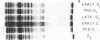Abstract
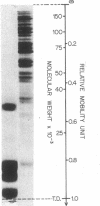
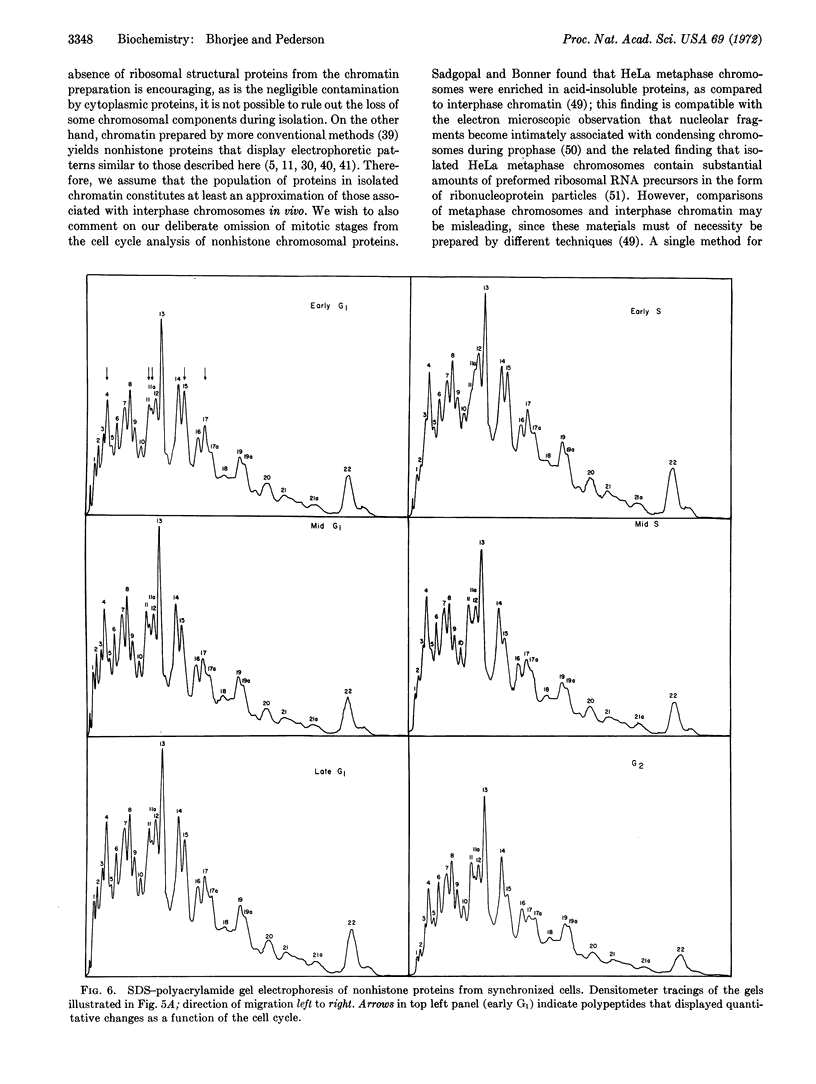
Chromatin was isolated from synchronized HeLa cells at different stages of the cell division cycle and fractionated into DNA, histones, and nonhistone proteins. Electrophoresis of the nonhistone proteins in sodium dodecyl sulfate-polyacrylamide gels revealed a highly reproducible pattern of 22 bands, having estimated molecular weights of 15,000-180,000, with 85% (by mass) over 40,000. The amounts of some nonhistone proteins varied during the cell cycle by as much as 50%, while others remained at a constant level. One group of nonhistone proteins (molecular weight 75,000) was greatly reduced just before the start of DNA replication (S-phase), then returned to normal levels in the mid-S phase. These results are discussed with regard to the possible role of nonhistone proteins in regulating chromosome structure and function.
