Abstract
Background
Enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli are important causes of morbidity and mortality worldwide. These enteric pathogens contain a type III secretion system (T3SS) responsible for the attaching and effacing (A/E) lesion phenotype. The T3SS is encoded by the locus of enterocyte effacement (LEE) pathogenicity island. The H-NS-mediated repression of LEE expression is counteracted by Ler, the major activator of virulence gene expression in A/E pathogens. A regulator present in EPEC, H-NST, positively affects expression of H-NS regulon members in E. coli K-12, although the effect of H-NST on LEE expression and virulence of A/E pathogens has yet-to-be determined.
Results
We examine the effect of H-NST on LEE expression and A/E lesion formation on intestinal epithelial cells. We find that H-NST positively affects the levels of LEE-encoded proteins independently of ler and induces A/E lesion formation. We demonstrate H-NST binding to regulatory regions of LEE1 and LEE3, the first report of DNA-binding by H-NST. We characterize H-NST mutants substituted at conserved residues including Ala16 and residues Arg60 and Arg63, which are part of a potential DNA-binding domain. The single mutants A16V, A16L, R60Q and the double mutant R60Q/R63Q exhibit a decreased effect on LEE expression and A/E lesion formation. DNA mobility shift assays reveal that these residues are important for H-NST to bind regulatory LEE DNA targets. H-NST positively affects Ler binding to LEE DNA in the presence of H-NS, and thereby potentially helps Ler displace H-NS bound to DNA.
Conclusions
H-NST induces LEE expression and A/E lesion formation likely by counteracting H-NS-mediated repression. We demonstrate that H-NST binds to DNA and identify arginine residues that are functionally important for DNA-binding. Our study suggests that H-NST provides an additional means for A/E pathogens to alleviate repression of virulence gene expression by H-NS to promote virulence capabilities.
Introduction
The histone-like nucleoid structuring protein (H-NS) of Escherichia coli is the prototype of an important family of regulatory proteins that repress transcription of numerous genes in Gram-negative bacteria [1], [2]. H-NS helps bacteria respond to a wide range of environmental conditions such as changes in pH, osmolality and temperature [3]. In E. coli K-12, H-NS is a small, 15.9 kDa protein composed of 137 amino acids. H-NS-mediated modulation of gene expression can involve multiple mechanisms including binding of H-NS to regulatory regions of H-NS regulon genes to block association of RNA polymerase or by preventing open-complex formation after RNAP has already associated with the promoter [1], [4]–[6]. These mechanisms can be augmented or countered by other nucleoid-associated proteins such as Hha, YmoA, Fis, HU, and IHF [1], [6]. The N-terminal coiled-coil region of H-NS functions in oligomerization, either forming multiple homo-oligomeric states or heteromers with H-NS paralogs such as StpA, and Hha/YmoA family of proteins [1], [4], [6]. The C-terminal region of H-NS is the DNA-binding domain. The H-NS family of proteins contains a conserved DNA-binding motif that shares preferences for curved AT-rich DNA targets [3], [7], [8].
In addition to modulating expression of backbone chromosomal genes in E. coli K-12 such as proU and bgl [3], [9], H-NS also plays a key role in regulating virulence factors of many bacterial pathogens, including Shigella, Salmonella, enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC) [1]. The majority of genes encoding virulence factors in these pathogens are contained in pathogenicity islands or other mobile genetic elements, which are AT-rich compared to chromosomal housekeeping genes. These DNA sequences thought to be obtained via lateral gene transfer are termed xenogenic (i.e., derived from a foreign source) [6]. Repression of such genes would presumably provide an evolutionary advantage in allowing these genes to be less likely to have a deleterious effect than if they were unregulated. H-NS, while encoded in the chromosomal backbone of these pathogens, can interact with other regulatory proteins encoded by pathogenicity islands to modulate virulence gene expression that allows pathogens to adapt to the host environment.
One group of gastrointestinal pathogens that illustrates this interaction of H-NS and pathogenicity island-encoded regulators is the attaching and effacing (A/E) pathogens [10], named for the pathognomonic intestinal histopathology characterized by intimate adherence of the bacteria to epithelial cells and effacement of microvilli. EPEC causes diarrhea, primarily in infants, while EHEC causes bloody diarrhea and the potentially fatal hemolytic uremic syndrome. In addition to these human pathogens, there are also A/E pathogens for rabbits (rabbit EPEC or REPEC) and for mice (Citrobacter rodentium) [11]. All of these pathogens contain the horizontally-acquired Locus of Enterocyte Effacement (LEE) pathogenicity island, which is primarily responsible for the A/E histopathology [12]–[19]. The LEE pathogenicity island contains 41 genes with the majority located in the five operons LEE1-5 [20]–[23] (figure 1A). The majority of LEE genes encode a type III secretion system (T3SS) that resembles a needle-like structure, with the EspA protein forming the filament and EspB/EspD forming the pore inserted into the host cell. Effector proteins are secreted through the needle-like structure into the host cell where they manipulate host signaling pathways to subsequently induce disease [24], [25]. Deletion of the hns gene encoding H-NS greatly increases transcription of many LEE genes [12], [16], [26].
