient and this was associated with a 2.4 fold increased risk for high blood pressure.167,169 Dong et al.168 conducted a 16-week randomized, blinded, clinical trial in 49 normotensive black boys and girls aged 16.3 ± 1.4 y to evaluate the effect of enhancing vitamin D intake from 400 IUs or 2000 IU vitamin D3 daily on arterial wall stiffness, determined by measuring carotid-femoral pulse wave velocity. The teenagers who received 400 IUs of vitamin D daily increased their circulating concentrations of 25(OH)D from 14 to 24 ng/mL and had an increased in the carotid-femoral pulse wave velocity (5.38 ± 0.53 m/sec to 5.71 ± 0.75 m/sec; p = 0.016). By contrast teenagers who received 2000 IUs of vitamin D daily for 4 mo not only increased their blood level of 25(OH)D from 13 to 34 ng/mL but also showed a significant decrease in carotid-femoral pulse wave velocity (5.41 ± 0.73 m/sec to 5.33 ± 0.79 m/sec; p = 0.031).
Two major contributing factors for cardiovascular disease are type 2 diabetes and obesity.170 It is well known both in children and adults that there is an inverse association with serum concentrations of 25(OH)D with body mass index (BMI) due to a sequestration and volumetric dilution of the lipophilic vitamin D in the fat tissue.73,171-174 Furthermore there is also an association with vitamin D deficiency and increased risk for type 2 diabetes.175,176 A similar observation was made in the Nurses’ Health Study where a combined daily intake of > 1200 mg calcium and > 800 IU vitamin D was associated with a 33% lower risk of type 2 diabetes.177 An inverse association between vitamin D status and diabetes was also shown in a study by Scragg et al.178 The odds ratio for diabetes in non-Hispanic whites and Mexican Americans who had 25(OH)D levels in the highest quartile compared with the lowest was reduced by up to 83%.178 However, this inverse association was not observed in non-Hispanic blacks.178
Several epidemiologic studies and prospective studies have reported a highly significant association with vitamin D deficiency with not only type 2 diabetes but also hypertension, hyperlipidemia and peripheral vascular disease all causative factors for coronary artery disease, myocardial infarction, heart failure and stroke.166,175,179 The prospective Intermountain Heart Collaborative Study revealed that in more than 40,000 participants a circulating concentration of 25(OH)D < 15 ng/mL compared with a concentration of > 30 ng/mL significantly increased all of these risk factors.175 A meta-analysis examining the association between vitamin D status or vitamin D supplementation revealed that adults with the highest circulating concentration of 25(OH)D had a 43% lower risk of developing cardiometabolic disorders compared with adults with low levels of 25(OH)D.180 Furthermore a prospective study following up with more than 2000 adults showed that the risk of progression from pretype 2 diabetes to type 2 diabetes was reduced by 48% in adults who had the highest circulating concentrations of 25(OH)D compared with those with the lowest.181
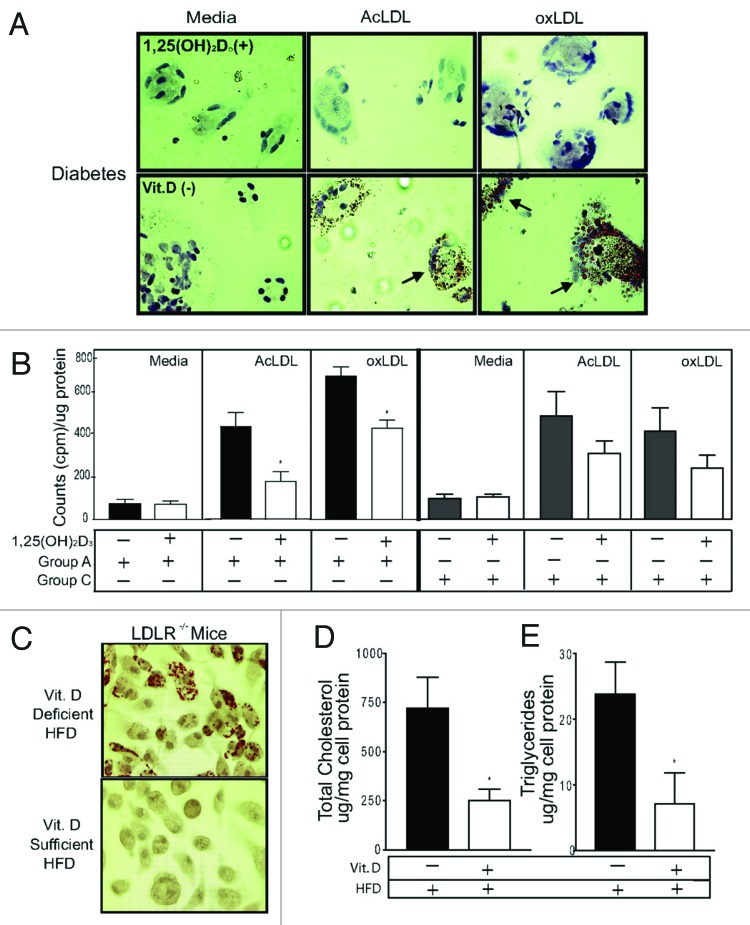
Beta islet cells in the pancreas have a VDR and 1,25(OH)2D3 is a potent stimulator of insulin production.182 Improvement in vitamin D status has also been associated with improvement in insulin sensitivity183 mediated by upregulation of insulin receptors.184 There is evidence that vascular smooth muscle and cardiomyocytes have a VDR73,166,171 and that 1,25(OH)2D3 causes vascular relaxation by suppressing the renin-angiotensin-aldosterone system73,163,185,186 and improves cardiomyocyte contractility.171 In addition 1,25(OH)2D3 inhibits macrophage cholesterol uptake and foam cell formation thereby reducing risk for atherosclerotic plaque formation (Fig. 71).73,166,187 Vitamin D deficiency negatively affects numerous physiological processes that are important in the pathogenesis of cardiovascular disease. This could explain why vitamin D deficiency is associated with an increased overall and cardiovascular mortality in patients with metabolic syndrome (Fig. 72).188
Figure 71. 1,25(OH)2D3 prevents foam cell formation. Macrophages stained with Oil Red O. (A) Diabetic subjects (group A). Top, 1,25(OH)2D3-treated cells; bottom, vitamin D–deficient cells. Arrowheads indicate foam cells. (B) Cholesteryl ester formation in macrophages from diabetics (group A) incubated in vitamin D–deficient (black bars) or 1,25(OH)2D3-supplemented (white bars) media or in macrophages from nondiabetic, vitamin D–deficient nondiabetic controls (group C) (n = 8 per group) incubated in vitamin D–deficient (gray bars) or 1,25(OH)2D3-supplemented (white bars) media (*p < 0.01 vs vitamin D deficient). (C) Oil Red O stain. (D) Cholesterol. (E) Triglycerides from peritoneal macrophages from LDR−/− mice fed vitamin D–deficient or –sufficient high-fat diet (n = 5 per group) (*p < 0.05 vs vitamin D deficient). Reproduced with permission from.187
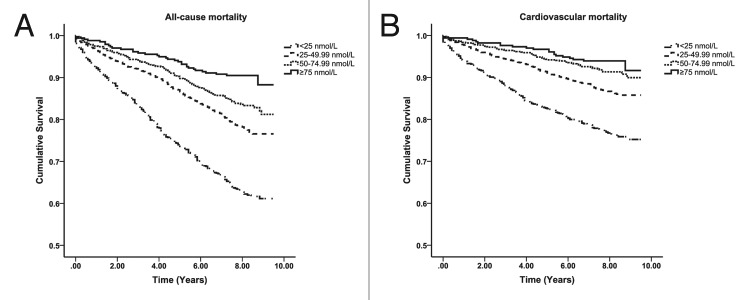
Figure 72. Kaplan-Meier plots for all-cause (left) and cardiovascular mortality (right) according to 25(OH)D groups in those with the metabolic syndrome. Log-rank analysis indicated a significant difference between all 25(OH)D groups (p = 0.001). Reproduced with permission from.188
Sunlight, Vitamin D and Mental Health
Schizophrenia has been associated with inadequate sun exposure and vitamin D deficiency (Fig. 73).189 Schizophrenia is more common in the Scandinavian countries.189,190 Winter births have been associated with an increased risk for developing schizophrenia later in life even in Australia.191,192 In British immigrants, incidence in schizophrenia is higher in children of immigrants from the Caribbean who moved to cities in countries farther North.193 Finnish male infants who received 2000 IUs of vitamin D daily during their first year of life reduced their risk of developing schizophrenia by 77% compared with infants who received less than 2000 IUs of vitamin D daily.194
Figure 73. Association between latitude and schizophrenia prevalence on several continents. Reproduced with permission from.189
Vitamin D could play an indirect role in the pathogenesis of schizophrenia. Several studies suggest that a prenatal influenza exposure increases the risk for schizophrenia later in life.195-197 The vitamin D status seems to influence the risk for an influenza infection respectively vitamin D supplementation has proven to decrease the risk for influenza infection. 2–4118,119,121
There are a variety of association studies relating vitamin D deficiency with increased risk for depression,198,199 Alzheimer disease,200 epilepsy,201 and neurocognitive decline.202,203 The brain not only has a VDR but also a 1-OHase.204 Evidence suggests that 1,25(OH)2D3 could increase calcium binding protein expression,205 although this could not be shown in all studies.206 1,25(OH)2D3 could also act by increasing serotonin levels in the brain.207,208 Furthermore 1,25(OH)2D3 has also been demonstrated to stimulate amyloid-β phagocytosis and clearance by macrophages in Alzheimer patients.209 This may help explain the association between neurocognitive decline,202,203 dementia,210 depression,198,199 and Alzheimer disease200 with a high prevalence of vitamin D deficiency.211 In a community setting depressed adults had significantly lower serum concentrations of 25(OH)D than those without depression.212
Approaches for Preventing and Treating Vitamin D Deficiency
The Institute of Medicine using a population model defined vitamin D deficiency for bone health as a circulating concentration of 25(OH)D < 20 ng/mL. They recommended that to satisfy 97.5% of the United States population’s needs for vitamin D that children 0–1 y, and adults 1–70 y and 70+ years require 400, 600, 800 IUs of vitamin D daily respectively (Fig. 74).213 The Endocrine Society used a medical model to make recommendations for the prevention and treatment of vitamin D deficiency [25(OH)D < 20 ng/mL] and vitamin D insufficiency [25(OH)D of 21–29 ng/mL] and concluded that a range rather than an absolute amount of vitamin D could be recommended for children 0–1 y, children 1–18 y and all adults of 400–1000 IUs, 600–1000 IUs and 1500–2000 IUs of vitamin D daily respectively (Fig. 74).24
Figure 74. Recommendations of the Institute of Medicine and the Endocrine Society Practice Guidelines for daily vitamin D supplementation to prevent vitamin D deficiency. Reproduced with permission from.24
Both the IOM213 and The Endocrine Society24 concluded that a circulating concentration of 25(OH)D up to 100 ng/mL was safe. They also found that most but not all of the literature supports the concept that vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25(OH)D.24,73,213-221
For almost 100 y a variety of strategies have been used to treat and prevent vitamin D deficiency especially in children.5,68,73,114 From 1930 through 1950s parents purchased a lamp at their local pharmacy that emitted vitamin D3 producing UVB radiation (Fig. 75).70,222,223 Children wearing eye protection had their arms, abdomen and legs were routinely exposed to a UV emitting lamp several times a week (Fig. 6).12,13 In Russia children in school in wintertime were routinely exposed to a mercury arc lamp placed in the center of the school room that emitted UVB radiation to prevent vitamin D deficiency rickets (Fig. 76).224
Figure 75. Various UVB lamps including Sperti and Sun Kraft used for vitamin D production and rickets prevention. Holick, copyright 2013. Reproduced with permission.
