Abstract
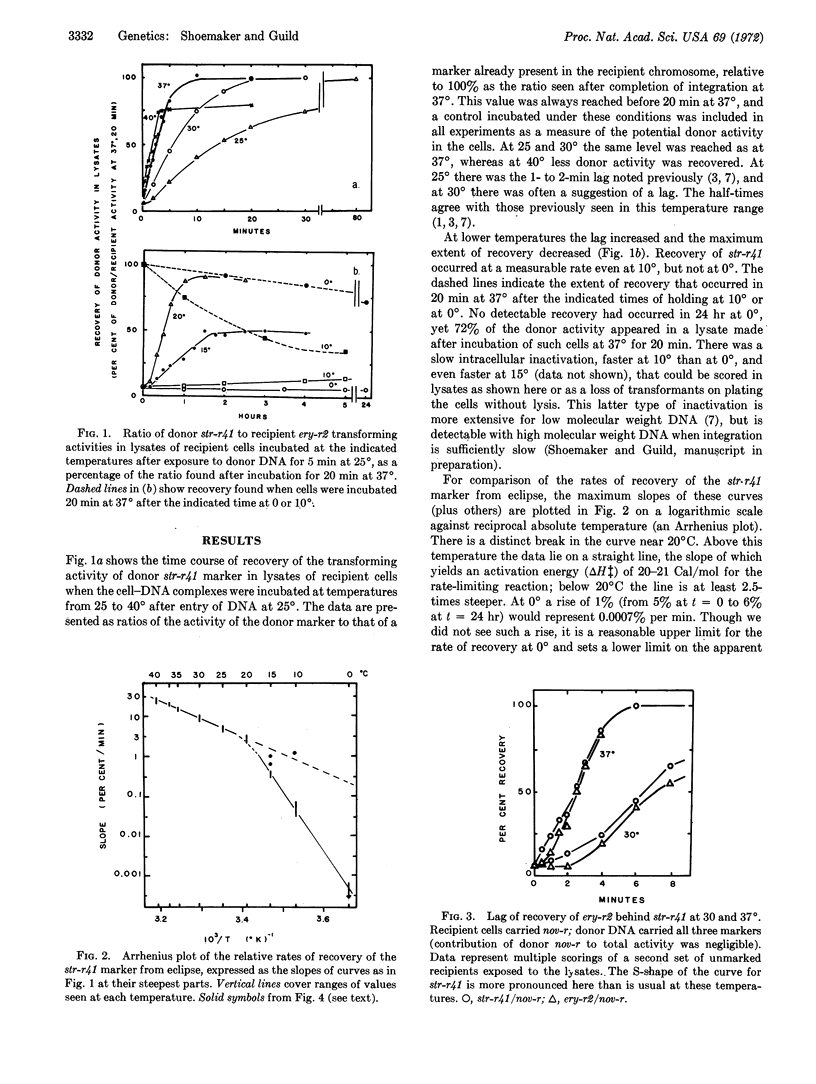
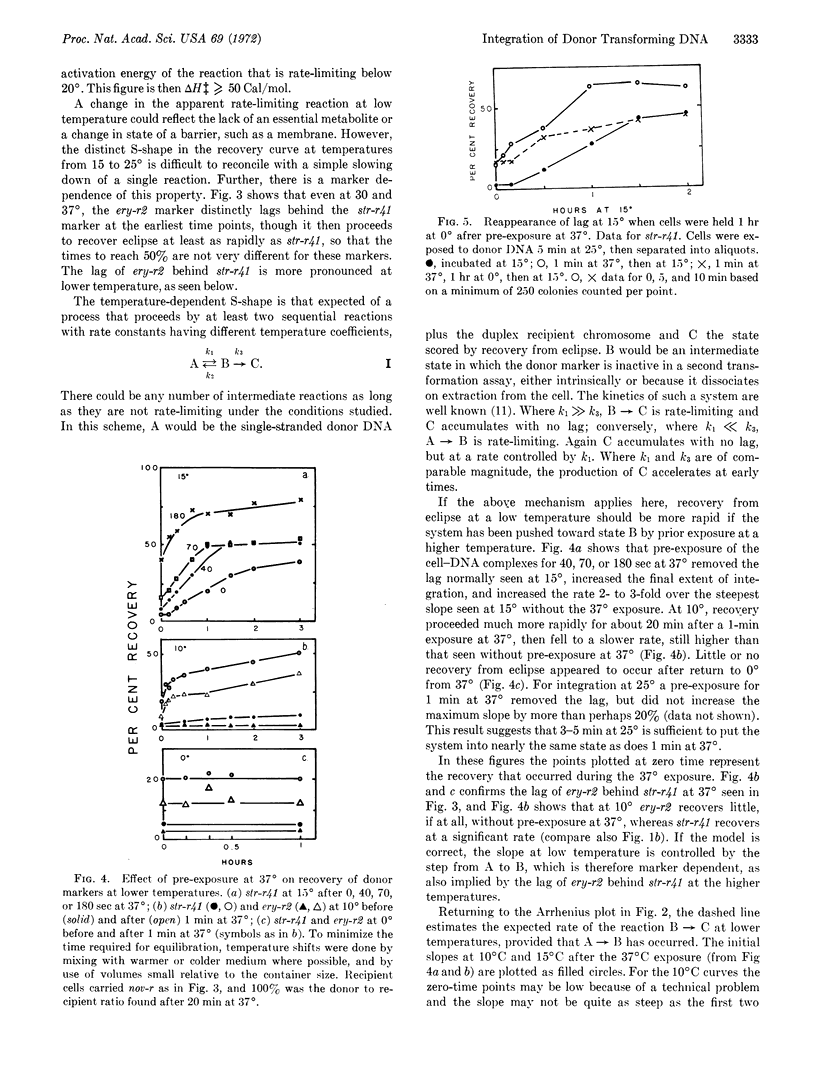
Integration of donor genes, as measured by recovery of their transforming activity from eclipse in lysates of newly transformed cells of pneumococcus, has been followed at temperatures from 0 to 40°. There is a lag in the recovery curve that is marker-dependent and increases as temperature falls. An Arrhenius plot of the rates shows a sharp break between 15 and 20°. Brief exposure of the system to 37° before incubation at 10 or 15° removes the lag and raises the subsequent rate of recovery. This activation is unstable, however, and disappears when the cells are held at 0° after the exposure at 37° and before incubation at 15°. The results are interpreted in terms of a reaction sequence A ⇌ B → C, with activation energies for the first forward rate-constant of the order of 50 Cal/mol, for the second, 20-21 Cal/mol, and for the reverse reaction, less than 20 Cal/mol. The properties of the first step, including its marker dependence, are the same as those observed earlier for stabilization of donor markers against intracellular inactivation, and it is suggested they may reflect an activation of the recipient chromosome prerequisite to synapsis.
Keywords: genetic recombination, recovery from eclipse, activation energies, sequential reactions, unstable intermediate