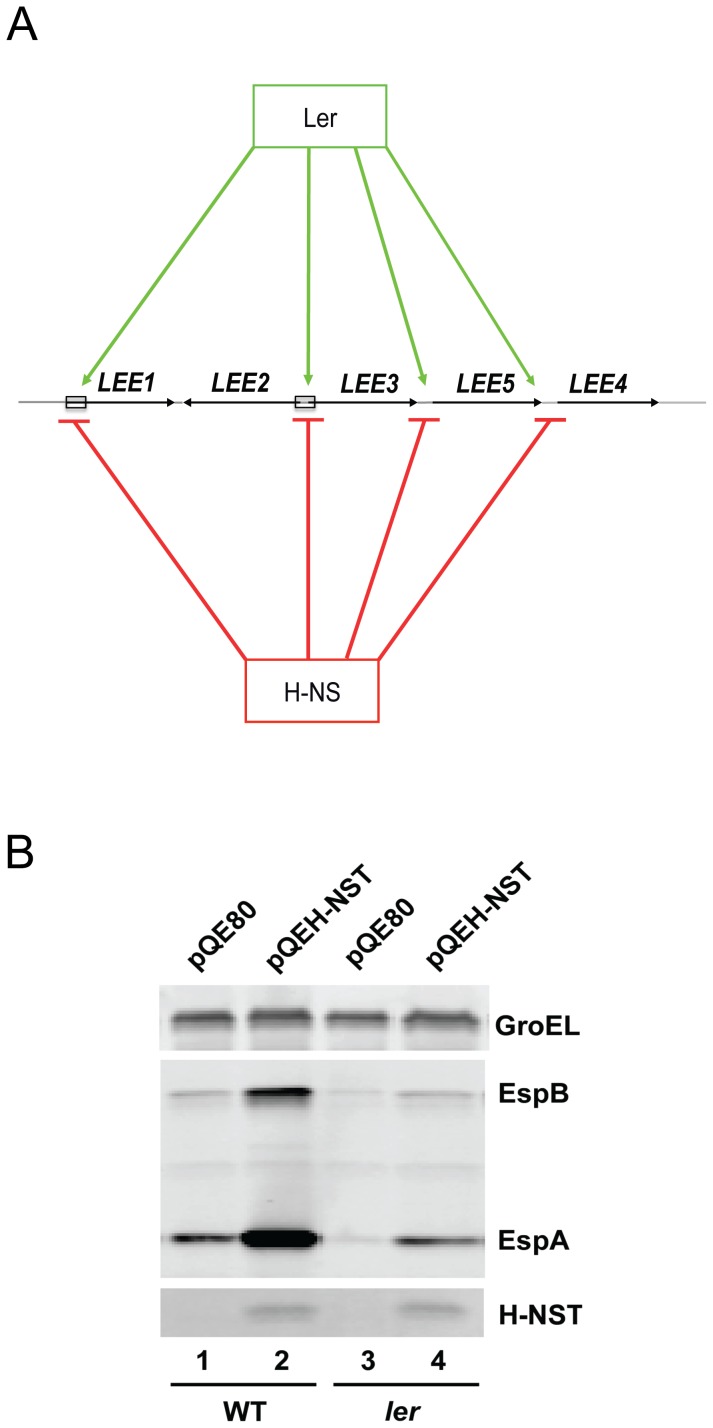
Figure 1. H-NST positively affects LEE-encoded protein levels.
(A) Regulation of the five major LEE operons, LEE1-5, by H-NS (red) and Ler (green). Positive regulation by Ler and repression by H-NS are indicated by open and blocked arrow heads respectively. Boxes indicate the approximate locations of the regulatory regions of LEE1 and LEE2/3 investigated in this study. (B) The effect of EPEC H-NST on the levels of LEE-encoded proteins in EHEC was determined by western analysis as described in Materials and Methods . Wild type EHEC TUV93-0 (lanes 1 and 2) and a ler-deleted derivative (lanes 3 and 4) containing the empty expression vector pQE80 (lanes 1 and 3) or pQE80H-NST (lanes 2 and 4) were grown in LB to a density of OD600∼0.5 followed by induction with 0.5 mM IPTG for 30 min. The LEE-encoded T3SS proteins EspA and EspB were detected by western analysis of total protein using polyclonal antisera against the respective proteins as indicated. His-tagged H-NST was detected using a tetra-His antisera. GroEL served as a loading control for total protein. Data shown are representative of four independent experiments.
The first gene in the LEE1 operon encodes the LEE-encoded regulator (Ler), an H-NS-like protein that shares 36% amino acid sequence identity to the DNA-binding C-terminal domain of H-NS. Ler, composed of 123 amino acids (14.3 kDa), is the master positive regulator of EPEC and EHEC LEE virulence genes such as espA and espB, as well as non-LEE-encoded virulence factors such as the long polar fimbria (lpf1) and a serine protease (stcE) [27]–[30]. As a positive regulator of virulence gene expression, Ler counteracts H-NS-mediated repression, probably by binding to DNA and displacing H-NS from regulatory regions of the Ler regulon [27], [31], [32]. Oligomerization of Ler, like H-NS, occurs through the coiled-coil region located in the N-terminus [30], [31]. DNA-binding activity of Ler involves the C-terminus, in particular the Arg90 residue lodged in the conserved DNA-binding motif of the H-NS family of proteins [1], [6], [25], [30], [33]–[35]. Ler preferentially binds to curved AT-rich DNA including a 10 bp long DNA sequence of the LEE2/LEE3 regulatory region, which was identified as a binding target for the Ler C-terminal DNA-binding domain [30], [36]. The specific binding to the LEE2/LEE3 target DNA involves the side chain of Arg90 being inserted into a narrow minor groove while Arg93 helps in stabilizing the DNA protein complex [36]. Both oligomerization and DNA binding are essential for Ler antagonism of the H-NS repression. Antagonizing H-NS repression leading to increased gene expression is not exclusive to Ler, since other H-NS-modulating proteins have this effect by a different mechanism of forming dominant-negative oligomers [1], [2], [6], [37]–[39].