Figure 76. Russian children who are being exposed to UVB radiation. Reproduced with permission from.272
The Sperti lamp which originally was designed with a single mercury arc lamp225,226 was commonly used in the United States in the 1940s and 1950s to prevent rickets and children (Fig. 75).8,70,114 This lamp was also effective in improving the circulating concentrations of 25(OH)D in individuals who had cystic fibrosis and who were unable to absorb vitamin D from dietary and supplemental sources (Fig. 77A and B).227,228 Because the lamp produced a lot of heat the Sperti lamp was redesigned and the mercury arc lamp was replaced with 4 fluorescent lamps (Fig. 78) that emitted UVB radiation and produced previtamin D3 (Fig. 79).229 This lamp was effective in raising circulating concentrations of 25(OH)D in healthy adults with skin types 2 and 3 (Fig. 80).227,228
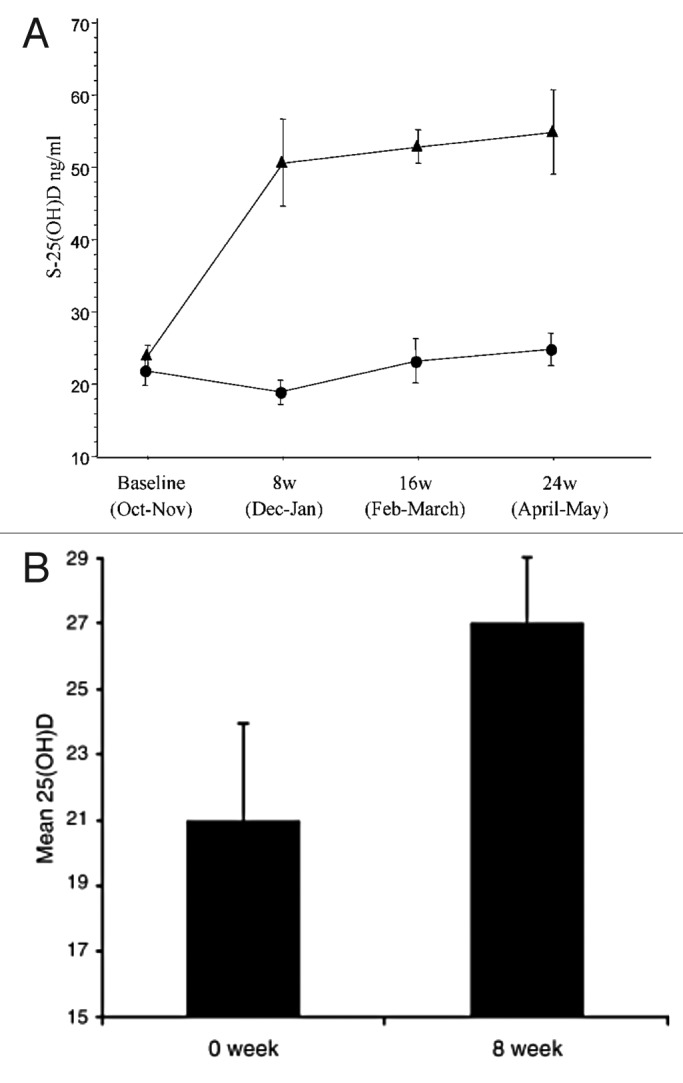
Figure 77. (A) Mean (SEM) serum levels of 25(OH)vitamin D in patients with cystic fibrosis, treated with UVB (▲) (n = 9), and non-treated CF patients as controls (●) (n = 14) at baseline and after 8, 16 and 24 weeks. There were significant differences between the groups at all time points except at baseline (ANOVA, p < 0.0001). (B) Mean (± SEM) serum 25-hydroxyvitamin D concentration (ng/mL) before and after 8 weeks of UV light to cystic fibrosis (CF) subjects. Serum 25-hydroxyvitamin D [25(OH)D] levels in the five CF subjects at baseline were 21 ± 3 ng/ml, which increased to 27 ± 4 ng/ml at the end of 8 weeks (p = 0.05). Reproduced with permission from.227

Figure 78. Photograph of a current version of the Sperti lamp with four fluorescent lamps. Holick, copyright 2013. Reproduced with permission.
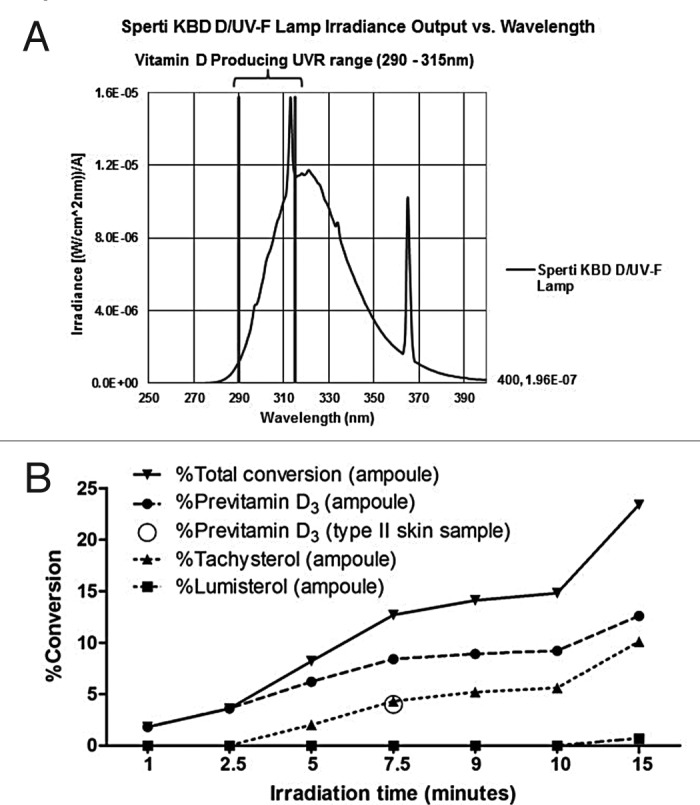
Figure 79. (A) The Sperti KBD D/UV-F lamp irradiance output overlaps with UV wavelengths necessary for cutaneous vitamin D3 production (290–315nm). (B) Relationship between UV irradiation time and conversion of 7-DHC to previtamin D3, lumisterol, and tachysterol in borosilicate glass ampoules containing 7-DHC. Conversion of 7-DHC to previtamin D3 in a type II human skin sample is represented by the open circle. Reproduced with permission from.229
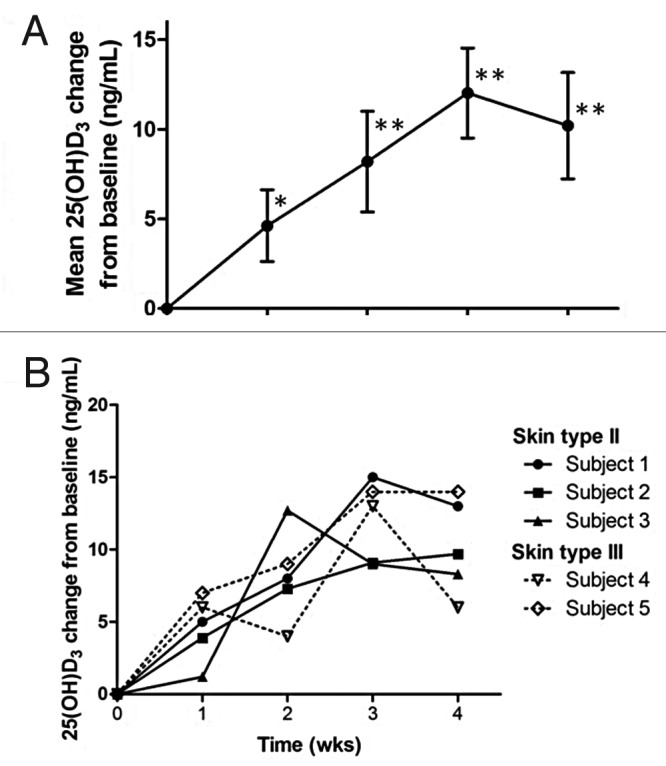
Figure 80. (A) Mean change in serum 25(OH)D3 levels (ng/mL) compared with baseline among the five subjects during the study, error bars represent standard deviation. (*) denotes p < 0.01 and (**) denotes p < 0.005 compared with baseline serum 25(OH)D3. (B) Changes in serum 25(OH)D3 (ng/mL) in each individual subject compared with baseline. Reproduced with permission from.229
Tanning beds which emit UVB radiation (estimated about 95% of tanning beds in the United States) can be a good source of vitamin D especially for patients with malabsorption syndromes.227,228 A patient with Crohn's disease and only 2 feet of her small intestine remaining had severe debilitating osteomalacic bone discomfort. Supplementing 400 IU dietary vitamin D from a multivitamin and 200 IU vitamin D from total parenteral nutrition couldn’t correct her severe vitamin D deficiency. Exposure to a tanning bed emitting UVB radiation was effective in improving her circulating concentration of 25(OH)D and as a result markedly improved her bone discomfort (Fig. 81).230 Tanners in Boston who frequented a tanning salon at least once a week had robust healthy circulating concentrations of 25(OH)D on average 46 ng/mL compared with age and sex matched controls whose blood level on average was 24 ng/mL (Fig. 82). Furthermore an evaluation of their bone mineral density revealed that the tanners had a significantly higher bone mineral density in their hip compared with the control group.231
Figure 81. Serum 25(OH)D, PTH and calcium levels in a patient with Crohn’s disease who had whole-body UVB exposure for 10 min 3 times a week for 6 mo. Reproduced with permission from.230
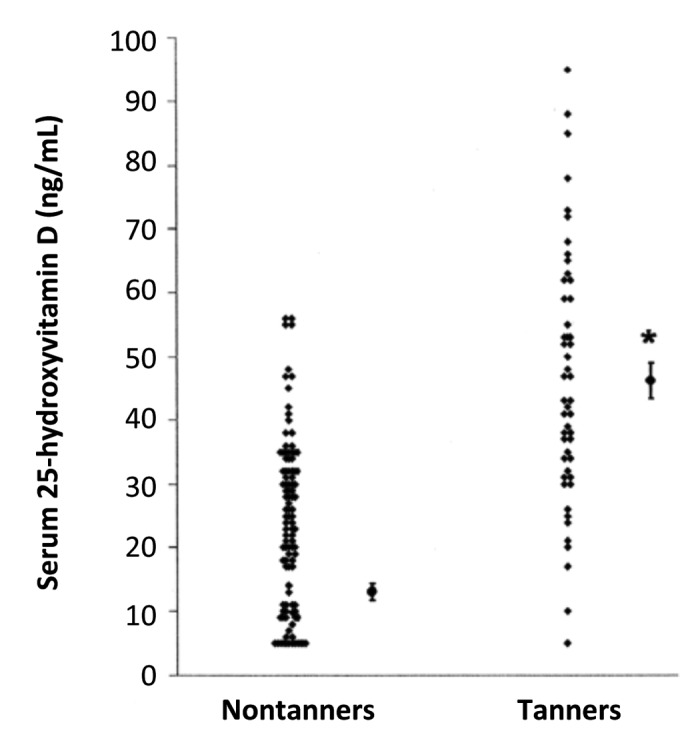
Figure 82. Mean (± SEM) serum 25-hydroxyvitamin D concentrations in tanners and nontanners. Single points for each category are means ± SEMS. *Significantly different from nontanners, p < 0.001. Reproduced with permission from.231
Sensible sun exposure can also be an excellent source of vitamin D for both children and adults.232,233 Sensible means never to be exposed to an amount of sunlight that would cause a sunburn since this is the major cause for both melanoma and non-melanoma skin cancer.234-237 Studies conducted worldwide using the in vitro ampule model39 and measuring 25(OH)D levels after quantitative UVB exposure in a tanning bed have been used to develop guidelines for sensible sun exposure based on latitude, season, time of day, altitude, and skin sensitivity, i.e., degree of skin pigmentation.22,46,51,66,231–233,238,2397–14 The rule of thumb is to be exposed to an amount of sunlight that is about 50% of what it would take to cause a mild sunburn i.e., slight pinkness to the skin 24 h later (minimal erythemal dose) followed by good sun protection i.e., clothing, hat and or sunscreen.22,239 The “rule of nines” helps to estimate the percentage of skin exposed to sunlight or UVB radiation and can be used to calculate the amount of vitamin D3 being produced. The face accounts for 9% of the body surface, each arm for 9%, each leg for 18%, and the abdomen and the back for 18% each.240 Exposure of the whole body in a bathing suit to 0.5 MED of UVB radiation is approximately equivalent to ingesting about 7000–10,000 IUs of vitamin D2.22,25,31 Therefore exposing 20% of the body surface to an amount of sunlight equal to 0.5 MED is equivalent to ingesting approximately 1400–2000 IUs of vitamin D3. This is effective for all skin types and the increase in serum 25(OH)D attained from exposure to UVB radiation is often more effective than ingesting 1000 IU vitamin D2 or vitamin D3 daily (Fig. 83).241 Always protect the face with a hat or sunscreen since it provides very little vitamin D3 and is most sun exposed and more prone to skin damage and skin cancer from sun exposure.242-244
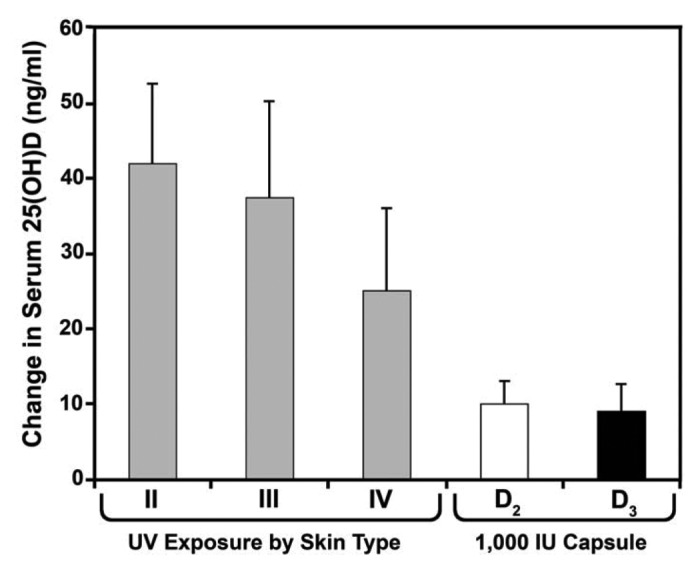
