Abstract
Novel metabolic pathways initiated by the enzymatic action of CYP11A1 on 7DHC (7-dehydrocholesterol), ergosterol, vitamins D3 and D2 were characterized with help of chemical synthesis, UV and mass spectrometry and NMR analyses. The first pathway follows the sequence 7DHC→22(OH)7DHC → 20,22(OH)27DHC → 7DHP (7-dehydropregnenolone), which can further be metabolized by steroidogenic enzymes. The resulting 5,7-dienes can be transformed by UVB to corresponding, biologically active, secosteroids. Action of CYP11A1 on vitamin D3 and D2 produces novel hydroxyderivatives with OH added at positions C17, C20, C22, C23 and C24, some of which can be hydroxylated by CYP27B1 and/or by CYP27A1 and/ or by CYP24A1.The main products of these pathways are biologically active with a potency related to their chemical structure and the target cell type. Main products of CYP11A1-mediated metabolism on vitamin D are non-calcemic and non-toxic at relatively high doses and serve as partial agonists on the vitamin D receptor. New secosteroids are excellent candidates for therapy of fibrosing, inflammatory or hyperproliferative disorders including cancers and psoriasis.
Keywords: skin; keratinocytes; melanocytes; melanoma cells; dermal fibroblasts; vitamin D; 5,7-dienes
New Secosteroidal Systems
An overview
While defining neuroendocrine activities of the skin,1-3 we discovered novel metabolic pathways initiated by the enzymatic action of cytochrome P450scc (CYP11A1) on 7-dehydrocholesterol (7DHC; pro-vitamin D3),4-6 ergosterol,7,8 vitamin D39-16 and vitamin D2.17,18 These substrates have structural similarity to the well characterized substrate for CYP11A1, cholesterol, where cleavage of its side chain between C20 and C22 producing pregnenolone represents the initial reaction in the synthesis of steroid hormones.19,20 We have now established that P450scc can hydroxylate vitamin D3 in a sequential manner at positions C17, C20, C22 and C23 to produce 20-hydroxyvitamin D3 (20(OH)D3), 20,23(OH)2D3, 20,22(OH)2D3 and 17,20,23(OH)3D3 as the main products, with additional production of 17(OH)D3, 22(OH)2D3, 23(OH)D3 and 17,20(OH)2D3. Some of these product can be hydroxylated, by CYP27B115,16 to produce 1,20(OH)2D3 and 1,20,23(OH)3D3, by CYP27A114 to produce 20,25(OH)2D3 or 20,26(OH)D3 or by CYP24A121 to produce 20,24(OH)2D3 or 20,25(OH)2D3. In addition, products of P450scc activity on vitamin D2 include 20(OH)D2, 17,20(OH)2D2 and 17,20,24(OH)3D217,18 with 20(OH)D2 also being hydroxylated by CYP27B1.22 The main products of these pathways, are biologically active with a potency defined by their chemical structure and the cell lineage.21-31
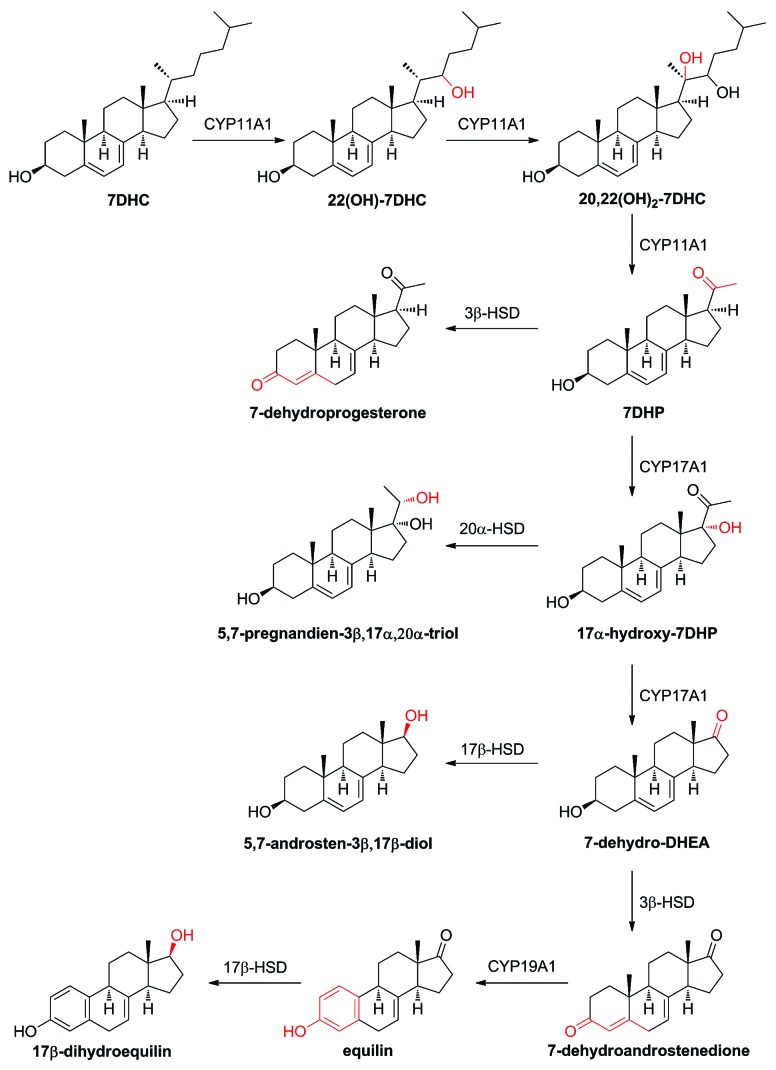
Cleavage of the side chain of 7DHC generates 7-dehydropregnenolene (7DHP), which can be further metabolized by classical steroidogenic enzymes to produce 5,7-diene steroids that are identifiable in pathological conditions [Smith Lemli Opitz syndrome (SLOS)32,33], or ex vivo in adrenal glands,5 placenta or keratinocytes6 incubated with 7DHC. These 5,7-diene steroids can be converted by UV B radiation (UVB) to androsta-calciferols (aD) and pregna-calciferols (pD), i.e., vitamin D compounds with a short or absent side chain, which are indeed biologically active in skin cells.34-38
In this review we will focus on ∆7 steroids and photolytically generated secosteroids, and describe their biological activity and methods for their synthesis. The review will be complemented by a description of ∆7 steroidogenic pathways.
General overview of ∆7 steroidogenic pathways
Studies on the metabolism of ∆7-steroids by purified enzymes, organelles and tissue fragments
7DHC can serve as the substrate for the synthesis of a range of ∆7 steroids including ∆7 pregnenes, androgens and estrogens. Pathways leading to ∆7-steroids have been elucidated in part by studies with purified enzymes, subcellular organelles and tissue fractions, and in part from the analysis of steroids present in patients with Smith-Lemli-Opitz Syndrome (SLOS).
Steroidogenesis in tissues such as the adrenal cortex and gonads starts with the transport of cholesterol from the outer to the inner mitochondrial membrane mediated by the Steroidogenic Acute Regulatory (StAR) protein.20 This transfer represents the rate-limiting step in the synthesis of progesterone, androgens or corticosteroids in these tissues and is regulated by the tropic hormones.20 Purified StAR protein can mediate the transfer of 7DHC between phospholipid vesicle membranes with comparable efficiency to that for cholesterol.5 The addition of purified StAR protein to placental mitochondria resulted in a 7.5-fold stimulation of 7DHC metabolism by the mitochondria with the rate being higher than that for cholesterol metabolism under similar conditions.6 It is therefore clear that the StAR protein can efficiently transport 7DHC to the inner mitochondrial membrane for steroid synthesis.