Inhibition of H-NS activity is also seen with the gene 5.5-encoded protein of T7 phage (gp 5.5) and the H-NS truncated protein (H-NST) of EPEC, both of which have been shown to interact with H-NS and hinder its repressive activity [37], [39]–[42]. In E. coli K-12 the gp 5.5 protein has been shown to relieve H-NS-mediated repression of the proU promoter in vivo [38], and was shown to diminish H-NS binding to the bglG promoter region via interaction with the H-NS oligomerization domain, forming a dominant-negative oligomer [39]. Although DNA-binding activity has yet-to-be demonstrated for gp5.5, it was reported to form a complex with H-NS and tRNA to mask tRNA priming in T7 DNA replication [40]. The 80-residue (10.5 kDa) protein H-NST is not present in E. coli K-12 or EHEC but is encoded in the chromosome of some isolates such as EPEC E2348/69, uropathogenic E. coli (UPEC) strain CFT073 and C. rodentium. H-NST from EPEC is encoded by a pathogenicity island located at the asnW locus. Though H-NST exhibits an overall low amino acid sequence similarity to H-NS of only 29%, the first 43 residues of H-NST share 40% similarity to H-NS [37]. Williamson et al demonstrated that EPEC H-NST negatively affects H-NS-mediated repression of the E. coli housekeeping genes proU and bgl by forming dominant-negative hetero-oligomers with H-NS that render H-NS inactive when tested in a E. coli K-12 background. These authors further demonstrated that Ala16 of H-NST is important for oligomerization and thereby activity [37]. DNA-binding activity of H-NST has not been demonstrated nor has relief of H-NS-mediated repression by H-NST yet been shown for virulence factor genes in pathogenic E. coli.
In this study, we assess the effect of H-NST on H-NS-mediated regulation of LEE expression. We show that H-NST positively affects levels of LEE-encoded proteins and A/E lesion formation. We demonstrate that H-NST specifically binds to LEE regulatory DNA regions and further show that Ala16 is required for H-NST-mediated increase in the levels of LEE-encoded proteins and induction of A/E lesion formation. Additionally, we determine H-NST Arg60 and Arg63 residues to be important for the ability of H-NST to bind DNA, resulting in the induction of LEE expression and A/E lesion formation. Further, we demonstrate that H-NST is conserved among many human and plant bacterial pathogens, suggesting a global role of H-NST in regulating the expression of the H-NS regulon.
Materials and Methods
Standard procedures
Standard DNA techniques, liquid media and agar plates were used as described [43]. Restriction endonucleases, T4 DNA kinase- and ligase were used as recommended by the manufacturer (New England Biolabs). DNA used for cloning purposes was PCR amplified using the high-fidelity DNA polymerases Phusion Flash (Fermentas) or Easy-A (Agilent). DNA oligonucleotides were obtained from Intergrated DNA Technologies and DNA sequencing was performed by the University of Maryland Biopolymer-Genomics Core Facility. Bacteria were grown at 37°C in LB or DMEM (Invitrogen #11885) media supplemented with ampicillin (100 µg/ml) (Sigma) as needed. HeLa cells (ATTC #CCL-2) were cultured in DMEM/F12 (Invitrogen #11330) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen) at 37°C in 7% CO2.
Plasmid constructions
Oligonucleotide sequences used for plasmid constructions are listed in table 1.
Table 1. Oligonucleotides used in this study.
| Application/name | Oligonucleotide sequence (5′ to 3′) |
| Plasmid construction | |
| QEH-NST F | AGCAGGATCCATGATTGATGAATTTCATGTGATGTATATGTAT |
| QEH-NST R | CACCGAAGCTTCAGTCAATGAGATCTTCTGGCGAAAC |
| QEH-NS F | AGCAGGATCCATGAGCGAAGCACTTAAAATTCTGAACAAC |
| QEH-NS R | CACCGAAGCTTATTGCTTGATCAGGAAATCGTCGAG |
| Site-directed mutagenesis | |
| H-NSTA16V F | TATATGTATAAAAAAATCCAAGTAGAAGCCGCAACCACTGACCTC |
| H-NSTA16V R | GAGGTCAGTGGTTGCGGCTTCTACTTGGATTTTTTTATACATATA |
| H-NSTA16L F | TATATGTATAAAAAAATCCAAGCAGAAGCCGCAACCACTGACCTC |
| H-NSTA16L R | GAGGTCAGTGGTTGCGGCTTCTGCTTGGATTTTTTTATACATATA |
| H-NSTR60Q F | CGTAAGTTGAAAATGAAACAAGCACAAAGATTACTTGAG |
| H-NSTR60Q R | CTCAAGTAATCTTTGTGCTTGTTTCATTTTCAACTTACG |
| H-NSTR60Q/R63Q F | TTGAAAATGAAACAAGCACAACAATTACTTGAGAAAATGGCATGTGACCGGG |
| H-NSTR60Q/R63Q R | CCCGGTCACATGCCATTTTCTCAAGTAATTGTTGTGCTTGTTTCATTTTCAA |
| EMSA DNA fragments | |
| LEE1P2 F | TTAAGGTGGTTGTTTGATGA |
| LEE1P2 R | TTTGGATTCAGCAAA |
| LEE1P1P2 F | GCAATGAGATCTATCTTATAAAGAGAAACGC |
| LEE3 F | GTTGAAGAGTTTTTAAGATTGTTGG |
| LEE3 R | ATAAATAATCTCCGCATGCT |
| rssB F | TGCAAGTCGAACGGTAACAG |
| rssB R | AGTTATCCCCCTCCATCAGG |
pQEH-NST: A 262 bp DNA fragment encoding hnsT was PCR amplified from EPEC E2348/69 gDNA using the primer set QEH-NST F/QEH-NSTF R, digested with BamHI and HindIII and cloned into the corresponding sites in pQE80 (QIAGEN). Plasmid pQEH-NST encodes a recombinant C-terminal His-tagged H-NST.
