Abstract
A membrane-bound adenosine triphosphatase (EC 3.6.1.3) that requires Mg++ and that is stimulated by monovalent ions has been purified 7- to 8-fold from homogenates of oat (Avena sativa L. Cult. Goodfield) roots by discontinuous sucrose-gradient centrifugation. The enzyme was substrate specific; adenosine triphosphate was hydrolyzed 25 times more rapidly than other nucleoside triphosphates. The membrane fraction containing adenosine triphosphatase was enriched in plasma membranes, which were identified by the presence of a glucan synthetase (EC 2.4.1.12), a high sterol to phospholipid ratio, and by a stain consisting of periodic acid, chromic acid, and phosphotungstic acid that is specific for plant plasma membranes. Oat-root plasma membranes and the associated adenosine triphosphatase were purified on either a 6-layer discontinuous sucrose gradient or on a simplified gradient consisting of only two sucrose layers.
These results represent the first demonstration that plant plasma membranes contain an adenosine triphosphatase that is activated by monovalent ions, and this finding further implicates the enzyme in the absorption of inorganic ions by plant roots.
Keywords: transport, phospholipid, sterols, plasma membrane stain, glucan synthetase
Full text
PDF
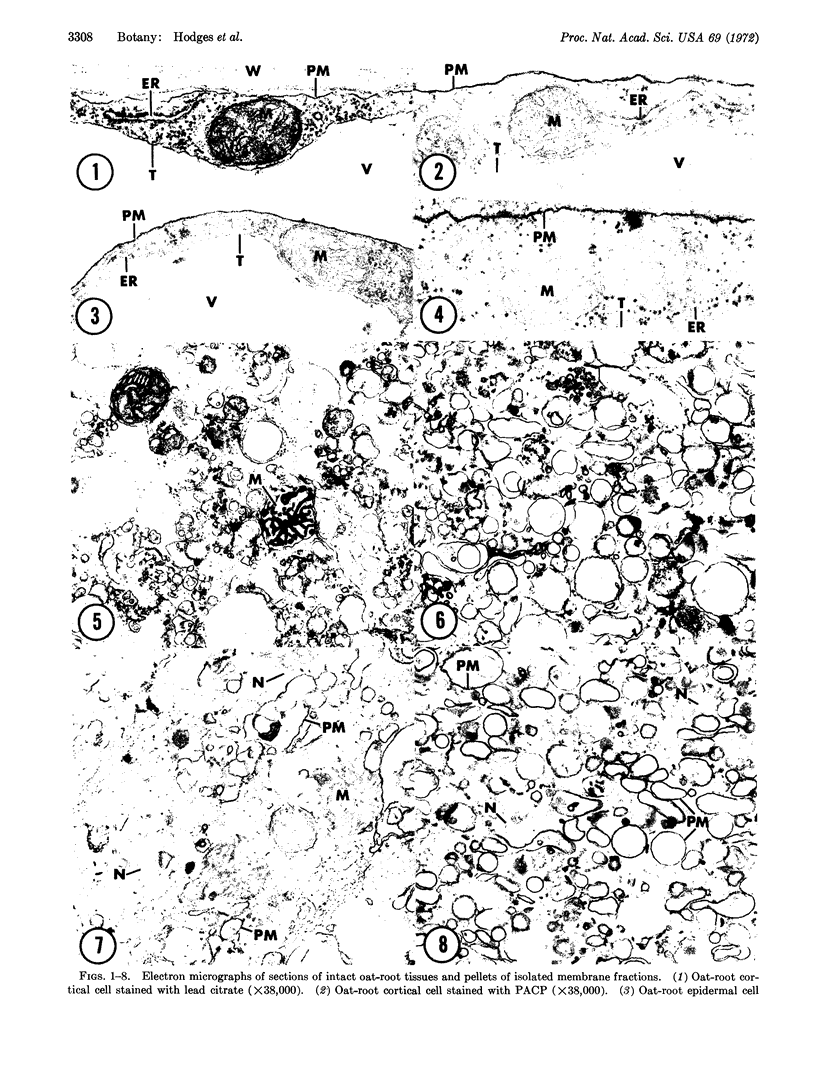
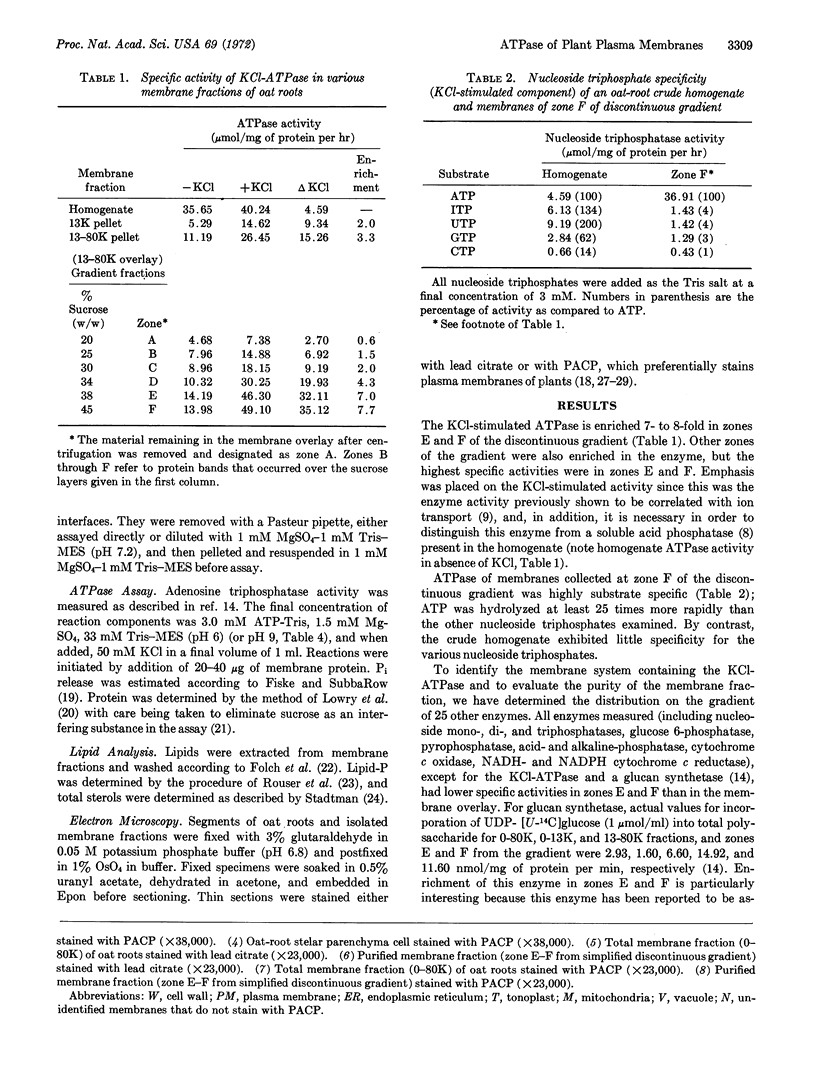
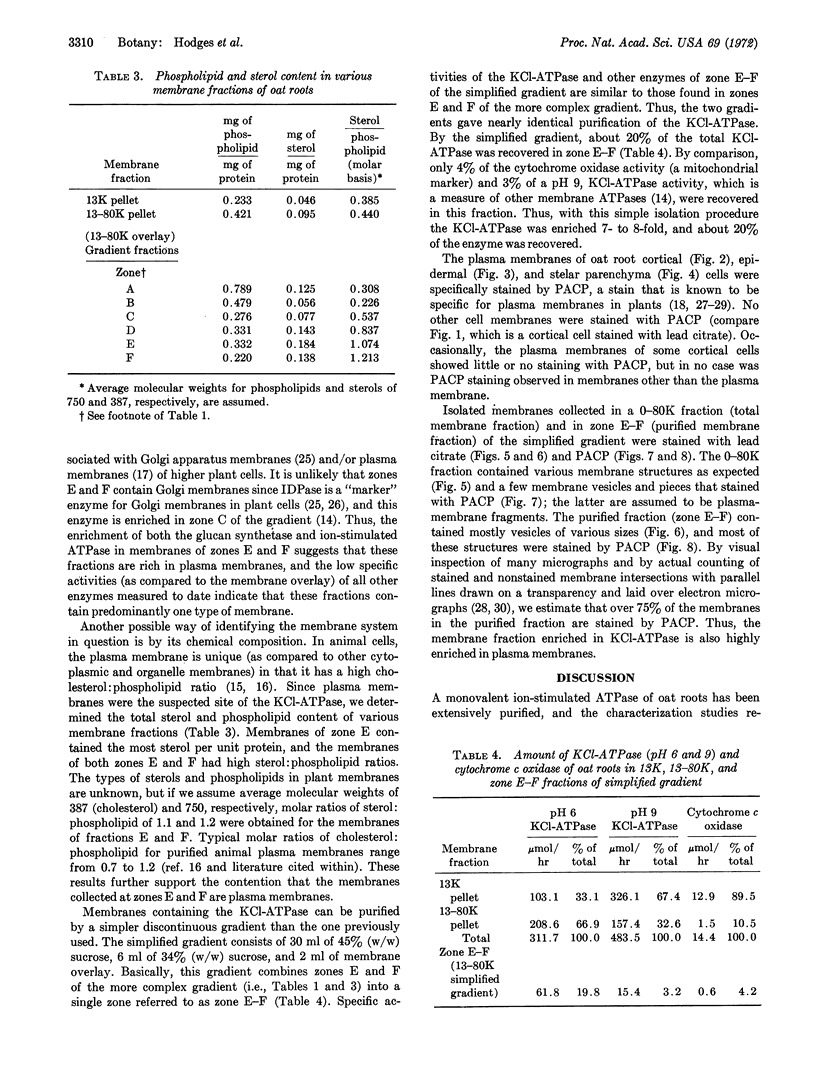

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bledsoe C., Cole C. V., Ross C. Oligomycin inhibition of phosphate uptake and ATP labeling in excised maize roots. Plant Physiol. 1969 Jul;44(7):1040–1044. doi: 10.1104/pp.44.7.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Dauwalder M., Whaley W. G., Kephart J. E. Phosphatases and differentiation of the Golgi apparatus. J Cell Sci. 1969 Mar;4(2):455–497. doi: 10.1242/jcs.4.2.455. [DOI] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B., Beevers H. Influence of sucrose on protein determination by the Lowry procedure. Anal Biochem. 1968 Aug;24(2):337–339. doi: 10.1016/0003-2697(68)90187-5. [DOI] [PubMed] [Google Scholar]
- Hall J. L. Cytochemical localization of ATP-ase activity in plant root cells. J Microsc. 1971 Jun;93(3):219–225. doi: 10.1111/j.1365-2818.1971.tb02284.x. [DOI] [PubMed] [Google Scholar]