The ability of CYP11A1 to catalyze the removal of the side chain of 7DHC and thus initiate ∆7-steroidogenic pathways starting from 7DHC is now well established.4-6,39 Studies with purified bovine and human CYP11A1 have shown that both can catalyze the side chain cleavage of 7DHC producing 7-dehydropregnenolone (7DHP), with a catalytic efficiency slightly higher than that for cholesterol conversion to pregnenolone.4 Conversion of 7DHC to 7DHP by human placental mitochondria has also been demonstrated, with inhibition by 22R-hydroxycholesterol, a tight-binding CYP11A1 intermediate,19 clearly demonstrating that the reaction is mediated by CYP11A1.6 Fragments of rat, dog, pig and rabbit adrenal glands incubated with 7DHC ex vivo also converted 7DHC to 7DHP as did mitochondria from rat skin.5
Conversion of 7DHC to 7DHP by human CYP11A1 is accompanied by accumulation of the two reaction intermediates, 22-hydroxy-7DHC (22(OH)7DHC) and 20,22-dihydroxy-7DHC (20,22(OH)27DHC). These intermediates accumulate well above the concentration of CYP11A during incubations with 7DHC and therefore should be considered as reaction products, not enzyme-bound reaction intermediates.6 Other minor, monohydroxylated 7DHC derivatives that remain to be identified are also produced in this reaction.6 Production of (20,22(OH)27DHC) and other mono-hydroxylated products was also observed in incubations of human placental mitochondria with 7DHC,6 as well as for adrenal fragments from rat, dog, rabbit and pig incubated with 7DHC ex vivo.5
Another ∆7 steroid found in mammals which is of dietary origin is ergosterol, a fungal sterol which is the precursor of vitamin D2.40,41 This sterol can be metabolised by CYP11A1 but unlike cholesterol and 7DHC, no cleavage of the side chain occurs.7,8 While the products cannot enter the classical steroidogenic pathways, some of the products do display antiproliferative activity on skin cells.8 Human CYP11A1 catalyzes both epoxidation and hydroxylation of the ergosterol side chain producing 20-hydroxy-22,23-epoxy-22,23-dihydroergosterol as a major metabolite.7
Studies with partially purified 3β-hydroxysteroid dehydrogenase/∆5-4 isomerase (3βHSD) type 1 from the human placenta show that this enzyme can act on 7DHP with a catalytic efficiency 40% of that measured for pregnenolone. The lower efficiency was largely due to an increase in the Km for substrate.5 The product of 3βHSD action on 7DHP was identified as 7-dehydroprogesterone (4,7-pregnadien-3-20-dione) by collecting the product from incubations of placental microsomes with 7DHP and subjecting it to structural analysis by mass spectrometry and NMR.6 7-Dehydroprogesterone proved to be unstable, with considerable conversion to several unidentified products on storage at room temperature for a few hours. 7-Dehydroprogesterone was also produced by adrenal fragments from several mammalian species, including the pig, incubated with 7DHC or 7DHP.5,6 Pig adrenal fragments also produced a metabolite tentatively identified as 17-hydroxy-7DHP, suggestive of CYP17A1 action on 7DHP.
∆7 Estrogen synthesis and metabolism in the pregnant mare
The urine and blood of the pregnant mare contain a high concentration of the B-ring unsaturated estrogen, equilin (3-hydroxy-1,3,5(10),7-estratetraen-17-one) and 17-dihydroequilin, both of which contain a ∆7 double bond, and 17-dihydroequilinin (3β,17-dihydroxy-1,3,5(10),6,8-estrapentaen), which contains double bonds at carbons 6 and 8 of the B-ring.42,43 The former two can be made by the equine placenta incubated with 5,7-androstadiene-3β,17β-diol, in a pathway proposed to involve 17βHSD, 3βHSD and CYP19A1 (aromatase). Dihydroequilinin appears to arise from dehydrogenation of dihydroequilin.44 In parallel studies on microsomes from the horse and human placenta, both were able to convert the ∆7 androgen, 3-hydroxy-3,5,7-androstatrien-17-one to equilin,45 illustrating the ability of both human and equine CYP19A1 to act on ∆7 substrates. The perfused human placenta also made equilin and 17β-dihydroequilin from 3β-hydroxy-5,7-androstadien-17-one (7-dehydro-DHEA).46
The androgen precursors for the synthesis of B-ring unsaturated estrogens in the pregnant mare are from the fetal gonads. 7-Dehydro-DHEA has been identified in the gonadal veins of fetal ovaries and testes in situ.47 The horse fetal gonad has been shown to convert 7DHC to 7DHP and then to 7-dehydro-DHEA. Thus, the equine feto-placental unit displays a complete ∆7 steroidogenic pathway from 7DHC right through to estrogens.42,44,47 The high concentration of B-ring unsaturated estrogens in the pregnant mare compared with other mammalian species appears to arise from the ability of the fetal gonads to produce a high proportion of ∆7 androgens, perhaps reflecting limited 7DHC reductase activity. The pathways in the pregnant mare provide strong evidence for the ability of all the enzymes in the estrogen biosynthetic pathway, including CYP17A1 and aromatase, to work on the ∆7 isoforms of their substrates.
Pathways of 7DHC metabolism indicated by studies on Smith-Lemli-Opitz Syndrome patients
Smith-Lemli-Opitz Syndrome (SLOS) is caused by a deficiency in the enzyme 7-dehydrocholestrol reductase (DHCR7) which catalyzes the last step in cholesterol synthesis, the conversion of 7DHC to cholesterol.48 This syndrome is associated with decreased cholesterol levels and markedly elevated 7DHC levels. The ∆7 steroids identified in the urine of these patients provide a good indicator of the ability of various steroidogenic enzymes to act on ∆7 steroids.33,49 Interestingly, corresponding ∆8 steroids are also observed in these samples indicating conversion of ∆7 steroids to ∆8 steroids occurs in these patients, either enzymatically or non-enzymatically. The identification of 5,7-pregnadiene-3β,17α,20α-triol and other 17α-hydroxy-7DHP derivatives in SLOS patients clearly supports the above studies with purified enzymes, organelles and tissue fractions demonstrating the sequential involvement of the StAR protein and CYP11A1 in ∆7 steroid synthesis.