pQEH-NS: A 434 bp DNA fragment encoding hns was PCR amplified from EPEC E2348/69 gDNA using primer set QEH-NS F/QEH-NS R, digested with BamHI and HindIII and cloned into the corresponding sites in pQE80 (QIAGEN). Plasmid pQEH-NS encodes a recombinant C-terminal His-tagged H-NS.
H-NST mutant derivatives were constructed by site-directed mutagenesis of pQEH-NST using the QuickChange XL Site-directed Mutagenesis Kit (Agilent) according to manufacturer's instructions. The plasmids pQEH-NST A16V, pQEH-NST A16L, pQEH-NST R60Q and pQEH-NST R60Q/R63Q encoding H-NST substitution mutants were generated using the primer sets H-NSTA16V F/H-NSTA16V R, H-NSTA16L F/H-NSTA16L R, H-NSTR60Q F/H-NSTR60Q R and H-NSTR60Q/R63Q F/H-NSTR60Q/R63Q R respectively.
BLAST searches and multiple amino acid sequence alignment
BLAST searches were used to identify H-NST present among non-redundant protein sequences in the NCBI database using the BLASTp program with an expect threshold of 10 and the scoring parameters: Blosum 62 matrix, gap cost was 11 for existence and 1 for extension, and conditional compositional score matrix adjustment (www.ncbi.nlm.nih.gov). H-NST from EPEC strain E2348/69 (YP 002329609) was used as query sequence. Proteins identified at a threshold e-value of 2×10−26 or less with sequence coverage of at least 77% were considered. In addition, a BLASTn search of a database containing a collection of 114 A/E E. coli isolates and 24 reference strains [44] was performed using a threshold e-value of 1×10−15 to identify genes encoding hnsT. The multiple amino acid sequence alignment of H-NST was prepared using ClustalW2 [45], [46].
H-NST proteins from the following 65 strains were used to generate the multiple sequence alignment: C. rodentium ICC168 (YP003365612), Dickeya zeae (WP019843943), Enterobacter sp. SST3 (EJO48231), E. coli 113303 (ESA61347), E. coli 2362-75 (EFR16544), E. coli 2848050 (EMW14361), E. coli 3003 (EII86141), E. coli 536 (YP669831), E. coli C262-10 (AIAP01000001.1), E. coli C639-08 (AIBH01000001.1), E. coli C844-97 (AIBZ01000001.1), E. coli C93-11 (AICD01000002.1), E. coli CFT073 (NP754305), E. coli CUMT8 (EIL77087), E. coli DEC1A (EHU10545), E. coli DEC1B (EHU13728), E. coli DEC1C (EHU11552), E. coli DEC1D (EHU23639), E. coli DEC1E (EHU27188), E. coli DEC2A (EHU30556), E. coli DEC2B (EHU39793), E. coli DEC2C (EHU44940), E. coli DEC2D (EHU46227), E. coli DEC12A (EHX20918), E. coli DEC12E (EHX46652), E. coli DEC15B (EHX98907), E. coli DEC15C (EHX01733), E. coli DEC15D (EHX09544), E. coli DEC15E (EHX13659), E. coli E2348/69 (YP002329609), E. coli E851/71 (ALNX00000000), E. coli HVH 125 (4-2634716) (EQR42309), E. coli HVH HVH 225 (4-1273116) (EQV31905), E. coli HVH 154 (4-5636698) (EQS65207), E. coli HVH 158 (4-3224287) (EQS57084), E. coli KTE100 (EOW07218), E. coli KTE157 (ELJ10809), E. coli KTE16 (ELC28364), E. coli KTE192 (ELH08668), E. coli KTE227 (ELH91245), E. coli MS 21-1 (EFK17805), E. coli MS 57-2 (EGB75116), E. coli MS 115-5 (EFJ95479), E. coli MS 200-1 (EFJ59489), E. coli 042 (CBG35676), E. coli OK1357 (EFZ69143), E. coli TA124 (EHN89354), E. coli TW07793 (EII95637), E. coli UMEA 3022-1 (EQW12951), E. coli UMEA 3108-1 (EQW66083), E. coli UMEA 4076-1 (ERA50029), Klebsiella oxytoca 10-5245 (EHT00587), K. pneumoniae UCICRE 7 (ESM00810), Pantoea ananatis PA13 (YP005993219), Pectobacterium atrosepticum SCRI1043 (CAG74542), Pectobacterium wasabiae CFBP 3304 (WP005969703), Salmonella enterica subsp. arizonae serovar 62:z4,z23:- strain RSK2980 (YP001569976), S. enterica subsp. diarizonae serovar 60:r:e,n,x,z15 strain 01-0170 (ESJ14503), S. enterica subsp. enterica serovar Anatum str. ATCC BAA-1592 (ESJ09538), S. enterica enterica subsp. enterica serovar Hvittingfoss strain A4-620 (EHC52821), S. enterica subsp. enterica serovar Indiana strain ATCC 51959 (ESG993750, S. enterica subsp. enterica serovar Muenster str. 660 (ESB61545), S. enterica subsp. enterica serovar Nchanga strain CFSAN001091 (ESJ38946), S. enterica subsp. enterica serovar Sloterdijk str. ATCC 15791 (ESF40572), and Yersinia rohdei ATCC 43380 (WP004713599).
