Abstract
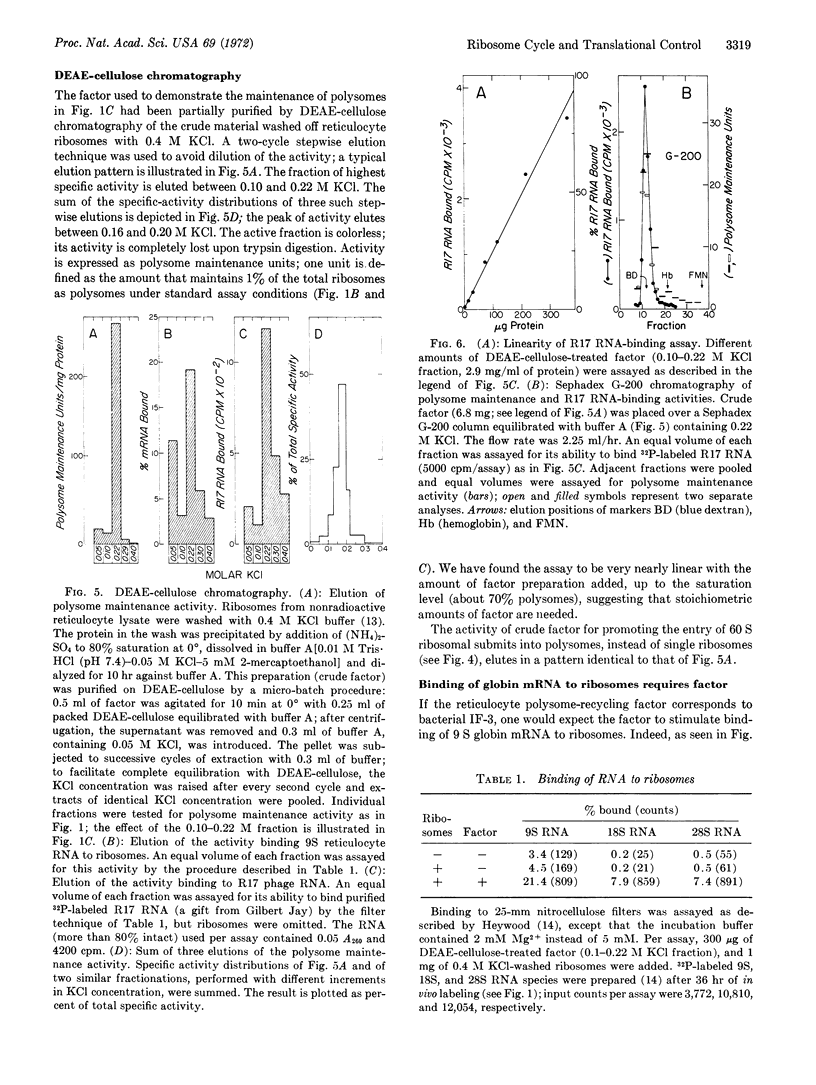
The continued recycling of ribosomes during protein synthesis in rabbit reticulocyte lysates at 37° requires an initiation factor whose activity is rapidly lost in the absence of added heme. Partially purified factor (i) fully maintains the polysomes; (ii) inhibits the association of 40S and 60S ribosomal subunits into single ribosomes; (iii) promotes the quantitative entry of added 60S subunits into polysomes; (iv) allows the accumulation of ribosomal subunits, instead of single ribosomes, when initiation is blocked with aurin tricarboxylate; and (v) is absolutely required for the binding of globin messenger RNA to ribosomes.
These properties suggest that this mammalian initiation factor functions analogously to bacterial IF-3. In addition, the translational control of globin synthesis by heme is exerted, directly or indirectly, through this factor.