The finding that many of the ∆7 steroids identified in SLOS patients possess a 17α-hydroxyl group indicates that CYP17A1 displays 17α-hydroxylase activity on 5,7-pregnadiens. Many of the products including 5β-pregn-7-ene-3α,17α,20α-triol and 5,7-pregnadiene-3β,17α,20α-triol and other 17α-hydroxy 7DHP derivatives possess a 20α-hydroxy group. Thus the involvement of 20α-hydroxysteroid dehydrogenase in converting the ketone group at C20 of 7DHP and/or 17-hydroxy 7DHP is indicated. ∆7-Androgens (and ∆8 analogs) such as 7-dehydro-DHEA and 5,7-androstene-3β,17β-diol, have also been identified in the urine of SLOS patient indicating that CYP17A1 can catalyze the C17-C20 lyase reaction on 17-hydroxy ∆7 steroids. The ∆7 17-hydroxy products accumulate relative to the usual steroids (lacking ∆7 unsaturation) more than the ∆7 androgens accumulate, suggesting that CYP17A1 displays higher hydroxylase activity than lyase activity on ∆7 C21 steroids.33
No ∆7 steroids with a 21-hydroxyl group or an 11β-hydroxyl group have been identified in the urine of SLOS patients.33 Thus it would appear that neither CYP21A2, CYP11B1 nor CYP11B2 can act on ∆7 steroids. Interestingly, no steroids with a 4,7-diene structure as in 7-dehyroprogesterone have been identified in SLOS patients. As mentioned above, we have found that this ∆7 steroid is unstable,6 which could potentially prevent the isolation and identification of it or its hydroxy derivatives in the urine of SLOS patients. It is likely to be unstable in vivo as well as in vitro, with the possibility that the resulting non-enzymatic products have adverse physiological effects in SLOS. C21 steroids with only a double bond in the ∆7 position such as 5β-pregn-7-ene-3α,17α,20α-triol have been isolated from SLOS patients. These are believed to have arisen from 7-dehydroprogesterone (or its derivatives that retain the 3-oxo-4,7-diene structure) since the action of 5β-reductase and 3α-hydroxysteroid dehydrogenase requires the 3-oxo-∆4 structure.50
Estriol levels are low in women pregnant with SLOS-affected fetuses, presumably due to decreased cholesterol synthesis. 7-Dehydroestrogens, especially 16α-hydroxy-17β-dihydroequilin (7-dehydroestriol) and the 8-dehydro isomer have been isolated from the urine of a woman carrying a SLOS fetus, further supporting that human CYP19A1(aromatase) can convert ∆7-androgens to ∆7-estrogens.48,49,51
In summary, studies with purified enzymes, organelles and tissues, together with studies on equilin synthesis by pregnant mares and on ∆7 steroids in SLOS patients, clearly demonstrate that 7DHC can be converted to 7DHP, 17-hydroxy-7-DHP, 7-dehydroprogestrone, 7-dehydro-DHEA and the estrogens equilin and 17-dihydroequilin, by the same steroidogenic enzymes involved in the classical steroidogenic pathways. No ∆7 corticosteroids have been detected suggesting that C21 steroids with a ∆7 double bond cannot serve as substrates for CYP21A2. Studies with pregnant mares and SLOS patients suggest that the availability of ∆7 substrates, such as 7DHC and 7-dehydro-DHEA determines the flux through ∆7 pathways. An overview of ∆7 steroidogenic pathways with the enzymes involved and major product is shown in Figure 1.
Figure 1. ∆7 Steroidogenic pathways with the enzymes involved and major products.
Local ∆7 steroidogenesis in the skin
Skin is the largest organ with powerful metabolic and neuroendocrine activities,2,52,53 and has a relatively high content of 7DHC which serves as the precursor for vitamin D3 formation.40,41 Although skin expresses CYP11A1 at relatively low levels, it has been predicted to be a site of ∆7 steroid synthesis under non-pathological conditions because of the availability of 7DHC to this enzyme.4 The first support for this hypothesis came from the incubation of mitochondria isolated from rat skin with 7DHC, which showed that 7DHC is metabolized to 7DHP.5 Interestingly, rat skin extracts metabolized 7DHP to a more polar 5,7-diene (most likely a hydroxyderivative), while the skin microsomal fraction transformed 7DHP to two products with modified A and B rings, presumably one of them representing a 4,6-diene.5
Recently we provided evidence for this novel cutaneous pathway using cultured epidermal keratinocytes and LC/MS techniques.6 Specifically, immortalized human HaCaT keratinocytes transformed exogenously added 7DHC to 22(OH)7DHC, 20,22(OH)27DHC and 7DHP.6 In addition, epidermal keratinocytes from pig skin which contain high concentration of endogenous 7DHC, were able to produce 22(OH)7DHC, 20,22(OH)27DHC and 7DHP without the addition of exogenous substrate.6 These studies suggest that skin can metabolize 7DHC, although at a low level, with the following sequence 7DHC → 22(OH)7DHC → 20,22(OH)27DHC → 7DHP→ 7-dehydroprogesterone and hydroxyl 7DHP. Possible production of 7-dehydroprogesterone and hydroxyl 7DHP is indicated by the expression of 3βHSD and other steroidogenic enzymes in the skin,54-56 as well as the detection of 7DHP metabolism by rat skin extracts or microsomes.5 Such cutaneous 5,7-dienes can be photolytically transformed to the corresponding secosteroids. However, isomerisation to a 4,7-diene as in 7-dehydroprogesterone or to a 4,6-diene, which could potentially occur in the skin, would prevent such phototransformation.
Chemical synthesis of steroidal 5,7-dienes
In order to confirm the chemical nature of the isolated metabolites from tissues as well as to test their biological activity several 5,7-diene precursors of secosteroid analogs were synthesized as outlined in Figure 2 and reported previously.27,34-36,38 Generally, compounds 1(a, d) were protected by acetylation at C-3 to give compounds 2(a- d) to be later brominated at the C-7 position followed by dehydrobromination to afford compounds 3(a-d) with an additional double bond at C7-C8. Compounds 3(a-d) were used for the generation of the subsequent products 4(a, b, R/S), 5(a-d) and 6c under various reaction conditions

Figure 2. The synthesis of the 5,7-diene precursors of secosteroid analogs.
The synthesis of 20R(OH)7DHC is shown in Figure 3. As described in our previous publication57 pregnenolone acetate was oxidized to etienic acid 8 by freshly prepared NaOBr from bromine and sodium hydroxide solution. Compound 8 was converted to the Weinreb amide 9 in the presence of HBTU/DIPEA, and HNMeOMe•HCl salt. The 3β-hydroxyl group of compound 9 was protected by tERT-butyldiphenylsilylation with TBDPSCl to yield compound 10. The Weinreb amide moiety of compound 10 was then reacted with (4-methylpentyl)magnesium bromide to produce compound 11. Removal of the TBDPS group from compound 11 followed by acetylation generated compound 12. Bromination-dehydrobromination of compound 12 provided the 5, 7-diene 13. Finally, compound 13 was reacted with methylmagnesium bromide to achieve 20R(OH)7DHC (14).

Figure 3. The synthesis of 20R(OH)7DHC.
The plausible synthetic route of 20(OH)ergosterol is shown in Figure 4.22 The 2,3-dimethylbutyraldehyde 16 will be converted to the iodo compound 17 followed by reacting with magnesium to produce the Grignard reagent 18.58 The coupling of 7DHP acetate with the Grignard reagent 18 will provide 20(OH)ergosterol.