Western blot analysis
The effect of expressing H-NST from pQEH-NST on the production of T3SS-associated proteins was determined in the EHEC O157:H7 EDL933 Δstx1Δstx2 strain TUV93-0 [47] and AMH101 [48], which is TUV93-0 containing an in-frame deletion of ler. Wild type H-NST and H-NST mutants were produced from pQEH-NST, pQEH-NST A16V, pQEH-NSTA16L, pQEH-NSTR60Q and pQEH-NSTR60Q/R63Q in TUV93-0. Cultures were grown in LB containing ampicillin at 37°C to a density of OD600∼0.5 followed by induction of H-NST expression with 0.5 mM IPTG for 1h. Total cellular protein was precipitated with 5% (vol/vol) trichloric acid, washed with acetone, resuspended in 1× Next Gel sample loading buffer (Amresco). Proteins were resolved on a 4–20% Tris-HCl Criterion precast protein gels (BioRad) and transferred onto an Immobilon-FL polyvinylidene difluoride membrane (Millipore) using a Trans-Blot SD Semi-Dry Transfer Cell (BioRad). The membrane was blocked in Odyssey blocking buffer (Li-Cor Biosciences), exposed to polyclonal antibodies specific to EspA, EspB and GroEL (Sigma), and subsequently to Alexa Fluor 680-conjugated goat anti-rabbit (Invitrogen). A monoclonal Tetra-His anti-mouse antisera (QIAGEN) and Alexa Fluor 680-conjugated goat anti-mouse (Invitrogen) were used to detect His-tagged H-NST. Detection of GroEL served as a loading control for total protein. Proteins were visualized and quantified using an Odyssey Infrared Imaging System with application software version 3.0 (Li-Cor Biosciences) as recommended. The levels of LEE-encoded proteins were normalized to that of GroEL. The western analyses were carried out on four independent biological samples for each strain.
Fluorescent actin staining assay
The effects of wild type H-NST and H-NST mutants on the ability of EHEC O157:H7 TUV93-0 to induce A/E lesion formation on HeLa cell monolayers was evaluated using the fluorescent actin staining assay (FAS) [49]. Wild type H-NST and the H-NST substitution mutants A16V, A16L, R60Q and R60Q/R63Q were produced from pQEH-NST, pQEH-NSTA16V, pQEH-NSTA16L, pQEH-NSTR60Q and pQEH-NSTR60Q/R63Q respectively. Bacterial strains carrying the vector pQE80 or plasmids encoding hnsT and its mutant derivatives were inoculated from freezer stocks into LB medium containing ampicillin and grown statically for about 18 h at 37°C. Cultures were then diluted 1∶3 in DMEM containing 0.2% mannose, ampicillin and 0.3 mM IPTG to induce expression of hnsT, and grown statically at 37°C in 7% CO2 for 1 h. Levels of wild type and mutant H-NST produced in the preinduced TUV93-0 strains were confirmed by western blot analysis. Semi-confluent HeLa cell monolayers grown on glass coverslips to ∼80% confluence were co-cultured with an initial number of ∼1×106 bacteria in DMEM supplemented with 2% FBS. After 3 h of infection cells were washed with Hanks buffer (Invitrogen), fresh media was added, and cells were incubated for an additional hour. At 4 h post infection cell monolayers were washed once with Hanks buffer, and fixed in 4% formamide in 1× PBS. Coverslips were washed three times with 1× PBS, cells were permeabilized with 0.1% Triton X-100 in 1× PBS, and F-actin was stained using Alexa Fluor 488 phalloidin (Invitrogen) diluted 1∶50. Coverslips were mounted on slides using Prolong Gold Antifade Reagent (Invitrogen). FAS assays were independently conducted at least three times for each strain. Actin-stained cells were visualized using an AxioSkop microscope equipped with a 40× objective and images were captured with an AxioCam MR3 digital camera using AxioVision v. 4.8 software (Carl Zeiss MicroImaging Inc).
Protein production and purification
Recombinant H-NS, H-NST, and H-NS mutant derivatives were produced from pQEH-NS, pQEH-NST, pQEH-NSTA16V, pQEH-NSTA16L, pQEH-NSTR60Q and pQEH-NSTR60Q/R63Q in BL21-DE3 (pLysS) (Novagen). Cells were grown in 1 L of LB medium at 37°C to an optical density of OD600 ∼0.5 prior to the induction with 0.5 mM IPTG. Proteins were produced for 1 h and cells were then harvested by centrifugation at 7,667 g for 20 min at 4°C. Cell pellets were suspended in binding buffer (50 mM NaH2PO4, 300 mM NaCl, 10% glycerol, 40 mM imidazole, pH 8) to a final volume of 40 ml. Cells were lysed by two passages through a Microfluidics LV1 micro-fluidizer, and then the lysed cell suspension was centrifuged at 26,536 g for 70 min at 4°C. The supernatant of the lysate was then filtered through a 0.2 µm pore size filter (Millipore) and applied to a HisTrap FF column (GE Healthcare) coupled to an ÄKTAprime plus system (GE Healthcare). After sample application the column was first washed with 30 ml of binding buffer at a flow rate of 2 ml/min and then washed with 50 ml of 6% elution buffer (50 mM NaH2PO4, 300 mM NaCl, 10% glycerol, 500 mM imidazole, pH 8) in binding buffer. Protein was eluted with 100% elution buffer and eluates were buffer exchanged into storage buffer (50 mM NaH2PO4, 300 mM NaCl, 10% glycerol, pH 7.4) and concentrated using an Amicon Ultra Centrifugal Filter Device with a 3 kDa MW cut-off value (Millipore). Protein samples were analyzed by SDS-PAGE using a 4–20% Tris-HCl Criterion precast gel (BioRad), and visualization with GelCode Blue Stain Reagent (Thermo Scientific), a technique that provides nanogram-level detection. The purity of the H-NST preparation was about 95% as estimated from the stained gel.