Figure 83. Comparison of the percentage increase in serum 25(OH)D levels of healthy adults who were in a bathing suit and exposed to suberythemal doses (0.5 MED) of UV B radiation once a week for 3 mo with healthy adults who received either 1000 IU of vitamin D2 or 1000 IU of vitamin D3 daily during the winter and early spring for a period of 11 weeks. Fifty percent increase represented approximately 10 ng/ml from baseline 18 ± 3 to 28 ± 4 ng/ml. Skin type is based on the Fitzpatrick scale: Type II always burns, sometimes tans; type III always burns, always tans; type IV sometimes burns, always tans; type V never burns, always tans. Data are means ± SEM. Holick, copyright 2008. Reproduced with permission.
Because foods contain very little vitamin D it is difficult to obtain enough vitamin D from dietary sources even when consuming foods fortified with vitamin D.22,51,246 The exception is indigenous populations including Inuits who consumed foods with high content of vitamin D such as oily fish, seal and whale blubber and polar bear liver.247 Therefore it is necessary without adequate sun exposure to improve childrens’ and adults’ vitamin D status by encouraging them to take a vitamin D supplement (Fig. 74).22,24,213
Infants should receive 400 IUs of vitamin D soon after they are born. This has been endorsed by the American Academy of Pediatrics, the Endocrine Society and the Institute of Medicine.24,213,248 However infants who are vitamin D deficient should be aggressively treated with pharmacologic doses of vitamin D in order to build up the body stores and quickly correct the vitamin D deficiency. The best method to treat and cure rickets is to give a total dose of 5–15 mg (200,000–600,000 IUs) of vitamin D2 or vitamin D3 orally with adequate dietary calcium.8,249 These doses can be given safely either as a single-day therapy or as daily doses of 2000–4000 IUs/day (50–100 µg/d) for 3–6 mo.8
Children one year and older should receive at least 600 IUs of vitamin D daily. The Endocrine Society recommends at least 600 IUs and up to 1000 IUs daily is safe and effective to prevent vitamin D deficiency and insufficiency.24 Infants and toddlers who received 50,000 IUs of vitamin D2 once a week or 2000 IUs of vitamin D2 or vitamin D3 daily for 6 weeks corrected their vitamin D deficiency without any untoward side effects.8,24,217 A study done in the young Lebanese girls who received 14,000 IUs of vitamin D weekly for one year were able to maintain their blood level of 25(OH)D in what is considered to be a healthy physiologic range above 30 ng/mL.250
The Endocrine Society recommends that all adults receive 1500–2000 IUs of vitamin D daily.15 A study in healthy adults in Boston who had a baseline serum concentration of 25(OH)D ~18 ng/mL in the winter revealed that ingesting 1000 IUs of vitamin D2 or vitamin D3 was ineffective in raising and maintaining blood levels 25(OH)D above 30 ng/mL (Fig. 84).215 This is not at all unexpected since it is documented that for every 100 IUs of vitamin D ingested the circulating concentration of 25(OH)D increases by approximately 0.6–1.0 ng/mL.215,251
Figure 84. Mean (± SEM) serum 25(OH)D levels after oral administration of vitamin D2 and/or vitamin D3. Healthy adults recruited at the end of the winter received placebo (•; n = 14), 1000 IU vitamin D3 (D3, ■; n = 20), 1000 IU vitamin D2 (D2, ▴; n = 16), or 500 IU vitamin D2 and 500 IU vitamin D3 [D2 and D3, ♦; n = 18) daily for 11 weeks. The total 25(OH)D levels are demonstrated over time. *, p = 0.027 comparing 25(OH)D over time between vitamin D3 and placebo; **, p = 0.041 comparing 25(OH)D over time between 500 IU vitamin D3 plus 500 IU vitamin D2 and placebo; ***, p = 0.023 comparing 25(OH)D over time between vitamin D2 and placebo. Reproduced with permission from.249
Because vitamin D is fat soluble upon its ingestion or production in the skin vitamin D3 gets incorporated into the body fat and is also transported to the liver to be converted to 25(OH)D.22,73,171-174 As a result to treat vitamin D deficiency and prevent recurrence, vitamin D can be given daily, weekly and even monthly with the same outcome i.e., improvement in circulating concentrations of 25(OH)D.24 One strategy that is effective to quickly fill up the empty vitamin D tank is to give 50,000 IUs of vitamin D2 or 50,000 IUs of vitamin D3 once a week for 8 weeks.252 This is equivalent to ingesting approximately 6600 IUs of vitamin D daily.253 To prevent recurrence of vitamin D deficiency patients have been instructed to take 50,000 IUs of vitamin D2 (equivalent to 3300 IUs of vitamin D daily) once every 2 weeks forever. This strategy has been effective in maintaining blood levels of 25(OH)D in the range of 40–60 ng/mL for up to 6 y without any toxicity (Fig. 85).253 Other strategies that have been equally effective have been to take 50,000 IUs of vitamin D daily for several days followed by maintenance therapy. Patients who have a BMI > 30 often need 3–5 times as much vitamin D to both treat and prevent recurrence of vitamin D deficiency.24 Patients with malabsorption syndromes or who have had gastric bypass surgery may require 50,000 IUs of vitamin D at least up to 7 times a week.22 Monitoring serum levels of 25(OH)D is important to prevent toxicity. Patients on glucocorticoids, anti-seizure medications and AIDS medications may also need more vitamin D to both treat and prevent vitamin D deficiency.22 Patients however with granulomatous disorders such as sarcoidosis have a hypersensitivity to vitamin D because of the uncontrolled conversion of 25(OH)D to 1,25(OH)2D by activated macrophages within the granulomas. This can also occur in patients with some lymphomas.22,24
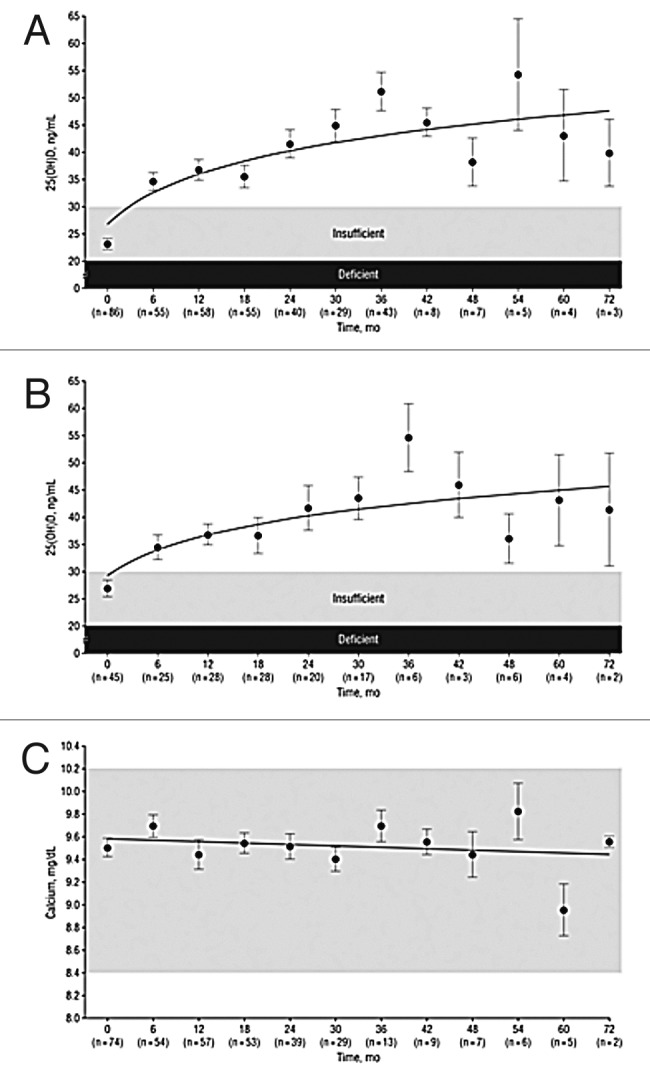
Figure 85. Mean serum 25-hydroxyvitamin D (25[OH]D) and calcium levels. Results are given as mean (SEM) values averaged over 6-mo intervals. Time 0 is initiation of treatment. (A) Mean 25(OH)D levels in all patients treated with 50 000 IU of ergocalciferol (vitamin D2) every 2 weeks (maintenance therapy, n = 86). Forty-one of the patients were vitamin D insufficient or deficient and first received 50 000-IU ergocalciferol weekly for 8 weeks before being placed on maintenance therapy of 50 000 IU of ergocalciferol every 2 weeks. The mean 25(OH)D level of each 6-mo interval was compared with initial mean 25(OH)D level and showed a significant difference of p < 0.001 for all time points. To convert 25(OH)D to nanomoles per liter, multiply by 2.496. (B) Mean serum 25(OH)D levels in patients receiving maintenance therapy only. There were 38 patients who were vitamin D insufficient (25[OH]D levels < 21–29 ng/mL and 7 patients who were vitamin D sufficient (25[OH]D levels ≥ 30 ng/mL) who were treated only with maintenance therapy of 50 000 IU of ergocalciferol (vitamin D2) every 2 weeks. The mean 25(OH)D levels in each 6-mo interval were compared with mean initial 25(OH)D levels and showed a significant difference of p < 0.001 for all time points up to 48 mo. The data for interval months 60 and 72 were pooled, and there was a significant difference of p < 0.01 compared with the baseline value. (C) Serum calcium levels. Results for all 86 patients who were treated with 50 000 IU of ergocalciferol (vitamin D2). The reference range for serum calcium level is 8.5 to 10.2 mg/dL (to convert to millimoles per liter, multiply by 0.25). Reproduced with permission from.252