Keywords: IF-3, mammalian ribosome cycle, R17 RNA binding
Full text
PDF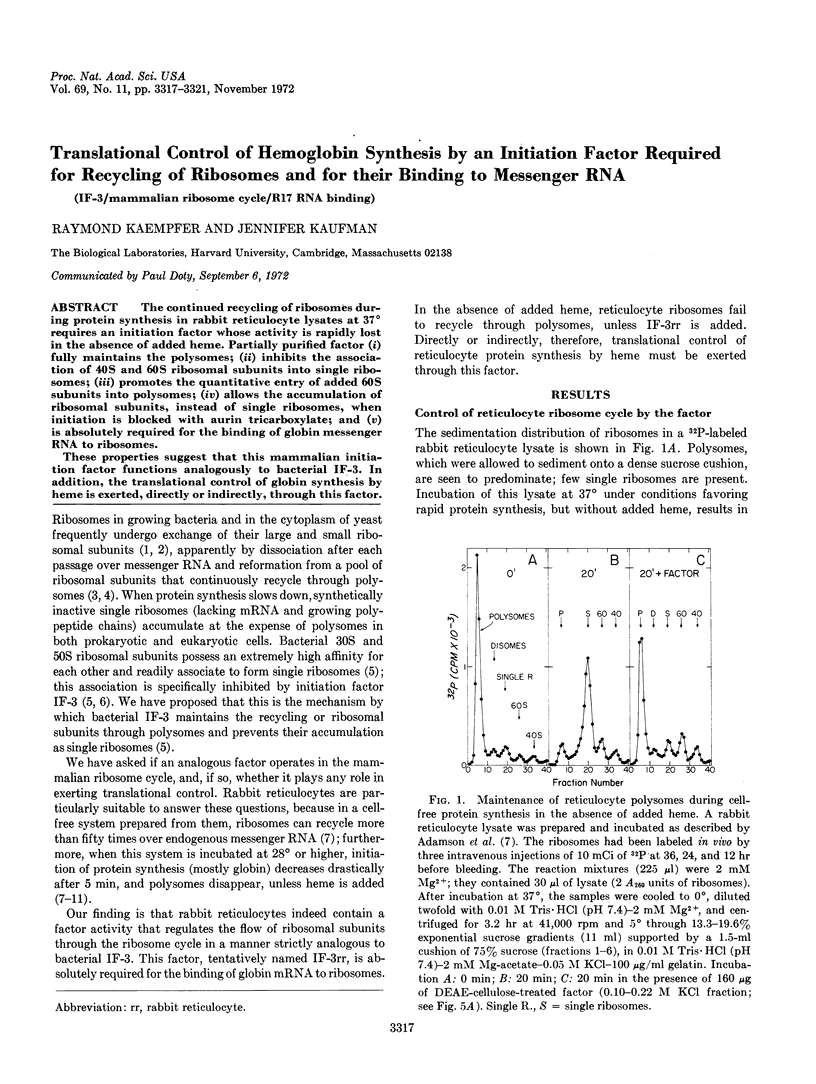



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Adamson S. D., Herbert E., Kemp S. F. Effects of hemin and other porphyrins on protein synthesis in a reticulocyte lysate cell-free system. J Mol Biol. 1969 Jun 14;42(2):247–258. doi: 10.1016/0022-2836(69)90041-2. [DOI] [PubMed] [Google Scholar]
- GIRARD M., LATHAM H., PENMAN S., DARNELL J. E. ENTRANCE OF NEWLY FORMED MESSENGER RNA AND RIBOSOMES INTO HELA CELL CYTOPLASM. J Mol Biol. 1965 Feb;11:187–201. doi: 10.1016/s0022-2836(65)80050-x. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M. Specificity of mRNA binding factor in eukaryotes. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1782–1788. doi: 10.1073/pnas.67.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L., Korner A. Ribosomal subunits of Landschütz ascites cells during changes in polysome distribution. Biochim Biophys Acta. 1968 Nov 20;169(1):129–138. doi: 10.1016/0005-2787(68)90014-2. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Subunit recycling during translation in a reticulocyte cell-free system. J Biol Chem. 1970 Nov 25;245(22):6237–6239. [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. I. Origin and significance of the subribosomal particles. J Mol Biol. 1965 Sep;13(2):496–510. doi: 10.1016/s0022-2836(65)80112-7. [DOI] [PubMed] [Google Scholar]
- Kabat D., Rich A. The ribosomal subunit--polyribosome cycle in protein synthesis of embryonic skeletal muscle. Biochemistry. 1969 Sep;8(9):3742–3749. doi: 10.1021/bi00837a038. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Meselson M., Raskas H. J. Cyclic dissociation into stable subunits and re-formation of ribosomes during bacterial growth. J Mol Biol. 1968 Jan 28;31(2):277–289. doi: 10.1016/0022-2836(68)90444-0. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Control of single ribosome formation by an initiation factor for protein synthesis. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2458–2462. doi: 10.1073/pnas.68.10.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R. Dissociation of ribosomes on polypeptide chain termination and origin of single ribosomes. Nature. 1970 Nov 7;228(5271):534–537. doi: 10.1038/228534a0. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Ribosomal subunit exchange during protein synthesis. Proc Natl Acad Sci U S A. 1968 Sep;61(1):106–113. doi: 10.1073/pnas.61.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R. Ribosomal subunit exchange in the cytoplasm of a eukaryote. Nature. 1969 Jun 7;222(5197):950–953. doi: 10.1038/222950a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Picciano D. J., Laycock D. G., Anderson W. F. Translation of exogenous messenger RNA for hemoglobin on reticulocyte and liver ribosomes. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2752–2756. doi: 10.1073/pnas.68.11.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol S., Sillero M. A., Iwasaki K., Ochoa S. Purification and properties of initiation factor F3. Nature. 1970 Dec 26;228(5278):1269–1273. doi: 10.1038/2281269a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Shafritz D. A., Laycock D. G., Anderson W. F. Puromycin-peptide bond formation with reticulocyte initiation factors M1 and M2. Proc Natl Acad Sci U S A. 1971 Feb;68(2):496–499. doi: 10.1073/pnas.68.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba A. J., Iwasaki K., Miller M. J., Sabol S., Sillero M. A., Vasquez C. Initiation of protein synthesis in Escherichia coli. II. Role of the initiation factors in polypeptide synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:291–299. doi: 10.1101/sqb.1969.034.01.035. [DOI] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]