Recombinant Ler-Myc-His protein was produced from pVS45 in DH5α [30]. Cultures were grown in LB medium at 37°C to a density of O.D600 ∼0.5 before the expression of ler was induced with 0.2% arabinose for 2 h. Cells were harvested by centrifugation at 7,667 g at 4°C for 20 min. Purification of Ler-Myc-His was as described for H-NST with the exception that 10% glycerol was omitted from all buffers. Purified Ler was buffer exchanged and concentrated using Amicon Ultra Centrifugal Filter Device with a 10 kDa MW cut-off value.
Preparation of fluorescently-labeled DNA fragments and Electrophoretic Mobility Shift Assays (EMSA)
Preparation of fluorescently-labeled DNA fragments
Fluorescently-labeled oligonucleotides used for PCR amplification of DNA fragments containing LEE regulatory regions were prepared as described [50]. Briefly, 5′-amine-modified oligonucleotides (Integrated DNA Technologies) were resuspended in 300 µl of dH2O, chloroform extracted three times, ethanol precipitated in 125 µM NaCl, and then resuspended in 300 µl dH2O. The oligonucleotides (5 µg) were fluorescently labeled in 100 µl reactions using a ten-fold molar excess of Alexa Fluor® 790 Carboxylic Acid, Succinimidyl Ester, penta (triethylammonium) Salt (Life Technologies) in 100 mM Sodium Tetraborate (pH 8.5). Reactions were allowed to proceed overnight at room temperature in the dark. Labeled oligonucleotides were purified using G-25 spin columns (GE Healthcare) according to manufacturer's directions.
DNA fragments encoding LEE1 P2, LEE1 P1P2 and LEE3 regulatory regions were PCR amplified from EHEC strain TUV93-0 gDNA using the fluorescently-labeled primer sets LEE1P2 F/LEE1P2 R, LEE1P1P2 F/LEE1P2 R and LEE3 F/LEE3 R respectively. The LEE1 P2 and LEE1 P1P2 DNA fragments contain the LEE1 regulatory region ranging from positions −112 to +33 and −301 to +33 relative to the transcription initiation site for the proximal LEE1 promoter respectively. The LEE3 DNA fragment contains the sequence from +83 to +210 relative to the transcription initiation site for LEE3. The primer set rssB F/rssB R was used to amplify a 99 bp long unlabeled DNA fragment containing part of rssB, which served as nonspecific DNA target. Amplified DNA products were purified using G-50 spin columns (GE Healthcare) according to manufacturer's directions.
Electrophoretic mobility shift assay
Purified H-NS, Ler, H-NST and mutant H-NST derivatives (concentrations as indicated in the figure legends) were incubated for one minute prior to addition of 24 ng of fluorescently-labeled DNA fragment containing the LEE1 P2, LEE1 P1P2 or LEE3 regulatory regions and then incubated for 20 min at 30°C in binding buffer (10 mM Tris-HCL, 1 mM EDTA, 50 mM KCl, 10% glycerol, 50 µg/mL, 1 mM DTT, pH 7.4). Unlabeled LEE DNA fragments added in 6-fold excess and unlabeled rrsB DNA fragment added in 12-fold excess served as specific and nonspecific competitor DNA, respectively. Binding reactions containing both H-NST and H-NS were preincubated for 10 min at room temperature to allow interaction between proteins before the DNA fragment was added. Further, binding experiments where Ler was added to reactions already containing H-NST and/or H-NS bound to DNA were first incubated for 10 min at room temperature, and then Ler was added followed by 20 min of incubation at 30°C. DNA fragments were separated by polyacrylamide gel electrophoresis (PAGE) using a 4–20% TBE Criterion precast gel run in 1× TBE at 52 amps for 60 min (BioRad). Fluorescently-labeled DNA fragments were visualized using an Odyssey Imaging System at 800 nm with application software version 3.0 (Li-Cor Biosciences). The EMSA analyses were carried out at least three times using proteins from at least two different protein purification preparations.