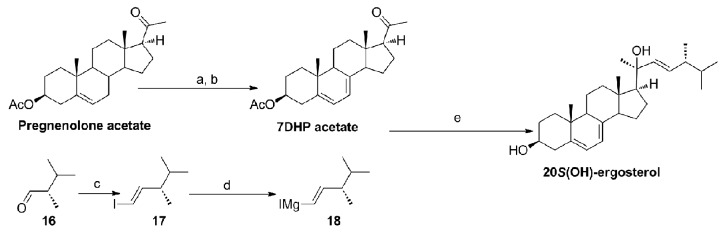
Figure 4. The plausible synthetic route of 20(OH)ergosterol
UVB-induced transformation of steroidal 5,7-dienes to secosteroids (Fig. 5)
Figure 5. UVB-induced transformation of steroidal 5,7-dienes to secosteroids
The UVB-induced photolysis of the B-ring of 7DHC is one of the most fundamental reactions in photobiology.40,59,60 It was also shown that other 5,7-dien-3β-ols can also be transformed to the corresponding secosteroids, generating a new family of secosteroids (Fig. 5, pro-D)36,38,61,62 (Table 1). The initial fast UVB-dependent reaction to break the B ring in 7DHC is followed by a relatively slow isomerization of pre-D3 to D3-like, tachysterol-like and lumisterol-like analogs,59 with similar reactions being involved in the generation of novel secosteroids from their intact B-ring precursors36,38,61,62 (Fig. 5). Prolonged UVB-exposure of 7DHC may lead to further transformation into 5,6-transvitamin D3, suprasterol I and suprasterol II.63 On the other hand, isomerization of tachysterol-like compounds may lead to isotachysterol-like compounds and their peroxide or hydroperoxide derivatives.38 Furthermore, UV irradiation is sufficient for the auto-catalytic conversion of 5,7-dien-3β-ols with or without a short side chain into the corresponding 5,7,9(11)-trien-3β-ols.61
Table 1. List of new secosteroids with shortened side chain and their 5, 7-diene precursors.
| Short name | Parental 5–7 diene | Photoderivative | |
|---|---|---|---|
| 7DHP | 3β-hydroxypregna-5,7-dien-20-one | ||
| pD | (5Z,7E)-3β-hydroxy-9,10-secopregna-5,7,10(19)-trien-20-one | ||
| pT | (6E)-3β-hydroxy-9,10-secopregna-5(10),6,8-trien-20-one | ||
| pL | 3β-hydroxy-9β,10α-pregna-5,7-dien-20-one | ||
| 17α(OH)7DHP | 3β,17α-dihydroxypregna-5,7-dien-20-one | ||
| 17α(OH)pD | (5Z,7E)-3β,17α-dihydroxy-9,10-secopregna-5,7,10(19)-trien-20-one | ||
| 17α(OH)pT | (6E)-3β,17α-dihydroxy-9,10-secopregna-5(10),6,8-trien-20-one | ||
| 17α(OH)pL | 3β,17α-dihydroxy-9β,10α-pregna-5,7-dien-20-one | ||
| 20(OH)7DHP | pregna-5,7-diene-3β,20-diol | ||
| 20(OH)pD | (5Z,7E)-9,10-secopregna-5,7,10(19)-triene-3β,20-diol | ||
| 20(OH)pL | 9β,10α-pregna-5,7-diene-3β,20-diol | ||
| 20(OH)pT | (6E)- 9,10-secopregna-5(10),6,8-triene-3β,20-diol | ||
| 7DHEA | androsta-5,7-dien-3β-ol | ||
| aD | (5Z,7E)-9,10-secoandrosta-5,7,10(19)-trien-3β-ol | ||
| aL | 9β,10α-androsta-5,7-dien-3β-ol | ||
| aT | (6E)- 9,10-secoandrosta-5(10),6,8-trien-3β-ol | ||
| 17α(OH)7DHEA | androsta-5,7-diene-3β,17α-diol | ||
| 17α(OH)aD | (5Z,7E)-9,10-secoandrosta-5,7,10(19)-triene-3β,17α-diol | ||
| 17α(OH)aL | 9β,10α-androsta-5,7-diene-3β,17α-diol | ||
| 17α(OH)aT | (6E)-9,10-secoandrosta-5(10),6,8-triene-3β,17α-diol | ||
| 17-COOH | (5Z,7E)-3β-hydroxy-androsta-5,7-diene-17β-carboxylic acid | ||
| 17-COOH aD3 | (5Z,7E)-3β-hydroxy-9,10-secoandrosta-5,7,9(10)-triene-17β-carboxylic acid | ||
| 17-COOH aT3 | (6E)-3β-hydroxy-9,10-secoandrosta-5(10),6,8-triene-17β-carboxylic acid | ||
| 17-COOH aL3 | (5Z,7E)-3β-hydroxy-9β,10α-androsta-5,7-diene-17β-carboxylic acid |
Biological Activity of ∆7 Secosteroids with a Short Side Chain
Cells of epidermal origin
Normal keratinocytes and melanocytes
Having established chemical/photochemical routes of synthesis for steroids/secosteroids with a short (2C) side chain or no side chain at all at C17, we tested their biological activity in normal skin cells. Specifically, 7DHP and pregnenolone inhibited the proliferation of epidermal HaCaT keratinocytes in a dose-dependent fashion with similar potency, while 20-oxopregnacalciferol (pD) was less potent.5,62 Similar effects were observed for cultures of immortalized normal epidermal melanocytes (PIG1)6,64 and normal primary melanocytes (see below).
We also tested the effect of 7DHP, pregnenolone and pD in comparison to 1,25(OH)2D3, on NFκB activity in HaCaT keratinocytes. All of these compounds inhibited NFκB activity with 7DHP being more efficient than pD, however, pD had similar potency to pregnenolone and 1,25(OH)2D3.5 This observation indicates the potential of these compounds to exhibit anti-inflammatory properties similar to 1,25(OH)2D3
We also tested in detail another compound that was unexpectedly discovered during synthesis of 21(OH)7DHP, namely (5Z,7E)-3β-hydroxyandrosta-5,7-diene-17β-carboxylic acid (17-COOH-7DA). It resulted from the oxidative cleavage of the side chain in a reaction dependent on the presence of oxygen.34 17-COOH-7DA showed high antiproliferative potency, without toxicity.34 For example it inhibited keratinocytes proliferation at doses as low as 10−11 M. Also, it inhibited proliferation of normal epidermal melanocytes and melanoma cells, and induced leukemia differentiation, however with lower potency than in keratinocytes. The corresponding secosteroidal derivatives remain to be tested.
Melanomas
Vitamin D analogs with a short (2C) side chain or the side chain absent possess proven or predicted low hypercalcemic activity.65-67 Therefore, secosteroids with a shortened and modified side chain were generated (Table 1) and their biological activity tested using a variety of human, mouse and hamster melanoma lines (for recent review see ref. 68). For instance, pD inhibited growth of human SKMEL-188 and hamster AbC1 cell lines in soft agar.5,62 In addition, vitamin D-like and lumisterol-like derivatives hydroxylated at C-21: (5Z,7E)-3β,21-dihydroxy-9,10-secopregna-5,7,10(19)-trien-20-one (21(OH)pD) and 3β,21-dihydroxy-9β,10α-pregna-5,7-dien-20-one (21(OH)pL) inhibited growth of human SKMEL-188 melanoma cells in a dose dependent manner, with a potency similar to or even higher than that of 1,25(OH)2D3.38 Moreover, 3β,21-dihydroxy-9β,10α-pregna-5,7-dien-20-one (21(OH)7DHP) precursor and its oxidized isotachysterol-like derivative (21(OH)oxy-piT) showed stronger antiproliferative activity against pigmented SKMEL-188 melanoma cells, while treatment with the parental compound, 21(OH)7DHP, and its UV-photoproduct 21(OH)pD, had similar impact on pigmented and nonpigmented cells.38 Finally, 21(OH)pD was shown to inhibit colony formation of SKMEL-188 melanoma cells.