Keywords: chromatin, SDS-gel electrophoresis, cell division cycle
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin W., Gellhorn A. Acidic proteins of mammalian nuclei: isolation and characterization. Proc Natl Acad Sci U S A. 1968 Jan;59(1):262–268. doi: 10.1073/pnas.59.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J., Dahmus M. E., Fambrough D., Huang R. C., Marushige K., Tuan D. Y. The Biology of Isolated Chromatin: Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968 Jan 5;159(3810):47–56. doi: 10.1126/science.159.3810.47. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock C. J., Prescott D. M., Kirkpatrick J. B. An evaluation of the double thymidine block for synchronizing mammalian cells at the G1-S border. Exp Cell Res. 1971 Sep;68(1):163–168. doi: 10.1016/0014-4827(71)90599-4. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Callan H. G. The organization of genetic units in chromosomes. J Cell Sci. 1967 Mar;2(1):1–7. doi: 10.1242/jcs.2.1.1. [DOI] [PubMed] [Google Scholar]
- DALY M. M., ALLFREY V. G., MIRSKY A. E. Uptake of glycine-N15 by components of cell nuclei. J Gen Physiol. 1952 Nov;36(2):173–179. doi: 10.1085/jgp.36.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGMAN C. W., SPORN M. B. STUDIES ON CHROMATIN. I. ISOLATION AND CHARACTERIZATION OF NUCLEAR COMPLEXES OF DEOXYRIBONUCLEIC ACID, RIBONUCLEIC ACID, AND PROTEIN FROM EMBRYONIC AND ADULT TISSUES OF THE CHICKEN. J Biol Chem. 1964 Oct;239:3483–3492. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Bonner J. Limited heterogeneity of the major nonhistone chromosomal proteins. Biochemistry. 1970 Oct 27;9(22):4440–4447. doi: 10.1021/bi00824a027. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of synthesis and processing of nucleolar components in metaphase-arrested cells. J Mol Biol. 1971 Jul 14;59(1):27–42. doi: 10.1016/0022-2836(71)90411-6. [DOI] [PubMed] [Google Scholar]
- Graziano S. L., Huang R. C. Chromatographic separation of chick brain chromatin proteins using a SP-sephadez column. Biochemistry. 1971 Dec 7;10(25):4770–4777. doi: 10.1021/bi00801a026. [DOI] [PubMed] [Google Scholar]
- Hill R. J., Poccia D. L., Doty P. Towards a total macromolecular analysis of sea urchin embryo chromatin. J Mol Biol. 1971 Oct 28;61(2):445–462. doi: 10.1016/0022-2836(71)90392-5. [DOI] [PubMed] [Google Scholar]
- Holoubek V., Crocker T. T. DNA-associated acidic proteins. Biochim Biophys Acta. 1968 Apr 22;157(2):352–361. doi: 10.1016/0005-2787(68)90089-0. [DOI] [PubMed] [Google Scholar]
- Johns E. W., Forrester S. Studies on nuclear proteins. The binding of extra acidic proteins to deoxyribonucleoprotein during the preparation of nuclear proteins. Eur J Biochem. 1969 Apr;8(4):547–551. doi: 10.1111/j.1432-1033.1969.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Kumar A., Warner J. R. Characterization of ribosomal precursor particles from HeLa cell nucleoli. J Mol Biol. 1972 Jan 28;63(2):233–246. doi: 10.1016/0022-2836(72)90372-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Simpson R. T., Sober H. A. Fractionation of chromatin components. Biochemistry. 1972 Apr 25;11(9):1547–1554. [PubMed] [Google Scholar]
- Martini O. H., Gould H. J. Enumeration of rabbit reticulocyte ribosomal proteins. J Mol Biol. 1971 Dec 14;62(2):403–405. doi: 10.1016/0022-2836(71)90435-9. [DOI] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Template properties of liver chromatin. J Mol Biol. 1966 Jan;15(1):160–174. doi: 10.1016/s0022-2836(66)80218-8. [DOI] [PubMed] [Google Scholar]