Concern about Vitamin D Intoxication
Vitamin D intoxication is one of the rarest medical conditions and is often caused by inadvertent or intentional ingestion of extremely high doses of vitamin D for prolonged periods of time. Vitamin D intoxication is associated with hypercalcemia, hyperphosphatemia, suppression of PTH that can lead to nephrocalcinosis and soft tissue calcification especially of blood vessels. Usually vitamin D intoxication is not observed until a 25(OH)D > 200 ng/mL.254-256
No matter how much sun exposure a person has this will never cause vitamin D intoxication because sunlight itself destroys any excess vitamin D and previtamin D.256 However there are several reports in adults that ingesting up to 1 million IUs of vitamin D3 daily for several months can raise blood levels of 25(OH)D > 500 ng/mL which was associated with hypercalcemia in the range of 15 mg/dL. Often simply removing all sources of vitamin D along with hydration can result in the serum calcium returning to normal within a relatively short period of time and with no sequelae.254-257 A recent report of a 3 mo old inadvertently receiving 14,000 IUs of vitamin D3 daily for 20 d (i.e., total of 280,000 IUs of vitamin D3) and achieving a circulating concentration of 25(OH)D of 425 ng/mL with suppression of PTH demonstrated no significant change in either the infant's serum calcium or phosphorus level and no change in kidney function demonstrates that short-term high doses of vitamin D resulting in very high serum concentrations of 25(OH)D > 400 ng/mL was well tolerated even in infants.258 Even pregnant women who received 4000 IU vitamin D/day through their pregnancy showed no change in either serum calcium or urinary calcium secretion.259 It is prolonged intake of extremely high doses of vitamin D for at least several months that not only markedly increases the circulating concentrations of 25(OH)D > 200 ng/mL but also results in hypercalcemia, hyperphosphatemia and if untreated can lead to kidney failure, soft tissue calcification and ultimately death.22,260
Conclusion and Perspective
Our ancestors routinely worshiped the sun for its life giving properties (Fig. 86).261,262 It is curious that some of the earliest photosynthetic life forms for more than 500 million years have been producing vitamin D and that throughout evolution most vertebrates including humans have depended on sun exposure for their skeletal health.263 The driver for the evolution of hypopigmented humans i.e., Caucasians is likely due to the need to have more vitamin D producing solar UVB radiation to penetrate into the skin to produce vitamin D3. Females born with vitamin D deficiency and suffering from infantile rickets resulted in them having a flat pelvis and a small pelvic outlet. These females although fertile would have had a difficult time, if not impossible, to give vaginal birth resulting in both maternal and fetal death.54,55 Indeed it was because of the vitamin D deficiency pandemic in late 1800s that Cesarean sectioning became common practice for the delivery of healthy children of mothers who had suffered from vitamin D deficiency in utero and during their first few years of life.8,54,55 Vitamin D deficiency in pregnant women today is still associated with a 400% increase in the predicted probability for a Cesarean section (Fig. 87).54
Figure 86. Egyptian painting showing the pharao and the queen being exposed to sunshine.
Figure 87. This graph shows the association between mother’s increasing 25(OH)D level in nmol/L, and decreasing predicted probability of having a Cesarean section vs. vaginal delivery, with a quadratically fit line. The predicted probabilities of Cesarean section are derived from a multivariate logistic regression model controlling for mother’s age, education, insurance status, and race. Additionally, the model controls for reporting ever drinking alcohol during pregnancy, as this was statistically significant in univariate analysis and remained statistically significant at the p < 0.05 level in multivariate analysis. Reproduced with permission from.54
It is remarkable that for more than 100 y investigators have been reporting an inverse association with latitude and many chronic illnesses including common cancers,85 several autoimmune diseases including type 1 diabetes and multiple sclerosis73,134-139 as well as hypertension.159 In addition the revelation that exposure to sunlight or UV radiation could cure and prevent rickets12,13 led to the widespread recommendation by health regulators and government agencies to encourage sensible sun exposure, i.e., amount of sun that would be beneficial for producing vitamin D and reducing risk for rickets while preventing sunburning (Fig. 8).
The global appreciation of the beneficial effects of vitamin D for health lead to widespread vitamin D fortification throughout Europe and the United States in the 1930s-1940s. Not only milk but hot dogs, soda, custard, bread, cereals and even beer was fortified with vitamin D (Fig. 9). Schlitz even promoted their vitamin D fortified beer in the winter with the slogan “if you want to keep sunny energy all winter long drink vitamin D fortified Schlitz beer.” (Fig. 88) They may have been correct now with the revelation that vitamin D deficiency was associated with depression, seasonal affective disorder and neurocognitive dysfunction.198-200,202,203,210
Figure 88. Schlitz Beer advertisement with the slogan “keep sunny energy all winter long drink vitamin D fortified Schlitz beer” from 1936.