Results
H-NST induces LEE-encoded protein levels independently of Ler
While H-NST encoded by EPEC is known to positively affect the expression of H-NS-controlled housekeeping genes in E. coli K-12 [37], the effect of H-NST on the expression of the LEE and the virulence-associated A/E lesion phenotype remains to be elucidated. To evaluate the effect of H-NST on LEE-encoded protein levels, we cloned hnsT from EPEC under the control of an IPTG-inducible tac promoter in pQE80 and produced H-NST from this construct in EPEC strain E2348/69. Production of H-NST from pQEH-NST in EPEC grown to the exponential phase in DMEM did not affect levels of LEE-encoded proteins (unpublished data), which might be due to the possibility that an effect of H-NST is masked by a relatively high basal level of LEE-encoded proteins present in EPEC. Since the abundance of LEE-encoded proteins in EHEC is minimal in the exponential phase [48], we assessed the regulatory effect of H-NST on the LEE using the EHEC O157:H7 strain TUV93-0, a stx-deleted derivative of EDL933 that exhibits Ler and H-NS-mediated regulation of the LEE similar to EPEC. Interestingly, production of H-NST from pQEH-NST in EHEC grown in LB to exponential phase (OD600∼0.4) induced the expression of the LEE as reflected by increased levels of the T3SS-secreted proteins EspA and EspB by 5.4-fold and 3.1-fold, respectively, as opposed to the vector control (figure 1B, lane 2). Moreover, production of C. rodentium H-NST, the amino acid sequence of which is 79% similar to that of EPEC H-NST, also increased levels of EspA and EspB in EHEC (unpublished data), suggesting that the H-NST proteins from both these A/E pathogens affect LEE expression. These results strongly suggest that production of H-NST has a dominant-negative effect on the H-NS-mediated repression of LEE expression in the exponential growth phase. Since our data indicated that H-NST positively affects levels of LEE-encoded proteins, we further investigated whether the LEE-encoded global virulence gene regulator Ler was required for regulation by H-NST. To assess this we provided H-NST from pQEH-NST in a ler-deleted derivative of EHEC TUV93-0, and found that production of H-NST increased EspA and EspB levels by 1.9-fold and 1.2-fold respectively (figure 1B, lane 4), indicating that H-NST positively affects levels of EspA independently of ler probably by hindering H-NS activity as previously suggested [37].
H-NST is conserved in various Enterobacteriaceae including A/E pathogens
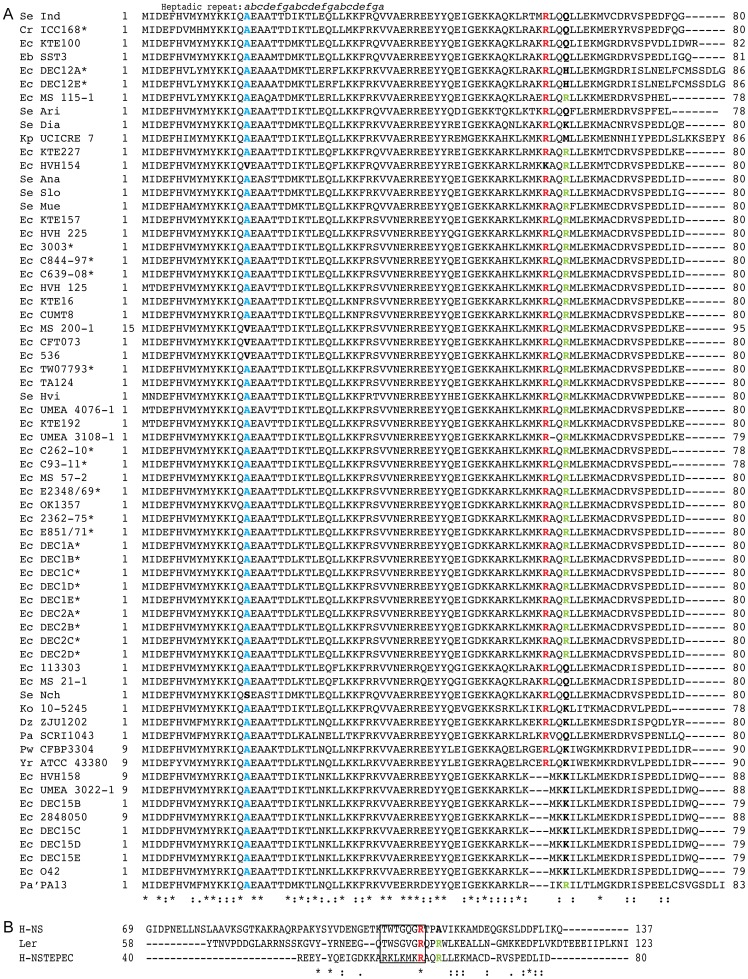
Since H-NST from EPEC E2348/69 positively affects the LEE we carried out BLAST searches to determine the presence of H-NST among A/E pathogens and other Enterobacteriaceae encoding H-NS as described in Material and Methods. We identified H-NST from 65 Enterobacteriaceae strains of which 32% contained the LEE (figure 2A). Those strains included 14 EPEC strains, C. rodentium and six LEE-positive, bfp- and stx- strains of unclassified phylogenetic lineage [44], which were serotypes O157:H45 (strains C844-97, C639-08 and 3003), O114:H49 (C262-10), O157:H39 (TW07793) and O157:H- (C93-11). H-NST homologs were not found in any Shiga toxin-producing strains such as EHEC O157:H7 and Shigella sp., which could be due to the fact that other pathogenicity islands occupy the asnW and serU loci of the H-NST-encoding P4-like phage in these strains as is the case of the CP933U island in EDL933 and Shigella flexneri. H-NST is also present in other E. coli pathotypes including numerous UPEC strains, enteroaggregative E. coli 042 and adherent invasive E. coli (CUMT8), the latter which has been associated with Crohn's disease [51]. We further identified H-NST in commensal E. coli including some reference strains from the Human Microbiome Project (NIH). Interestingly, H-NST also is conserved in other human pathogens such as Klebsiella pneumonia and K. oxytoca as well as in Salmonella enterica, where H-NST is present in three subspecies and eight serovars that are associated with food poisoning outbreaks worldwide [52]. Further, H-NST is present in phytopathogenic bacteria targeting potato and rice plants including Dickeya zeae, P. atrosepticum, Pectobacterium wasabiae and Pantoea ananatis, the latter of which is an opportunistic human pathogen causing bacteremia [53]. H-NST is present in some A/E pathogens, in other human pathogens such as Salmonella and in plant pathogens that utilize H-NS-mediated regulation of virulence factors. This widespread distribution suggests a global regulatory effect of H-NST, prompting us to further investigate the molecular basis for H-NST function on the regulation of LEE expression.