- Melli M., Pemberton R. E. New method of studying the precursor-product relationship between high molecular weight RNA and messenger RNA. Nat New Biol. 1972 Apr 12;236(67):172–174. doi: 10.1038/newbio236172a0. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M. Enzyme synthesis in synchronous cultures. Science. 1969 Aug 15;165(3894):657–663. doi: 10.1126/science.165.3894.657. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Pederson T. Chromatin structure and the cell cycle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2224–2228. doi: 10.1073/pnas.69.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Kumar A. Relationship between protein synthesis and ribosome assembly in HeLa cells. J Mol Biol. 1971 Nov 14;61(3):655–668. doi: 10.1016/0022-2836(71)90070-2. [DOI] [PubMed] [Google Scholar]
- Pederson T., Robbins E. A method for improving synchrony in the G2 phase of the cell cycle. J Cell Biol. 1971 Jun;49(3):942–945. doi: 10.1083/jcb.49.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Robbins E. Chromatin structure and the cell division cycle. Actinomycin binding in synchronized HeLa cells. J Cell Biol. 1972 Nov;55(2):322–327. doi: 10.1083/jcb.55.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T. Use of diphenylamine as a colorimetric reagent for ribonucleic acid. Anal Biochem. 1969 Apr 4;28(1):35–46. doi: 10.1016/0003-2697(69)90154-7. [DOI] [PubMed] [Google Scholar]
- Puck T. T. Phasing, Mitotic Delay, and Chromosomal Aberrations in Mammalian Cells. Science. 1964 May 1;144(3618):565–566. doi: 10.1126/science.144.3618.565-c. [DOI] [PubMed] [Google Scholar]
- Robbins E., Pederson T. On the role of ions in mitosis. In Vitro. 1971 Mar-Apr;6(5):323–334. doi: 10.1007/BF02619070. [DOI] [PubMed] [Google Scholar]
- STEELE W. J., BUSCH H. STUDIES ON ACIDIC NUCLEAR PROTEINS OF THE WALKER TUMOR AND LIVER. Cancer Res. 1963 Sep;23:1153–1163. [PubMed] [Google Scholar]
- Sadgopal A., Bonner J. Proteins of interphase and metaphase chromosomes compared. Biochim Biophys Acta. 1970 Apr 28;207(1):227–239. doi: 10.1016/0005-2795(70)90154-6. [DOI] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sonnenbichler J., Nobis P. The so-called non-histones from acid-treated calf thymus chromatin. Eur J Biochem. 1970 Sep;16(1):60–65. doi: 10.1111/j.1432-1033.1970.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. Role of chromatin in estrogen action in the uterus. II. Hormone-induced synthesis of nonhistone acidic proteins which restore histone-inhibited DNA-dependent RNA synthesis. Proc Natl Acad Sci U S A. 1969 Jun;63(2):465–472. doi: 10.1073/pnas.63.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T., Allfrey V. G. Studies of nuclear acidic proteins. Evidence for their phosphorylation, tissue specificity, selective binding to deoxyribonucleic acid, and stimulation effects on transcription. J Biol Chem. 1971 Jun 10;246(11):3597–3609. [PubMed] [Google Scholar]
- Thomas C. A., Jr, Hamkalo B. A., Misra D. N., Lee C. S. Cyclization of eucaryotic deoxyribonucleic acid fragments. J Mol Biol. 1970 Aug;51(3):621–632. doi: 10.1016/0022-2836(70)90012-4. [DOI] [PubMed] [Google Scholar]
- Vaughan M. H., Warner J. R., Darnell J. E. Ribosomal precursor particles in the HeLa cell nucleus. J Mol Biol. 1967 Apr 28;25(2):235–251. doi: 10.1016/0022-2836(67)90140-4. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R. Nascent ribosomes from HeLa cells. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1984–1990. doi: 10.1073/pnas.58.5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in HeLa cells. J Mol Biol. 1966 Aug;19(2):383–398. doi: 10.1016/s0022-2836(66)80012-8. [DOI] [PubMed] [Google Scholar]