Unfortunately in the early 1950s the outbreak of hypercalcemia in British infants, who also had birth defects which included altered facial features, mental retardation, and heart problems, was incorrectly attributed to be over fortification of milk with vitamin D since it was believed that these were signs of vitamin D intoxication.20,22 The more likely explanation is that these children had a syndrome which is associated with a hypersensitivity to vitamin D causing hypercalcemia and also with an elfin appearance and heart problems.20 However because this “outbreak” was associated with infants who had birth defects and mental retardation laws were quickly passed forbidding the fortification of not only foods but any consumer product including skin cream with vitamin D. This legislation was quickly adopted in most European countries and was used as a reason by other countries not to fortify milk with vitamin D.
Clearly the paranoia about food fortification with vitamin D causing toxicity needs to be reconsidered in light of observations that infants who consumed 2000 IU vitamin D per day during their first year of life not only did not have any evidence of toxicity but for the ensuing 31 y markedly decreased their risk for type 1 diabetes.145
In the 1970s sunscreens were first introduced as a way to prevent sunburning. The sunscreens contained UVB absorbing chemicals such as paraaminobezoic acid because it was believed that only UVB radiation damaged the skin and caused skin cancer. It is now realized that UVA radiation not only alters the immune system making it more immunotolerant but also increases risk for non-melanoma and melanoma skin cancers. Over the past four decades with very little thought as to its consequences, several national and international health organizations have condemned any direct sun exposure. The American Academy of Dermatology has taken the extreme position of recommending that no one should ever be exposed to direct sunlight without sun protection. This radical view of sunlight and UVB radiation has led to its designation as a carcinogen. To suggest that one should never be exposed to sunlight because excessive exposure to sunlight is linked to an increased risk for non-melanoma skin cancer is like suggesting that because breathing 100% oxygen can cause lung damage and death, that no one should breath an atmosphere that contains 20% oxygen.
The lack of appreciation of the importance of sensible sun exposure for providing children and adults with their vitamin D requirement has led to a worldwide vitamin D deficiency pandemic.22,173 In the United States the Center for Disease Control and Prevention (CDC) reported that 32% of children and adults have a circulating concentration of 25(OH)D < 20 ng/mL. Reports from Mexico, South America, Europe, Asia, India and even Africa suggest that more than 50% of the world population is at risk for vitamin D deficiency.22,265,266 Even in Australia, the skin cancer capital of the world, it is now recognized that the slip, slap, slop message has led to more than 40% of the population being vitamin D deficient.265-267 Even the Australian Dermatology Society now recommends sensible sun exposure as a source of vitamin D. A study of Australian dermatologists at the end of the summer revealed that 87% had a 25(OH)D < 20 ng/mL. More than 90% of the physicians in India were found to be vitamin D deficient.
The CDC concluded that vitamin D deficiency is becoming more prevalent in the US because of obesity, decrease in the consumption of vitamin D fortified milk and increased sun protection.269 Thus a three-part strategy should be employed worldwide to prevent vitamin D deficiency and its many negative health consequences (Fig. 89). Sensible sun exposure which is free, eating foods that naturally contain vitamin D or are fortified with vitamin D as well as taking a vitamin D supplement should guarantee vitamin D sufficiency.22,66 A global strategy to reduce the risk of vitamin D deficiency should be to consider not only increasing programs for food fortification not only of dairy products but also juice products, flour, and other commonly used food sources. There is no downside to increasing vitamin D intake and there could be a substantial upside, i.e., improvement not only of musculoskeletal health but overall health and welfare. It has been estimated that as much as 25% of health care dollars could be saved just by improving the world’s vitamin D status.78
Figure 89. A Schematic representation of the major causes for vitamin D deficiency and potential health consequences. Holick, copyright 2007. Reproduced with permission.
Acknowledgments
This work was supported in part by the National Institutes of Health Clinical Translational Institute Grant UL-1-RR-25711.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/24494
References
- 1.Holick M. Phylogenetic and evolutionary aspects of vitamin D from phytoplankton to humans. In: PKT Pang and MP Schreibman (eds), Verebrate Endocrinology: Fundamentals and Biomedical Implications Academic Press, Inc (Harcourt Brace Jovanovich) Orlando, FL 1989;3:7-43. [Google Scholar]
- 2.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 3.Pasanen AL, Yli-Pietila K, Pasanen P, Kalliokoski P, Tarhanen J. Ergosterol content in various fungal species and biocontaminated building materials. Appl Environ Microbiol. 1999;65:138–42. doi: 10.1128/aem.65.1.138-142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaras MD, Beelman RB, Holick MF, Elias RJ. Generation of potentially bioactive ergosterol-derived products following pulsed ultraviolet light exposure of mushrooms (Agaricus bisporus) Food Chem. 2012;135:396–401. doi: 10.1016/j.foodchem.2012.04.132. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, Tian XQ, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc Natl Acad Sci U S A. 1995;92:3124–6. doi: 10.1073/pnas.92.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian XQ, Chen TC, Matsuoka LY, Wortsman J, Holick MF. Kinetic and thermodynamic studies of the conversion of previtamin D3 to vitamin D3 in human skin. J Biol Chem. 1993;268:14888–92. [PubMed] [Google Scholar]
- 8.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennemann J, McQuarrie I. Brennemann's practice of pediatrics: W.F. Prior; 1946. [Google Scholar]
- 10.Mozołowski W. Jędrzej Sniadecki (1768-1838) on the Cure of Rickets. Nature. 1939;143:121. doi: 10.1038/143121a0. [DOI] [Google Scholar]
- 11.Palm TA. The geographical distribution and etiology of rickets. Practitioner. 1890;45:270–342. [Google Scholar]
- 12.Huldschinsky K. Heilung von Rachitis durch künstliche Höhensonne. Dtsch Med Wochenschr. 1919;45:712–3. doi: 10.1055/s-0028-1137830. [DOI] [Google Scholar]
- 13.Huldschinsky K. The ultra-violet light treatment of rickets. Alpine Press New Jersey, USA 1928;3–19. [Google Scholar]
- 14.Hess AF, Unger LJ. The cure of infantile rickets by sunlight. J Am Med Assoc. 1921;77:39. doi: 10.1001/jama.1921.02630270037013. [DOI] [Google Scholar]
- 15.Steenbock H, Black A. The reduction of growth-promoting and calcifying properties in a ration by exposure to ultraviolet light. J Biol Chem. 1924;61:408–22. doi: 10.1126/science.60.1549.224. [DOI] [PubMed] [Google Scholar]
- 16.Hess AF, Weinstock M. Antirachitic properties imparted to inert fluids and to green vegetables by ultra-violet irradiation. J Biol Chem. 1924;62:301–13. [Google Scholar]
- 17.Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes Obes. 2002;9:87–98. doi: 10.1097/00060793-200202000-00011. [DOI] [Google Scholar]
- 18.Rajakumar K, Greenspan SL, Thomas SB, Holick MF. SOLAR ultraviolet radiation and vitamin D: a historical perspective. Am J Public Health. 2007;97:1746–54. doi: 10.2105/AJPH.2006.091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holick MF. Biologic effects of light: historical and new perspectives. Biologic Effects of Light Edited by Holick MF and Jung EG Boston Kluwer Academic Publisher 1999:11-32. [Google Scholar]
- 20.Lightwood R, Sheldon W, Harris C. Hypercalcaemia in infants and vitamin D. BMJ. 1956;2:149. doi: 10.1136/bmj.2.4985.149. [DOI] [Google Scholar]
- 21.Tshibangu K, Oosterwijck K, Doumont-Meyvis M. Effects of massive doses of ergocalciferol plus cholesterol on pregnant rats and their offspring. J Nutr. 1975;105:741–58. doi: 10.1093/jn/105.6.741. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 23.Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–52. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 25.Holick M. Sunlight, UV-Radiation, Vitamin D and Skin Cancer: How Much Sunlight Do We Need? In: Reichrath J, ed. Sunlight, Vitamin D and Skin Cancer: Springer New York; 2008:1-15. [Google Scholar]
- 26.Tian XQ, Chen TC, Lu Z, Shao Q, Holick MF. Characterization of the translocation process of vitamin D3 from the skin into the circulation. Endocrinology. 1994;135:655–61. doi: 10.1210/en.135.2.655. [DOI] [PubMed] [Google Scholar]
- 27.Tian XQ, Holick MF. A liposomal model that mimics the cutaneous production of vitamin D3. Studies of the mechanism of the membrane-enhanced thermal isomerization of previtamin D3 to vitamin D3. J Biol Chem. 1999;274:4174–9. doi: 10.1074/jbc.274.7.4174. [DOI] [PubMed] [Google Scholar]
- 28.Tian XQ, Holick MF. Catalyzed thermal isomerization between previtamin D3 and vitamin D3 via β-cyclodextrin complexation. J Biol Chem. 1995;270:8706–11. doi: 10.1074/jbc.270.15.8706. [DOI] [PubMed] [Google Scholar]
- 29.Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–5. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holick MF. The D-lightful vitamin D for child health. JPEN J Parenter Enteral Nutr. 2012;36(Suppl):9S–19S. doi: 10.1177/0148607111430189. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF. McCollum Award Lecture, 1994: vitamin D--new horizons for the 21st century. Am J Clin Nutr. 1994;60:619–30. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–3. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 33.Havinga E. Vitamin D, example and challenge. Experientia. 1973;29:1181–93. doi: 10.1007/BF01935064. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs HJC, Boomsma F, Havinga E, van der Gen A. The photochemistry of previtamin D and tachysterol. Recueil des Travaux Chimiques des Pays-Bas. 1977;96:113–7. doi: 10.1002/recl.19770960406. [DOI] [Google Scholar]
- 35.Boomsma F, Jacobs HJC, Havinga E, van der Gen A. The “overirradiation products” of previtamin D and tachysterol: Toxisterols. Recueil des Travaux Chimiques des Pays-Bas. 1977;96:104–12. doi: 10.1002/recl.19770960405. [DOI] [Google Scholar]
- 36.Dauben WG, Baumann P. Photochemical transformations. IX. Total structure of suprasterol II. Tetrahedron Lett. 1961;2:565–72. doi: 10.1016/S0040-4039(01)91648-X. [DOI] [Google Scholar]
- 37.Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, et al. 1α,25(OH)₂-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila) 2011;4:1485–94. doi: 10.1158/1940-6207.CAPR-11-0165. [DOI] [PubMed] [Google Scholar]
- 38.Caldwell M, Flint S. Stratospheric ozone reduction, solar UV-B radiation and terrestrial ecosystems. Clim Change. 1994;28:375–94. doi: 10.1007/BF01104080. [DOI] [Google Scholar]
- 39.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 40.Spina C, Tangpricha V, Yao M, Zhou W, Wolfe MM, Maehr H, et al. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J Steroid Biochem Mol Biol. 2005;97:111–20. doi: 10.1016/j.jsbmb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Ladizesky M, Lu Z, Oliveri B, San Roman N, Diaz S, Holick MF, et al. Solar ultraviolet B radiation and photoproduction of vitamin D3 in central and southern areas of Argentina. J Bone Miner Res. 1995;10:545–9. doi: 10.1002/jbmr.5650100406. [DOI] [PubMed] [Google Scholar]
- 42.Bills CE. Antiricketic substances: VI. The distribution of vitamin D, with some notions on its possible origin. J Biol Chem. 1927;72:751–8. [Google Scholar]
- 43.Kenny DE, O'Hara TM, Chen TC, Lu Z, Tian X, Holick MF. Vitamin D content in Alaskan Arctic zooplankton, fishes, and marine mammals. Zoo Biol. 2004;23:33–43. doi: 10.1002/zoo.10104. [DOI] [Google Scholar]
- 44.Holick MF. Vitamin D: Physiology, molecular biology, and clinical applications: Humana Press; 2010. [Google Scholar]
- 45.Spina CS. Master of Arts Thesis. Novel vitamin D3 analogues, and colon cancer growth in mice: Boston University School of Medicine; 2004. [Google Scholar]
- 46.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 47.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–8. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 48.Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D. A preliminary study. Arch Dermatol. 1988;124:1802–4. doi: 10.1001/archderm.1988.01670120018003. [DOI] [PubMed] [Google Scholar]