Similar effects on colony formation by SKMEL-188 human melanoma cells were also observed for other vitamin D-like (17,20(OH)2pD) and lumisterol-like (17,20(OH)2pL) compounds derived from pregna-5,7-diene-3β,17α,20R-triol and pregna-5,7-diene-3β,17α,20S-triol, respectively, which were equally potent to or even more potent than 1,25(OH)2D3.35 We have also shown that 20(OH)pD3 and 20(OH)pL inhibit proliferation of SKMEL-188 human melanoma cells, and colony formation of AbC1 melanoma cells on soft agar, while (5Z,7E)-9,10-secoandrosta-5,7,10(19)-trien-3β-ol (aD) had a similar inhibitory effect on anchorage independent growth of SKMEL-188 melanoma cells.62 In addition, lumisterol derivatives including pL and 20(OH)pL inhibited growth of human melanoma in soft agar (Fig. 6). Interestingly, pD inhibited colony formation on soft agar by PC3 human prostate cancer cells.62
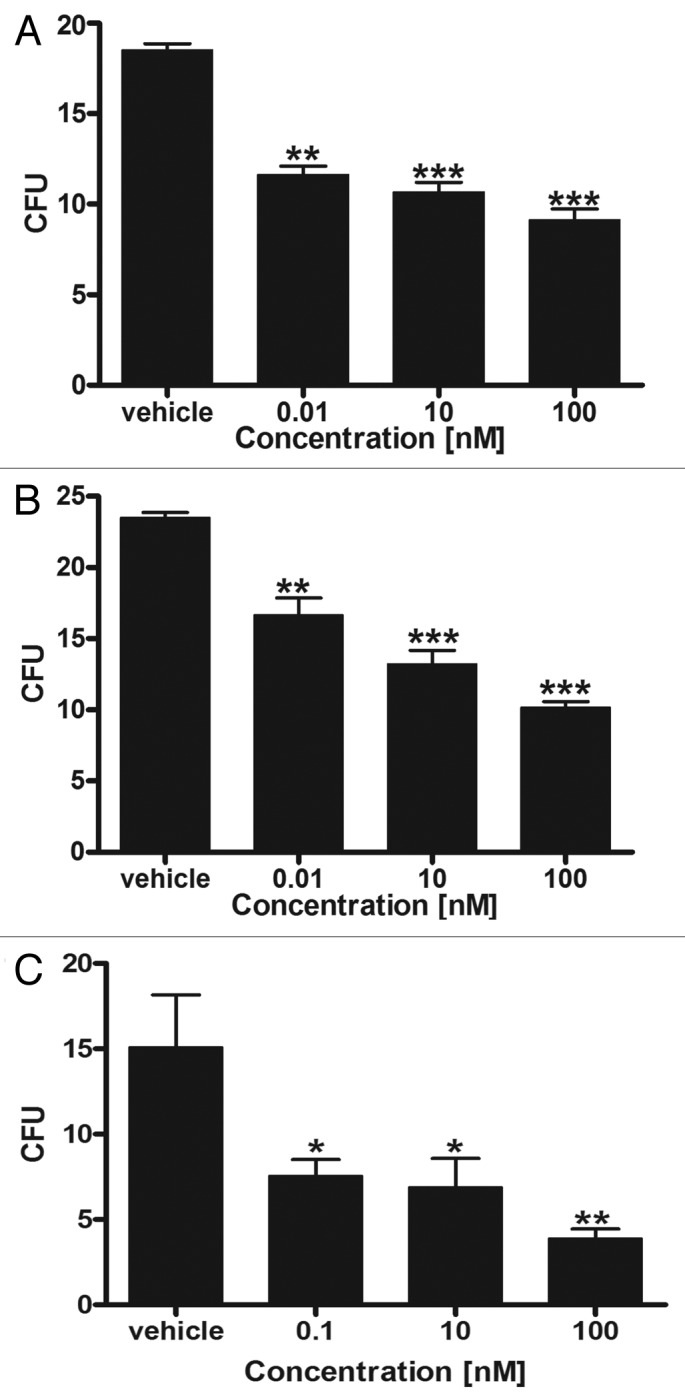
Figure 6. pL (3β-hydroxy-9β,10α-pregna-5,7-dien-20-one) and 20(OH)pL (9β,10α-pregna-5,7-diene-3β,20-diol) inhibit the anchorage independent growth (ability to form colonies in soft agar) of human melanoma cells in a dose-dependent manner. SKMel-188 human melanoma cells were grown in soft agar in the presence of graded concentrations of 20(OH)pL (A and B) or pL (C) as described previously.24 After three weeks colonies with a diameter larger than 0.2 mm (A) or 0.5 mm (B and C) were counted. Data are shown as mean ± SD (n = 4); statistical significance was estimated using the t-test and is presented as *p < 0.05, **p < 0.01 and ***p < 0.001.
Since vitamin D analogs with a short side chain were less efficient in inhibiting the proliferation of normal immortalized epidermal keratinocytes and melanocytes in comparison to melanoma cells,5,69 we further tested them on normal primary epidermal melanocytes and found that while 1,25(OH)2D3 and 20(OH)D3 inhibited cell proliferation at 10−7 M, the effect of 20(OH)pD or pD was minimal or absent (Fig. S1). Also, these compounds had no effect on melanin pigmentation and cell shape. However, when we used a continuous line of transformed melanocytes (PIG1) we observed inhibition of proliferation by 7DHP and pD, however, with lower efficiency in comparison to 25(OH)D3, 20(OH)D3, 20,23(OH)2D3 and 1,25(OH)2D3, indicating some sensitivity of immortalized melanocytes to pD (Fig. 7). Furthermore, 20(OH)pD inhibited proliferation of human melanoma cells without noticeable toxic effect (Fig. S2).
Figure 7. Comparison of the effects of vitamin D derivatives having a full-length site chain to those of 7DHP and pD, on the proliferation of the immortalized line, PIG1, of human melanocytes. The immortalized melanocytes were cultured as described previously.82 To study DNA synthesis, immortalized melanocytes were plated in 96-well plates at 10,000 cells/well. After overnight incubation, 20(OH)D3, 1,25(OH)2D3, 20,23(OH)2D3, 25(OH)D3, pD, 7DHP or ethanol as a control were added to the cells which were processed as described previously.22 Briefly, after 12 h of incubation [3H]-thymidine was added to a final concentration of 1 μCi/mL medium. After an additional 12 h, media were discarded, cells washed with cold PBS, lysed and processed for final measurement of the incorporated of 3H-radioactivity into DNA with a β counter.22 Significant differences between treated and non-treated (ethanol control) cells were measured using the t-test (*p < 0.05, **p < 0.01 and ***p < 0.001). Data are expressed as % of control.