- Higinbotham N. The Possible Role of Adenosine Triphosphate in Rubidium Absorption as Revealed by the Influence of External Phosphate, Dinitrophenol and Arsenate. Plant Physiol. 1959 Nov;34(6):645–650. doi: 10.1104/pp.34.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai Y. F., Thompson J. E. The preparation and properties of an isolated plant membrane fraction enriched in (Na plus-K plus)-stimulated ATPase. Biochim Biophys Acta. 1971 Mar 9;233(1):84–90. doi: 10.1016/0005-2736(71)90360-9. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Increased Membrane-bound Adenosine Triphosphatase Activity Accompanying Development of Enhanced Solute Uptake in Washed Corn Root Tissue. Plant Physiol. 1972 Mar;49(3):436–440. doi: 10.1104/pp.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON R. N., WILKINS M. J., WEEKS D. C. Studies in the metabolism of plant cells. IX. The effects of 2,4-dinitrophenol on salt accumulation and salt respiration. Aust J Sci Res B. 1951 Aug;4(3):248–264. doi: 10.1071/bi9510248. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- VanDerWoude W. J., Lembi C. A., Morré D. J. Auxin (2,4-D) stimulation (in vivo and in vitro) of polysaccharide synthesis in plasma membrane fragments isolated from onion stems. Biochem Biophys Res Commun. 1972 Jan 14;46(1):245–253. doi: 10.1016/0006-291x(72)90656-0. [DOI] [PubMed] [Google Scholar]