- 49.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 50.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–6. doi: 10.1016/S0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 51.Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–7. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armas LAG, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588–93. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Luxwolda MF, Kuipers RS, Kema IP, Dijck-Brouwer DA, Muskiet FAJ. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr. 2012;108:1557–61. doi: 10.1017/S0007114511007161. [DOI] [PubMed] [Google Scholar]
- 54.Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94:940–5. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potter EL. Maternal health in relation to infant mortality. Am J Nurs. 1945;45:122–4. [Google Scholar]
- 56.Sheikh A, Alam R. Red Hair: A mutation, a royal trait, and sometimes a curse. [Google Scholar]
- 57.Lalueza-Fox C, Römpler H, Caramelli D, Stäubert C, Catalano G, Hughes D, et al. A melanocortin 1 receptor allele suggests varying pigmentation among Neanderthals. Science. 2007;318:1453–5. doi: 10.1126/science.1147417. [DOI] [PubMed] [Google Scholar]
- 58.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–8. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–5. doi: 10.1016/S0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 60.Chuck A, Todd J, Diffey B. Subliminal ultraviolet-B irradiation for the prevention of vitamin D deficiency in the elderly: a feasibility study. Photodermatol Photoimmunol Photomed. 2001;17:168–71. doi: 10.1034/j.1600-0781.2001.170405.x. [DOI] [PubMed] [Google Scholar]
- 61.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 62.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sørensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(Suppl 1):S97–103. doi: 10.1079/BJN2001345. [DOI] [PubMed] [Google Scholar]
- 63.Holick M. Vitamin D and health: Evolution, biologic functions, and recommended dietary intakes for vitamin D. Clinical Review in Bone and Mineral Metabolism. 2009;7:2–19. doi: 10.1007/s12018-009-9026-x. [DOI] [Google Scholar]
- 64.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–21. doi: 10.1210/jc.86.3.1212. [DOI] [PubMed] [Google Scholar]
- 66.Holick MF. The Vitamin D Solution: A 3-Step Strategy to Cure Our Most Common Health Problem. Penguin Group US. 2010. [Google Scholar]
- 67.Wintzen M, Yaar M, Burbach JP, Gilchrest BA. Proopiomelanocortin gene product regulation in keratinocytes. J Invest Dermatol. 1996;106:673–8. doi: 10.1111/1523-1747.ep12345496. [DOI] [PubMed] [Google Scholar]
- 68.Battersby AJ, Kampmann B, Burl S. Vitamin D in Early Childhood and the Effect on Immunity to Mycobacterium tuberculosis. Clin Dev Immunol. 2012:430972. doi: 10.1155/2012/430972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.The Nobel Prize in Physiology or Medicine 1903. Niels Ryberg Finsen. 2012-09-08. URL:http://www.nobelprize.org/nobel_prizes/medicine/laureates/1903/ Accessed: 2012-09-08. (Archived by WebCite® at http://www.webcitation.org/6AWpWACU0). 1903. (Accessed at.
- 70.Albert MR, Ostheimer KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 3. J Am Acad Dermatol. 2003;49:1096–106. doi: 10.1016/S0190-9622(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 71.Albert MR, Ostheimer KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 2. J Am Acad Dermatol. 2003;48:909–18. doi: 10.1067/mjd.2003.272. [DOI] [PubMed] [Google Scholar]
- 72.Albert MR, Ostheimer KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 1. J Am Acad Dermatol. 2002;47:930–7. doi: 10.1067/mjd.2002.127254. [DOI] [PubMed] [Google Scholar]
- 73.Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111–48. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffman FL. The mortality from cancer throughout the world: Prudential Press; 1916. [Google Scholar]
- 75.Peller S, Stephenson CS. Skin Irritation and Cancer in the U. S. Navy. Am J Med Sci. 1937;194:326–33. doi: 10.1097/00000441-193709000-00004. [DOI] [Google Scholar]
- 76.Apperly FL. The Relation of Solar Radiation to Cancer Mortality in North America. Cancer Res. 1941;1:191–5. doi: 10.1158/0008-5472.CAN-15-3169. [DOI] [PubMed] [Google Scholar]
- 77.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–31. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 78.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–8. doi: 10.1016/S0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 79.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–75. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 80.Lefkowitz ES, Garland CF. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol. 1994;23:1133–6. doi: 10.1093/ije/23.6.1133. [DOI] [PubMed] [Google Scholar]
- 81.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–22. doi: 10.1016/0091-7435(90)90058-R. [DOI] [PubMed] [Google Scholar]
- 82.Mizoue T. Ecological study of solar radiation and cancer mortality in Japan. Health Phys. 2004;87:532–8. doi: 10.1097/01.HP.0000137179.03423.0b. [DOI] [PubMed] [Google Scholar]
- 83.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::AID-CNCR2820701224>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 84.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10(5A):1307–11. [PubMed] [Google Scholar]
- 85.Grant WB. Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 2012;32:223–36. [PubMed] [Google Scholar]
- 86.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 88.Luscombe CJ, Fryer AA, French ME, Liu S, Saxby MF, Jones PW, et al. Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet. 2001;358:641–2. doi: 10.1016/S0140-6736(01)05788-9. [DOI] [PubMed] [Google Scholar]
- 89.Chang ET, Smedby KE, Hjalgrim H, Porwit-MacDonald A, Roos G, Glimelius B, et al. Family history of hematopoietic malignancy and risk of lymphoma. J Natl Cancer Inst. 2005;97:1466–74. doi: 10.1093/jnci/dji293. [DOI] [PubMed] [Google Scholar]
- 90.Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:422–9. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- 91.Neale R, Russell A, Muller HK, Green A. Sun exposure, sunscreen and their effects on epidermal Langerhans cells. Photochem Photobiol. 1997;66:260–4. doi: 10.1111/j.1751-1097.1997.tb08652.x. [DOI] [PubMed] [Google Scholar]
- 92.Hersey P, Bradley M, Hasic E, Haran G, Edwards A, McCarthy WH. Immunological effects of solarium exposure. Lancet. 1983;1:545–8. doi: 10.1016/S0140-6736(83)92808-8. [DOI] [PubMed] [Google Scholar]
- 93.Nghiem DX, Kazimi N, Clydesdale G, Ananthaswamy HN, Kripke ML, Ullrich SE. Ultraviolet a radiation suppresses an established immune response: implications for sunscreen design. J Invest Dermatol. 2001;117:1193–9. doi: 10.1046/j.0022-202x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 94.Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–93. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28:4191–8. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freedman DM, Looker AC, Chang S-C, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 97.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–8. doi: 10.1016/S0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 98.Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307–9. doi: 10.1016/S0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 99.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971-1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 101.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 102.Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer--ready for prime time? N Engl J Med. 2011;364:1385–7. doi: 10.1056/NEJMp1102022. [DOI] [PubMed] [Google Scholar]
- 103.Shin M-H, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–11. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 104.Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–8. [PubMed] [Google Scholar]
- 105.Woo TCS, Choo R, Jamieson M, Chander S, Vieth R. Pilot study: potential role of vitamin D (Cholecalciferol) in patients with PSA relapse after definitive therapy. Nutr Cancer. 2005;51:32–6. doi: 10.1207/s15327914nc5101_5. [DOI] [PubMed] [Google Scholar]
- 106.Eaton CB, Young A, Allison MA, Robinson J, Martin LW, Kuller LH, et al. Prospective association of vitamin D concentrations with mortality in postmenopausal women: results from the Women’s Health Initiative (WHI) Am J Clin Nutr. 2011;94:1471–8. doi: 10.3945/ajcn.111.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94:1144–9. doi: 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gündüz M, Cacına C, Toptaş B, Yaylım-Eraltan I, Tekand Y, İsbir T. Association of vitamin D receptor gene polymorphisms with colon cancer. Genet Test Mol Biomarkers. 2012;16:1058–61. doi: 10.1089/gtmb.2012.0044. [DOI] [PubMed] [Google Scholar]
- 109.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Yamaji T, Iwasaki M, Sasazuki S, Sakamoto H, Yoshida T, Tsugane S. Association between plasma 25-hydroxyvitamin D and colorectal adenoma according to dietary calcium intake and vitamin D receptor polymorphism. Am J Epidemiol. 2012;175:236–44. doi: 10.1093/aje/kwr295. [DOI] [PubMed] [Google Scholar]
- 111.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:827–38. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 112.Hart PD. Chemotherapy of tuberculosis; research during the past 100 years. BMJ. 1946;2:805–49. [PubMed] [Google Scholar]
- 113.Everett D. On the Use of Cod-Liver Oil in Tubercular Disease. Prov Med Surg J. 1846;10:538–9. doi: 10.1136/bmj.s1-10.45.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roelandts R. The history of phototherapy: something new under the sun? J Am Acad Dermatol. 2002;46:926–30. doi: 10.1067/mjd.2002.121354. [DOI] [PubMed] [Google Scholar]
- 115.Muhe L, Lulseged S, Mason KE, Simoes EAF. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–4. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 116.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr. 2004;50:364–8. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 117.Phelan JJ. Calciferol in pulmonary tuberculosis. Lancet. 1947;1:764. doi: 10.1016/S0140-6736(47)91513-4. [DOI] [PubMed] [Google Scholar]
- 118.Hope-Simpson RE. The role of season in the epidemiology of influenza. J Hyg (Lond) 1981;86:35–47. doi: 10.1017/S0022172400068728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 122.Maxwell CS, Carbone ET, Wood RJ. Better newborn vitamin D status lowers RSV-associated bronchiolitis in infants. Nutr Rev. 2012;70:548–52. doi: 10.1111/j.1753-4887.2012.00517.x. [DOI] [PubMed] [Google Scholar]
- 123.Cohn ZA. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 125.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 126.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee PHA, Ohtake T, Zaiou M, Murakami M, Rudisill JA, Lin KH, et al. Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc Natl Acad Sci U S A. 2005;102:3750–5. doi: 10.1073/pnas.0500268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–6. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837–43. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laaksi I, Ruohola J-P, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–7. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 131.Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80:108–13. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 132.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001;111:452–6. doi: 10.1016/S0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 133.Miley DD, Garcia MN, Hildebolt CF, Shannon WD, Couture RA, Anderson Spearie CL, et al. Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol. 2009;80:1433–9. doi: 10.1902/jop.2009.090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grant WB. The prevalence of multiple sclerosis in 3 US communities: the role of vitamin D. Prev Chronic Dis. 2010;7:A89–, author reply A90. [PMC free article] [PubMed] [Google Scholar]
- 135.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127–36. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 136.Ponsonby A-L, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002;181-182:71–8. doi: 10.1016/S0300-483X(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 137.Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51:1391–8. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 138.Talley NJ, Abreu MT, Achkar JP, Bernstein CN, Dubinsky MC, Hanauer SB, et al. American College of Gastroenterology IBD Task Force An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106(Suppl 1):S2–25, quiz S26. doi: 10.1038/ajg.2011.58. [DOI] [PubMed] [Google Scholar]
- 139.Schultz M, Butt AG. Is the north to south gradient in inflammatory bowel disease a global phenomenon? Expert Rev Gastroenterol Hepatol. 2012;6:445–7. doi: 10.1586/egh.12.31. [DOI] [PubMed] [Google Scholar]
- 140.Noonan CW, Williamson DM, Henry JP, Indian R, Lynch SG, Neuberger JS, et al. The prevalence of multiple sclerosis in 3 US communities. Prev Chronic Dis. 2010;7:A12. [PMC free article] [PubMed] [Google Scholar]