Dermal fibroblasts
In contrast to normal epidermal cells, secosteroidal products with a short side chain significantly inhibited total collagen and hyaluronan synthesis in dermal fibroblasts induced by TGF-β.25 Interestingly, the S isomer of 17,20(OH)2pD was more potent than the R form or the corresponding 17,20-dihydroxypregnalumisterols (17,20(OH)2pL) or even 1,25(OH)2D3.25 We also tested the ability of 7DHP, pD and 17,20R(OH)27DHP and 17,20S(OH)27DHP (precursors to corresponding 17,20(OH)2pD and 17,20(OH)2pL) to inhibit collagen synthesis (Fig. 8). All of these compounds inhibited TGF-β-induced collagen synthesis, with pD being more potent than 7DHP. Thus, 7DHP and 17,20(OH)27DHP and their corresponding photoderivatives pD, 17,20(OH)2pD and 17,20(OH)2pL are identified as excellent inhibitors of collagen synthesis (Fig. 8).25

Figure 8. 7DHP, pD, 17,20R(OH)27DHP and 17,20S(OH)27DHP inhibit TGF-β-induced collagen synthesis. Human dermal fibroblasts grown from explant skin cultures at less than 10 subpassages were used as described previously.25 After a 2 h preincubation with the compounds being tested at concentrations of 10−9 or 10−10 M, or the vehicle control (ethanol), TGF-β1 (R and D systems) was added to each well (except control) at a final concentration of 5 ng/ml. After 48 h of culture, plate wells were pulsed with 1 μCi 3[H]-proline. After 24 h, culture supernatants were harvested and collagenase sensitive protein was determined.25,71 Data are shown as mean ± SD (n = 4); statistical significance was estimated using the t-test and showed that TGF-β1 stimulated collagen synthesis, while the 5,7-dienes and pD inhibited it (p < 0.001). pD was more potent than its precursor, 7DHP, at both concentrations: 1 nM (p < 0.01) and 0.1 nM (p < 0.05).
Leukemias
Selected compounds with a short side chain (7DHP, pD, pL, 20(OH)7DHP, 20(OH)pD) were also tested against leukemia cells in comparison to vitamin D3 hydroxy-derivatives with a full 8 carbon side-chain.26 In general, they inhibited proliferation and induced erythroid differentiation of K562 human chronic myeloid and MEL mouse leukemia cells, being only slightly or moderately less potent in comparison to novel vitamin D3 hydroxyderivatives with a full side-chain or to 1,25(OH)2D3. With HL-60 promyelocytic and U937 promonocytic human leukemia cells, pD and pL compounds were significantly less potent at inhibiting proliferation and stimulating monocytic differentiation in comparison to 20(OH)D3, 20,23(OH)2D3, 1,20(OH)2D3 and 1,25(OH)2D3.26
Overview of biological activity of secosteroids with a full-length side chain
The biological activity of vitamin D hydroxyderivatives with a full-length, 8 carbon side chain has been extensively studied in normal epidermal keratinocytes, melanocytes, fibroblasts and melanoma cells, as well as other malignant tumors.6,8,12,17,22,23,26,28-31,38,57,61,62,70,71 Below is short overview.
In keratinocytes, 20(OH)D3, 20,23(OH)2D3 and 17,20,23(OH)3D3 inhibited DNA synthesis and colony formation, caused cell cycle arrest, and stimulated the differentiation program with potencies comparable to that of 1,25(OH)2D3.29,31,62,70 20(OH)D3 and 20,23(OH)2D3 inhibited NFκB activity in normal and immortalized keratinocytes, and in melanoma,28-30 and had both anti-inflammatory and anti-fibrinogenic properties.30,62,71 In additional studies using normal human peripheral blood mononuclear cell (PBMC) cultures, we found that 20(OH)D3 markedly reduced TNFα production induced by LPS (10 pg/ml) [vehicle = 6002 ± 1479 pg/ml; 20(OH)D3 10−8 M = 2609 ± 1961 pg/ml p < 0.01] (description of methodology is in supplemental file).
20(OH)D3 and 20,23(OH)2D3 have potent anti-melanoma and anti-cancer activities.23,24,26,28,62 They act as partial agonists of the VDR, as demonstrated by gene silencing experiments.29,31 1α-Hydroxyderivatives of 20(OH)D3 and 20,23(OH)2D3 show similar inhibition of keratinocyte proliferation, and stimulation of differentiation and VDR expression to that of their precursors. The related CYP11A1-derived 22(OH)D3 and 20,22(OH)2D3 show antiproliferative and prodifferentiation effects, being less potent than 20(OH)D3 and 20,23(OH)2D3.12 Chemically synthesized 20S(OH)D3 has the same properties as that generated enzymatically27 but a different effect is exerted by 20R(OH)D3 at low concentrations.57 Interestingly, 20S(OH)7DHC (a precursor to 20S(OH)D3) also inhibited cell proliferation (Fig. 9).

Figure 9. 20S(OH)7DHC (precursor to 20S(OH)D3) inhibits proliferation of neonatal human epidermal keratinocytes (HEKn). HEKn in their third passage were treated with graded concentrations of 20S(OH)7DHC for 24 h (A) or 48 h (B), as described previously.57 Incorporation of radioactive thymidine (3H) into DNA was determined 4 h after incubation. Data are presented as mean ± SD (n = 4). DNA incorporation was calculated as a percentage of the control (ethanol treated cells). Statistical significance was measured using one-way ANOVA presented as *p < 0.05, **p < 0.01 and p < 0.001.
Studies on the structurally related secosteroid, 20(OH)D2, have also demonstrated its ability to induce the cell differentiation program mediated, at least in part, through activation of the VDR. This is illustrated by the attenuation of cell proliferation after silencing of the VDR, by enhancement of the inhibitory effect through stable overexpression of the VDR and by the demonstration that 20(OH)D2 induces time-dependent translocation of VDR from the cytoplasm to the nucleus at a comparable rate to 1,25(OH)2D3.22 20(OH)D2 did not require 1α-hydroxylation for biological activity. In addition, we have demonstrated that ergosterol hydroxyderivatives have the potential to inhibit proliferation and induce differentiation of keratinocytes.8
Importantly, 20(OH)D3 at a dose as high as 3.0 µg/kg had no calcemic activity in rats whereas 1,25(OH)2D3 at lower doses raised calcium to 16.0 ± 1.2 mg/dL.26 The addition of a 1α-hydroxyl group to 20(OH)D3 conferred some calcemic activity to the derivative.26 We repeated this testing and administered 20(OH)D3 doses as high as 30 µg/kg to C57BL/6 mice daily for 14 d and found no significant differences in sera Ca2+ levels compared with control mice and no toxicity as determined by serum chemistry and histological analyses of heart, liver, spleen and kidney.23 20(OH)D2 is also non-calcemic in rats at doses at least up to 4 μg/kg,22 and 20,23(OH)2D3 is non-calcemic in mice at 3 μg/kg.71
Secosteroids with long side chain are better ligands for the vitamin D receptor than secosteroids with short side chain
Using pLenti-CMV-VDR-EGFP-pgk-puro constructs,22,70 we tested the ligand-induced translocation of VDR from the cytoplasm to the nucleus.22 Vitamin D3 hydroxy-derivatives with a full-length (8C) side chain and hydroxy-secosteroids with a shortened side (2C) chain (pD) stimulated VDR translocation and inhibited proliferation, however, the former were more potent than pDs. Molecular modeling of the binding of secosteroids to the VDR genomic binding pocket (G-pocket) correlated well with the experimental data for VDR translocation. In contrast, docking scores for the non-genomic binding site [A-pocket] of the VDR were poor, suggesting that they do not act on the A-pocket.70 For example, 20(OH)D3 bound to the G-pocket in a manner that overlapped well with the native ligand 1,25(OH)2D3. Both of these secosteroids posed the side chains toward the surface of VDR and buried the secocholesta head deeply inside the pocket bottom. The glide score of 20(OH)D3 was −11.746 compared with that of −12.321 for 1,25(OH)2D3.