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cato A., Jr, Guild W. R. Transformation and DNA size. I. Activity of fragments of defined size and a fit to a random double cross-over model. J Mol Biol. 1968 Oct 14;37(1):157–178. doi: 10.1016/0022-2836(68)90080-6. [DOI] [PubMed] [Google Scholar]
- Collins C. J., Guild W. R. Events occurring near the time of synapsis during transformation in Diplococcus pneumoniae. J Bacteriol. 1972 Jan;109(1):266–275. doi: 10.1128/jb.109.1.266-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Fate of transforming deoxyribonucleate following fixation by transformable bacteria. Nature. 1960 Sep 17;187:1002–1006. doi: 10.1038/1871002a0. [DOI] [PubMed] [Google Scholar]
- GUILD W. R., ROBINSON M. Evidence for message reading from a unique strand of pneumococcal DNA. Proc Natl Acad Sci U S A. 1963 Jul;50:106–112. doi: 10.1073/pnas.50.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghei O. K., Lacks S. A. Recovery of donor deoxyribonucleic acid marker activity from eclipse in pneumococcal transformation. J Bacteriol. 1967 Mar;93(3):816–829. doi: 10.1128/jb.93.3.816-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Number of transformable units per cell in Diplococcus pneumoniae. J Bacteriol. 1969 Mar;97(3):1033–1035. doi: 10.1128/jb.97.3.1033-1035.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]