- 141.Wallin MT, Page WF, Kurtzke JF. Multiple sclerosis in US veterans of the Vietnam era and later military service: race, sex, and geography. Ann Neurol. 2004;55:65–71. doi: 10.1002/ana.10788. [DOI] [PubMed] [Google Scholar]
- 142.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 143.Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–5. doi: 10.1212/01.WNL.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 144.Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51:1391–8. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 145.Hyppönen E, Läärä E, Reunanen A, Järvelin M-R, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 146.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–9. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vieira VM, Hart JE, Webster TF, Weinberg J, Puett R, Laden F, et al. Association between residences in U.S. northern latitudes and rheumatoid arthritis: A spatial analysis of the Nurses’ Health Study. Environ Health Perspect. 2010;118:957–61. doi: 10.1289/ehp.0901861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG, Iowa Women’s Health Study Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 149.Nielen MMJ, van Schaardenburg D, Lems WF, van de Stadt RJ, de Koning MH, Reesink HW, et al. Vitamin D deficiency does not increase the risk of rheumatoid arthritis: comment on the article by Merlino et al. Arthritis Rheum. 2006;54:3719–20. doi: 10.1002/art.22191. [DOI] [PubMed] [Google Scholar]
- 150.Manolagas SC, Yu XP, Girasole G, Bellido T. Vitamin D and the hematolymphopoietic tissue: a 1994 update. Semin Nephrol. 1994;14:129–43. [PubMed] [Google Scholar]
- 151.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 153.Iho S, Takahashi T, Kura F, Sugiyama H, Hoshino T. The effect of 1,25-dihydroxyvitamin D3 on in vitro immunoglobulin production in human B cells. J Immunol. 1986;136:4427–31. [PubMed] [Google Scholar]
- 154.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 155.Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–7. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 156.Stio M, Bonanomi AG, d’Albasio G, Treves C. Suppressive effect of 1,25-dihydroxyvitamin D3 and its analogues EB 1089 and KH 1060 on T lymphocyte proliferation in active ulcerative colitis. Biochem Pharmacol. 2001;61:365–71. doi: 10.1016/S0006-2952(00)00564-5. [DOI] [PubMed] [Google Scholar]
- 157.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 2010;88:441–50. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–12. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 159.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6. doi: 10.1161/01.HYP.30.2.150. [DOI] [PubMed] [Google Scholar]
- 160.Krause R, Bühring M, Hopfenmüller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–10. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 161.Li YC, Kong J, Wei M, Chen Z-F, Liu SQ, Cao L-P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–32. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 163.Pilz S, Tomaschitz A, März W, Drechsler C, Ritz E, Zittermann A, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011;75:575–84. doi: 10.1111/j.1365-2265.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- 164.Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–33. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 165.Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D deficiency and supplementation and relation to cardiovascular health. Am J Cardiol. 2012;109:359–63. doi: 10.1016/j.amjcard.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 166.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Holick MF. Vitamin D deficiency in 2010: health benefits of vitamin D and sunlight: a D-bate. Nat Rev Endocrinol. 2011;7:73–5. doi: 10.1038/nrendo.2010.234. [DOI] [PubMed] [Google Scholar]
- 168.Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–91. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 169.Reis JP, von Mühlen D, Miller ER, 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124:e371–9. doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L, Slattery ML, et al. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA. 1999;282:1539–46. doi: 10.1001/jama.282.16.1539. [DOI] [PubMed] [Google Scholar]
- 171.Reid IR, Bolland MJ. Role of vitamin D deficiency in cardiovascular disease. Heart. 2012;98:609–14. doi: 10.1136/heartjnl-2011-301356. [DOI] [PubMed] [Google Scholar]
- 172.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 173.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 174.Drincic AT, Armas LAG, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–8. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 175.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Intermountain Heart Collaborative (IHC) Study Group Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 176.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–6. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 178.Scragg R, Sowers M, Bell C, Third National Health and Nutrition Examination Survey Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 179.Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, et al. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39:2611–3. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 180.Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, et al. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65:225–36. doi: 10.1016/j.maturitas.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 181.Deleskog A, Hilding A, Brismar K, Hamsten A, Efendic S, Östenson CG. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55:1668–78. doi: 10.1007/s00125-012-2529-x. [DOI] [PubMed] [Google Scholar]
- 182.Riachy R, Vandewalle B, Moerman E, Belaich S, Lukowiak B, Gmyr V, et al. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11:151–9. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- 183.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–55. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 184.Maestro B, Dávila N, Carranza MC, Calle C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223–30. doi: 10.1016/S0960-0760(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 185.Dong J, Wong SL, Lau CW, Lee HK, Ng CF, Zhang L, et al. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J. 2012;33:2980–90. doi: 10.1093/eurheartj/ehr459. [DOI] [PubMed] [Google Scholar]
- 186.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90:387–92. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 187.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–98. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thomas GN, ó Hartaigh B, Bosch JA, Pilz S, Loerbroks A, Kleber ME, et al. Vitamin D levels predict all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome: the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Diabetes Care. 2012;35:1158–64. doi: 10.2337/dc11-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kinney DK, Teixeira P, Hsu D, Napoleon SC, Crowley DJ, Miller A, et al. Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: a role for prenatal vitamin d deficiency and infections? Schizophr Bull. 2009;35:582–95. doi: 10.1093/schbul/sbp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Torrey EF. Prevalence studies in schizophrenia. Br J Psychiatry. 1987;150:598–608. doi: 10.1192/bjp.150.5.598. [DOI] [PubMed] [Google Scholar]
- 191.O’Callaghan E, Gibson T, Colohan HA, Walshe D, Buckley P, Larkin C, et al. Season of birth in schizophrenia. Evidence for confinement of an excess of winter births to patients without a family history of mental disorder. Br J Psychiatry. 1991;158:764–9. doi: 10.1192/bjp.158.6.764. [DOI] [PubMed] [Google Scholar]
- 192.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28:1–38. doi: 10.1016/S0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 193.Jarvis E. Schizophrenia in British immigrants: Recent findings, issues and implications. Transcult Psychiatry. 1998;35:39–74. doi: 10.1177/136346159803500102. [DOI] [Google Scholar]
- 194.McGrath J, Saari K, Hakko H, Jokelainen J, Jones P, Järvelin MR, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67:237–45. doi: 10.1016/j.schres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 195.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–80. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 196.Barr CE, Mednick SA, Munk-Jorgensen P. Exposure to influenza epidemics during gestation and adult schizophrenia. A 40-year study. Arch Gen Psychiatry. 1990;47:869–74. doi: 10.1001/archpsyc.1990.01810210077012. [DOI] [PubMed] [Google Scholar]
- 197.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–92. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 198.Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65:508–12. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- 199.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264:599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 200.Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin d concentrations in Alzheimer's disease: a systematic review and meta-analysis. Journal of Alzheimer's disease. JAD. 2013;33:659–74. doi: 10.3233/JAD-2012-121432. [DOI] [PubMed] [Google Scholar]
- 201.Holló A, Clemens Z, Kamondi A, Lakatos P, Szűcs A. Correction of vitamin D deficiency improves seizure control in epilepsy: a pilot study. Epilepsy Behav. 2012;24:131–3. doi: 10.1016/j.yebeh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 202.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032–40. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 203.Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–41. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 α-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 205.Jande SS, Maler L, Lawson DEM. Immunohistochemical mapping of vitamin D-dependent calcium-binding protein in brain. Nature. 1981;294:765–7. doi: 10.1038/294765a0. [DOI] [PubMed] [Google Scholar]
- 206.Clemens TL, McGlade SA, Garrett KP, Horiuchi N, Hendy GN. Tissue-specific regulation of avian vitamin D-dependent calcium-binding protein 28-kDa mRNA by 1,25-dihydroxyvitamin D3. J Biol Chem. 1988;263:13112–6. [PubMed] [Google Scholar]
- 207.Partonen T. Vitamin D and serotonin in winter. Med Hypotheses. 1998;51:267–8. doi: 10.1016/S0306-9877(98)90085-8. [DOI] [PubMed] [Google Scholar]
- 208.Gloth FM, 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3:5–7. [PubMed] [Google Scholar]
- 209.Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17:703–17. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 210.Buell JS, Dawson-Hughes B, Scott TM, Weiner DE, Dallal GE, Qui WQ, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74:18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nimitphong H, Holick MF. Vitamin D, neurocognitive functioning and immunocompetence. Curr Opin Clin Nutr Metab Care. 2011;14:7–14. doi: 10.1097/MCO.0b013e3283414c38. [DOI] [PubMed] [Google Scholar]
- 212.Anglin RES, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–7. doi: 10.1192/bjp.bp.111.106666. [DOI] [PubMed] [Google Scholar]
- 213.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK, et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr. 2010;91:1621–6. doi: 10.3945/ajcn.2009.27972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–81. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Thacher TD, Obadofin MO, O’Brien KO, Abrams SA. The effect of vitamin D2 and vitamin D3 on intestinal calcium absorption in Nigerian children with rickets. J Clin Endocrinol Metab. 2009;94:3314–21. doi: 10.1210/jc.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, et al. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 2008;93:2716–21. doi: 10.1210/jc.2007-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rapuri PB, Gallagher JC, Haynatzki G. Effect of vitamins D2 and D3 supplement use on serum 25OHD concentration in elderly women in summer and winter. Calcif Tissue Int. 2004;74:150–6. doi: 10.1007/s00223-003-0083-8. [DOI] [PubMed] [Google Scholar]
- 219.Armas LAG, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 220.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–8. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 221.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84:694–7. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- 222.Rollier A. Heliotherapy: Its Therapeutic, prophylactic and social value. Am J Nurs. 1927;27:815–23. [Google Scholar]
- 223.Huldschinsky K. Heilung von Rachitis durch künstliche Höhensonne. Dtsch Med Wochenschr. 1919;45:712–3. doi: 10.1055/s-0028-1137830. [DOI] [Google Scholar]
- 224.Iakovlev VI. Prevention of ultraviolet deficiency in children and adolescents. Gig Sanit. 1981:81–2. [PubMed] [Google Scholar]
- 225.Sperti G. United States Patent Office, Patent Number 1,956,599. In: Google Patents; 1934.