Anti-oxidative effects of 5,7-dienes
Since it has been proposed that cholesterol and vitamin D act as membrane anti-oxidants,72,73 we compared anti-oxidative properties of 7DHC, 7DHP, vitamin D3, vitamin D2 and cholesterol by measuring 2,4-dinitrophenylhydrazine (DNPH) reactive protein-bound carbonyls in isolated rat liver mitochondria treated with iron/ascorbate (for methodology see supplemental file). As shown in Figure 10A, 7DHC and 7DHP significantly attenuated oxidative protein modification being more effective than cholesterol, vitamin D3 and vitamin D2. 7DHP at concentrations of 1 μM and 10 μM decreased the protein carbonyl content by 26.7% (**p < 0,01) and 26.3% (**p < 0.01), respectively, compared with mitochondria treated only with iron/ascorbate. 7DHC at the same concentrations was more potent than 7DHP and attenuated oxidative protein modification by 37.8% (**p < 0.01) and 34.3% (**p < 0.01), respectively.
Figure 10. 7DHC and 7DHP attenuate oxidative protein damage in isolated rat liver mitochondria treated with iron/ascorbate. (A) 7DHC and 7DHP are more effective than cholesterol, vitamin D3 and vitamin D2 in attenuating oxidative protein modification. (B) Relative antioxidant abilities of 7-DHC and 7-DHP in comparison to GSH, melatonin and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine(AFMK) and its precursor, N-acetylserotonin (NAS). The carbonyl content of mitochondrial proteins following treatment with iron/ascorbate was measured as described in the supplemental file. Data are presented as means ± SEM (n = 3), statistical significance was estimated using the t-test. The basal level of protein-bound carbonyls in intact mitochondria was 2.20 ± 0.13 nmol/mg protein. The content of protein carbonyls increased 3.6 times over the basal level after incubation of mitochondria in the iron/ascorbate system for 45 min.
We also compared the antioxidant ability of 7DHC and 7DHP to that of the powerful mitochondrial antioxidants such as GSH, as well other endogenous anti-oxidants such as melatonin and its metabolites [6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK)]74-76 and its precursor N-acetylserotonin (NAS). While 6-hydroxymelatonin was the most effective in preventing oxidative damage to the proteins, 7DHC and 7DHP also showed prominent antioxidant properties, similar to that of AFMK and NAS (Fig. 10B). Furthermore, 7DHC and 7DHP were more effective than melatonin (a recognized anti-oxidant74,75) and GSH. 7DHC at concentrations 100 nM and 1 μM attenuated oxidative protein modification being 1.35 (*p < 0.05) and 1.31 (*p < 0.05) times more effective than melatonin, and in 1.42 (**p < 0.01) and 1.44 (**p < 0.01) times more effective than GSH. This finding opens an exciting possibility for defining a novel role for 7DHC and 7DHP as endogenous antioxidants in the skin, which are either equal (AFMK) or more potent than melatonin. AFMK and melatonin are considered as excellent endogenous or exogenous protectors of the skin against oxidative damage.77-81
Conclusions and perspective
We have discovered new pathways for the generation of new secosteroids with short and full-length side chains which are based on the catalytic activity of CYP11A1 on the side chain of 7DHC, ergosterol and vitamin D. Although production of 5,7-diene steroids was anticipated because of SLOS, our4 and Guryev and coworker’s39 studies were the first to document a crucial role of CYP11A1 in the generation of ∆7-steroids. Importantly, our laboratories were the first to indicate UVB induced transformation of such ∆7-steroids in the skin to androsta- and pregna- calciferol, lumisterol or tachysterol-like compounds that are biological active. Of equal significance is the P450scc-mediated generation of novel biologically active hydroxy-derivatives of vitamins D3 and D2. Recently, evidence was provided for their production in vivo under physiological conditions.6,13 We have also established biochemical and chemical routes of synthesis of these secosteroids and defined their structures.
Importantly, 20(OH)D3, 20,23(OH)2D3 and 20(OH)D2, as well as 17,20(OH)2pD, are non-toxic and non-calcemic in rodents. These and related novel secosteroids show anti-proliferative, pro-differentiation, anti-cancer, anti-fibrosing and anti-inflammatory properties that are determined by their chemical structure and the lineage of the target cells. Therefore, they should serve as excellent therapeutic agents for hyperproliferative, fibrosing or inflammatory disorders, or as major drugs or adjuvants in cancer therapy.
Although we have already provided evidence that these new secosteroids can act as partial agonists on the vitamin D receptor, the future challenge is to identify alternative nuclear receptors that are activated by these compounds. Furthermore, mechanism(s) of action of steroidal 5,7-dienes remain(s) to be clarified, since they also demonstrate antiproliferative and antifibrosing activities. Lastly, measurement of the relative concentrations of the new vitamin D analogs in body fluids as well as the CYP11A1 dependent rates of production of ∆7-steroids in the skin in vivo await further experimentation.
Supplementary Material
Acknowledgments
The work was supported by NIH grants R01AR052190 and R01AR056666 to A.S., by the University of Western Australia, by 1S10RR026377-01 and 1S10OD010678-01 to W.L., and by the College of Pharmacy at the University of Tennessee Health Science Center, and a grant from the Polish Ministry of Science and Higher Education, project no. N405 623238 to M.A.Z. and A.S.