- 226.Sperti G. Electric light source. In: Google Patents; 1936. [Google Scholar]
- 227.Gronowitz E, Larkö O, Gilljam M, Hollsing A, Lindblad A, Mellström D, et al. Ultraviolet B radiation improves serum levels of vitamin D in patients with cystic fibrosis. Acta Paediatr. 2005;94:547–52. doi: 10.1080/08035250410025276. [DOI] [PubMed] [Google Scholar]
- 228.Chandra P, Wolfenden LL, Ziegler TR, Tian J, Luo M, Stecenko AA, et al. Treatment of vitamin D deficiency with UV light in patients with malabsorption syndromes: a case series. Photodermatol Photoimmunol Photomed. 2007;23:179–85. doi: 10.1111/j.1600-0781.2007.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dabai NS, Pramyothin P, Holick MF. The effect of ultraviolet radiation from a novel portable fluorescent lamp on serum 25-hydroxyvitamin D3 levels in healthy adults with Fitzpatrick skin types II and III. Photodermatol Photoimmunol Photomed. 2012;28:307–11. doi: 10.1111/phpp.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Koutkia P, Lu Z, Chen TC, Holick MF. Treatment of vitamin D deficiency due to Crohn’s disease with tanning bed ultraviolet B radiation. Gastroenterology. 2001;121:1485–8. doi: 10.1053/gast.2001.29686. [DOI] [PubMed] [Google Scholar]
- 231.Tangpricha V, Turner A, Spina C, Decastro S, Chen TC, Holick MF. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr. 2004;80:1645–9. doi: 10.1093/ajcn/80.6.1645. [DOI] [PubMed] [Google Scholar]
- 232.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(Suppl):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 233.Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135:2739S–48S. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 234.MacKie RM. Incidence, risk factors and prevention of melanoma. Eur J Cancer. 1998;34(Suppl 3):S3–6. doi: 10.1016/S0959-8049(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 235.Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research 1996;1:136-42. [PubMed] [Google Scholar]
- 236.Kricker A, Armstrong BK, English DR, Heenan PJ. Does intermittent sun exposure cause basal cell carcinoma? a case-control study in Western Australia. Int J Cancer. 1995;60:489–94. doi: 10.1002/ijc.2910600411. [DOI] [PubMed] [Google Scholar]
- 237.Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN, Leiden Skin Cancer Study The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087–93. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 238.Chen TC. The Photobiology of Vitamin D. In: Holick MF, ed. Vitamin D – Physiology, Molecular Biology and Clinical Applications. Totowa, NJ: Humana Press; 1998:17-37. [Google Scholar]
- 239.Knaysi GA, Crikelair GF, Cosman B. The role of nines: its history and accuracy. Plast Reconstr Surg. 1968;41:560–3. doi: 10.1097/00006534-196806000-00008. [DOI] [PubMed] [Google Scholar]
- 240.Holick MF. Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol. 2008;3:1548–54. doi: 10.2215/CJN.01350308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Buettner PG, Raasch BA. Incidence rates of skin cancer in Townsville, Australia. Int J Cancer. 1998;78:587–93. doi: 10.1002/(SICI)1097-0215(19981123)78:5<587::AID-IJC10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 242.Warren R, Gartstein V, Kligman AM, Montagna W, Allendorf RA, Ridder GM. Age, sunlight, and facial skin: a histologic and quantitative study. J Am Acad Dermatol. 1991;25:751–60. doi: 10.1016/S0190-9622(08)80964-4. [DOI] [PubMed] [Google Scholar]
- 243.Cleaver JE, Crowley E. UV damage, DNA repair and skin carcinogenesis. Front Biosci. 2002;7:d1024–43. doi: 10.2741/A829. [DOI] [PubMed] [Google Scholar]
- 244.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 245.Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D intake in the United States. J Am Diet Assoc. 2004;104:980–3. doi: 10.1016/j.jada.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 246.Käkelä R, Hyvärinen H, Vainiotalo P. Fatty acid composition in liver and blubber of the Saimaa ringed seal (Phoca hispida saimensis) compared with that of the ringed seal (Phoca hispida botnica) and grey seal (Halichoerus grypus from the Baltic. Comp Biochem Physiol B. 1993;105:553–65. doi: 10.1016/0305-0491(93)90088-M. [DOI] [PubMed] [Google Scholar]
- 247.Wagner CL, Greer FR. Breastfeeding atSo, Nutrition Co. Prevention of Rickets and Vitamin D Deficiency in Infants, Children, and Adolescents. Pediatr. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 248.Shah BR, Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr. 1994;125:487–90. doi: 10.1016/S0022-3476(05)83303-7. [DOI] [PubMed] [Google Scholar]
- 249.El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–12. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 250.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 251.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/S0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 252.Pietras SM, Obayan BK, Cai MH, Holick MF. Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med. 2009;169:1806–8. doi: 10.1001/archinternmed.2009.361. [DOI] [PubMed] [Google Scholar]
- 253.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Koutkia P, Chen TC, Holick MF. Vitamin D intoxication associated with an over-the-counter supplement. N Engl J Med. 2001;345:66–7. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 255.Adams JS, Lee G. Gains in bone mineral density with resolution of vitamin D intoxication. Ann Intern Med. 1997;127:203–6. doi: 10.7326/0003-4819-127-3-199708010-00004. [DOI] [PubMed] [Google Scholar]
- 256.Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68:882–7. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- 257.Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2007;22(Suppl 2):V64–8. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 258.Rajakumar K, Reis EC, Holick MF. Dosing error with over-the-counter vitamin D supplement: a risk for vitamin D toxicity in infants. Clin Pediatr (Phila) 2013;52:82–5. doi: 10.1177/0009922812439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 261.Taylor JG. Yahweh and the sun: biblical and archaeological evidence for sun worship in ancient Israel: T&T Clark; 1993. [Google Scholar]
- 262.Srivastava VC, Basham AL, Sharma G. Sun-worship in ancient India: Indological Publications; 1972. [Google Scholar]
- 263.Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 264.Holick MF. Phylogenetic and evolutionary aspects of vitamin D from phytoplankton to humans. In: P.K.T. Pang and M.P. Schreibman (eds), Verebrate Endocrinology: Fundamentals and Biomedical Implications. Academic Press, Inc. (Harcourt Brace Jovanovich) Orlando, FL. 1989. 3:7-43. [Google Scholar]
- 265.Looker, AC, Johnson, CL, Lachner, DA, Pfeiffer, CM, Schleicher, RL and Sempos, CT. Vitamin D Status: United States, 2001-2006. NCHS Data Brief. 2011; 56. [PubMed]
- 266.Hossein-nezhad A, Holick MF. Optimize dietary intake of vitamin D: an epigenetic perspective. Curr Opin Clin Nutr Metab Care. 2012;15:567–79. doi: 10.1097/MCO.0b013e3283594978. [DOI] [PubMed] [Google Scholar]
- 267.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97:1153–8. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 268.Daly RM, Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Sikaris KA, et al. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin Endocrinol (Oxf) 2012;77:26–35. doi: 10.1111/j.1365-2265.2011.04320.x. [DOI] [PubMed] [Google Scholar]
- 269.Czarnecki D, Meehan CJ, Bruce F. The vitamin D status of Australian dermatologists. Clin Exp Dermatol. 2009;34:621–38. doi: 10.1111/j.1365-2230.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 270.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 271.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–82. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 272.A day in the life of the Soviet Union. Special edition. Time Magazine 1987 October 26th:83.