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/23938
References
- 1.Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug Discov Today Dis Mech. 2008;5:137–44. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87. doi: 10.1210/er.21.5.457. [DOI] [PubMed] [Google Scholar]
- 3.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012;44:2003–18. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuckey RC, Nguyen MN, Chen J, Slominski AT, Baldisseri DM, Tieu EW, et al. Human cytochrome P450scc (CYP11A1) catalyzes epoxide formation with ergosterol. Drug Metab Dispos. 2012;40:436–44. doi: 10.1124/dmd.111.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, et al. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol. 2005;12:931–9. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–90. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, et al. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2008;112:213–9. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–96. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, et al. Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos. 2011;39:1577–88. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EKY, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D₃ metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–15. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem Mol Biol. 2012;129:163–71. doi: 10.1016/j.jsbmb.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang EK, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, et al. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab Dispos. 2010;38:1553–9. doi: 10.1124/dmd.110.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang EK, Voo KJ, Nguyen MN, Tuckey RC. Metabolism of substrates incorporated into phospholipid vesicles by mouse 25-hydroxyvitamin D3 1alpha-hydroxylase (CYP27B1) J Steroid Biochem Mol Biol. 2010;119:171–9. doi: 10.1016/j.jsbmb.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin d2 to 17,20,24-trihydroxyvitamin d2 by cytochrome p450scc (CYP11A1) Drug Metab Dispos. 2009;37:761–7. doi: 10.1124/dmd.108.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–81. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tieu EW, Tang EK, Chen J, Li W, Nguyen MN, Janjetovic Z, et al. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem Pharmacol. 2012;84:1696–704. doi: 10.1016/j.bcp.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–41. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, et al. 20-hydroxyvitamin D₃ inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–46. [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski AT, Janjetovic Z, Kim T-K, Wright AC, Grese LN, Riney SJ, et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–42. [PMC free article] [PubMed] [Google Scholar]
- 25.Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011;131:1167–9. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, et al. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–35. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, et al. High basal NF-κB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–84. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr., Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–80. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcos J, Guo LW, Wilson WK, Porter FD, Shackleton C. The implications of 7-dehydrosterol-7-reductase deficiency (Smith-Lemli-Opitz syndrome) to neurosteroid production. Steroids. 2004;69:51–60. doi: 10.1016/j.steroids.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD. Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome) J Steroid Biochem Mol Biol. 2002;82:225–32. doi: 10.1016/S0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 34.Kim TK, Chen J, Li W, Zjawiony J, Miller D, Janjetovic Z, et al. A new steroidal 5,7-diene derivative, 3beta-hydroxyandrosta-5,7-diene-17beta-carboxylic acid, shows potent anti-proliferative activity. Steroids. 2010;75:230–9. doi: 10.1016/j.steroids.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, et al. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–28. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, et al. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci. 2008;7:1570–6. doi: 10.1039/b809005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, et al. Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2010 doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, et al. Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2010;76:193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100:14754–9. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 41.Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 42.Tait AD, Hodge LC, Allen WR. The biosynthesis of 3 beta-hydroxy-5,7-androstadien-17-one by the horse fetal gonad. FEBS Lett. 1985;182:107–10. doi: 10.1016/0014-5793(85)81164-9. [DOI] [PubMed] [Google Scholar]
- 43.Savard K. The estrogens of the pregnant mare. Endocrinology. 1961;68:411–6. doi: 10.1210/endo-68-3-411. [DOI] [PubMed] [Google Scholar]
- 44.Foster SJ, Marshall DE, Houghton E, Gower DB. Investigations into the biosynthetic pathways for classical and ring B-unsaturated oestrogens in equine placental preparations and allantochorionic tissues. J Steroid Biochem Mol Biol. 2002;82:401–11. doi: 10.1016/S0960-0760(02)00224-8. [DOI] [PubMed] [Google Scholar]
- 45.Numazawa M, Osawa Y. Equilin and equilenin biosynthesis. Stereochemistry of aromatization of 3-hydroxy-3,5,7-androstatrien-17-one by horse placenta. J Steroid Biochem. 1987;26:137–43. doi: 10.1016/0022-4731(87)90042-2. [DOI] [PubMed] [Google Scholar]
- 46.Stárka L, Breuer H, Cedard L. Biosynthesis of equilin and related ring B unsaturated oestrogens in perfused human placenta. J Endocrinol. 1966;34:447–56. doi: 10.1677/joe.0.0340447. [DOI] [PubMed] [Google Scholar]
- 47.Raeside JI, Renaud RL. Identification of 3 beta-hydroxy-5,7-androstadien-17-one as a secretory product of the fetal horse gonad in vivo and in vitro. J Endocrinol. 1985;107:415–9. doi: 10.1677/joe.0.1070415. [DOI] [PubMed] [Google Scholar]
- 48.Shackleton CH. Role of a disordered steroid metabolome in the elucidation of sterol and steroid biosynthesis. Lipids. 2012;47:1–12. doi: 10.1007/s11745-011-3605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shackleton CH, Roitman E, Kratz LE, Kelley RI. Equine type estrogens produced by a pregnant woman carrying a Smith-Lemli-Opitz syndrome fetus. J Clin Endocrinol Metab. 1999;84:1157–9. doi: 10.1210/jc.84.3.1157. [DOI] [PubMed] [Google Scholar]
- 50.Slominski A, Wortsman J, Foecking MF, Shackleton CH, Gomez-Sanchez CE, Szczesniewski A. Gas chromatography/mass spectrometry characterization of corticosteroid metabolism in human immortalized keratinocytes. J Invest Dermatol. 2002;118:310–5. doi: 10.1046/j.0022-202x.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- 51.Shackleton CH, Roitman E, Kratz L, Kelley R. Dehydro-oestriol and dehydropregnanetriol are candidate analytes for prenatal diagnosis of Smith-Lemli-Opitz syndrome. Prenat Diagn. 2001;21:207–12. doi: 10.1002/1097-0223(200103)21:3<207::AID-PD27>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 52.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v–, vii, 1-115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 54.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.02.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–9. doi: 10.1210/jc.81.7.2746. [DOI] [PubMed] [Google Scholar]
- 56.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265-266:143–9. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y, Chen J, Janjetovic Z, Michaels P, Tang EK, Wang J, et al. Design, synthesis, and biological action of 20R-hydroxyvitamin D3. J Med Chem. 2012;55:3573–7. doi: 10.1021/jm201478e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hua Z, Carcache DA, Tian Y, Li YM, Danishefsky SJ. The synthesis and preliminary biological evaluation of a novel steroid with neurotrophic activity: NGA0187. J Org Chem. 2005;70:9849–56. doi: 10.1021/jo051556d. [DOI] [PubMed] [Google Scholar]
- 59.Holick MF, Clark MB. The photobiogenesis and metabolism of vitamin D. Fed Proc. 1978;37:2567–74. [PubMed] [Google Scholar]
- 60.Holick MF, Tian XQ, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc Natl Acad Sci U S A. 1995;92:3124–6. doi: 10.1073/pnas.92.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, et al. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–28. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slominski A, Li W, Zbytek B, Tuckey RC, Zjawiony J, Nguyen MN, et al. Enzymatic production or chemical synthesis and uses for 5,7-dienes and UVB conversion products thereof. US2011/0118228A1. 2011 [Google Scholar]
- 63.Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68:882–7. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- 64.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plum LA, Prahl JM, Ma X, Sicinski RR, Gowlugari S, Clagett-Dame M, et al. Biologically active noncalcemic analogs of 1alpha,25-dihydroxyvitamin D with an abbreviated side chain containing no hydroxyl. Proc Natl Acad Sci U S A. 2004;101:6900–4. doi: 10.1073/pnas.0401656101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murari MP, Londowski JM, Bollman S, Kumar R. Synthesis and biological activity of 3 beta-hydroxy-9,10-secopregna-5,7,10[19]-triene-20-one: a side chain analogue of vitamin D3. J Steroid Biochem. 1982;17:615–9. doi: 10.1016/0022-4731(82)90562-3. [DOI] [PubMed] [Google Scholar]
- 67.Holick MF, Garabedian M, Schnoes HK, DeLuca HF. Relationship of 25-hydroxyvitamin D3 side chain structure to biological activity. J Biol Chem. 1975;250:226–30. [PubMed] [Google Scholar]
- 68.Szyszka P, Zmijewski MA, Slominski AT. New vitamin D analogs as potential therapeutics in melanoma. Expert Rev Anticancer Ther. 2012;12:585–99. doi: 10.1586/era.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zmijewski MA, Li W, Chen J, Kim T-K, Zjawiony JK, Sweatman TW, et al. Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76:193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, et al. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol. 2012;361:143–52. doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab. 2013;98:E298–303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith LL. Another cholesterol hypothesis: cholesterol as antioxidant. Free Radic Biol Med. 1991;11:47–61. doi: 10.1016/0891-5849(91)90187-8. [DOI] [PubMed] [Google Scholar]
- 73.Wiseman H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326:285–8. doi: 10.1016/0014-5793(93)81809-E. [DOI] [PubMed] [Google Scholar]
- 74.Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, et al. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34:75–8. doi: 10.1034/j.1600-079X.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- 75.Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–97. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 76.Tan DX, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, et al. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001;15:2294–6. doi: 10.1096/fj.01-0309fje. [DOI] [PubMed] [Google Scholar]
- 77.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 78.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152–66. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008;17:713–30. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 80.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–6. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 81.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–94. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 82.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–6. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.