Figure 2. H-NST is conserved in various enteropathogens.
(A) ClustalW2 sequence alignment of 65 H-NST homologs from 65 Enterobacteriaceae. The following strain abbreviations were used: Cr (C. rodentium), Dz (Dickeya zeae), Eb (Enterobacter), Ec (E. coli), Ko (Klebsiella oxytoca), Kp (K. pneumonia), Pa' (Pantoea ananatis), Pa (Pectobacterium atrosepticum), Pw (P. wasabiae), Se Ana (Salmonella enterica subsp. enterica serovar Anatum), Se Ari (S. enterica subsp. arizonae serovar 62:z4,z23:-), Se Dia (S. enterica subsp. diarizonae serovar 60:r:e,n,x,z15), Se Hvi (S. enterica enterica subsp. enterica serovar Hvittingfoss), Se Ind (S. enterica subsp. enterica serovar Indiana), Se Mue (S. enterica subsp. enterica serovar Muenster), Se Nch (S. enterica subsp. enterica serovar Nchanga), Se Slo (S. enterica subsp. enterica serovar Sloterdijk), and Yr (Yersinia rohdei). An asterix following the strain name indicates strains containing the LEE. Residues of potential importance for H-NST function are indicated in color or bold, where the Ala16 residue previously reported to be important for H-NST activity is shown in blue, the Arg60 and Arg63 residues that could be involved in DNA-binding by H-NST are shown in red and green respectively. The conserved arginine residue shown in red is involved in DNA-binding of H-NS and Ler, while the arginine in green present in Ler and H-NST is involved in DNA-binding by Ler [36]. An asterisk represents identical amino acids, a colon represents a conserved amino acid substitution and a dot indicates a semi-conserved amino acid substitution. The heptadic repeat defined for H-NSTEPEC is indicated above the sequence alignment. The letters a and d represent hydrophobic residues, e and g represent charged residues, whereas positions b and c can be occupied by any residue in the repeat. (B) Sequence alignment of the C-terminal regions of Ler, H-NS and H-NST generated by ClustalW. The boxed region indicates the conserved DNA-binding motif for the H-NS family of proteins. The annotation used for the alignment is as described for panel A.
Identification of residues important for H-NST-mediated induction of LEE-encoded proteins and A/E lesion formation
The current literature suggests that H-NST, like Ler, functions by negatively affecting H-NS-mediated repression of gene expression probably by displacing H-NS bound to regulatory DNA sites [37]. However, it remains to be determined whether H-NST, like Ler and H-NS, binds to DNA. To gain further insight into the molecular mechanism by which H-NST regulates gene expression, we identified residues of potential functional interest based on sequence alignment of H-NST, Ler and H-NS. We used ClustalW2 [45], [46] to align the C-terminal regions of Ler and H-NS that contain the DNA-binding motif with the C-terminal half of H-NST, since only the first 40 residues of H-NST share a high similarity with the N-terminus of H-NS. The alignment revealed that the conserved DNA-binding motif of the H-NS family of proteins including H-NS and Ler is absent from H-NST (figure 2B). Interestingly, despite the absence of the DNA-binding motif present in the H-NS family of proteins, H-NST contains an arginine residue at position 60 that aligned with the Ler Arg90 and H-NS Arg114, which are essential for DNA-binding activity of those regulators [36], [54], [55]. Also, the Ler Arg93 residue that is associated with stabilization of the Ler-LEE DNA complex, and was suggested to play a role in Ler regulation of LEE expression [36], is conserved in H-NST as Arg63 (figure 2B). Indeed, H-NST residues Ala16 and Arg60 are conserved as indicated in the multiple sequence alignment (figure 2A). To assess the role of Arg60 and Arg63, which potentially could be part of a DNA-binding domain, we performed site-directed mutagenesis on pQEH-NST to substitute the arginine residues with glutamine resulting in plasmids encoding the H-NST mutants R60Q and R60Q/R63Q as described in Materials and Methods .
The H-NST Ala16 residue was previously identified as essential for H-NST function based on the finding that a H-NST A16V mutant was incapable of regulating proU expression and that H-NST from UPEC containing valine at position 16 is nonfunctional [37]. The authors speculated that the loss of activity was caused by the inability of the H-NST A16V mutant to engage in higher order oligomeric protein-protein interactions with H-NS. To determine whether H-NST Ala16 also is important for the H-NST-mediated regulation of LEE expression and the A/E lesion phenotype, we constructed the H-NST A16V mutant using site-directed mutagenesis of pQEH-NST. Evaluation of the sequence around Ala16 revealed a potential heptadic repeat within the coiled-coil element of H-NST of which Ala16 is the first residue (figure 2A). A canonical heptadic repeat is a seven amino acid residue long sequence that forms coiled-coil secondary structures, which are involved in protein-protein interactions [56]. The proposed repeat in H-NST contains the characteristics of a classic heptadic repeat since positions a and d occupy hydrophobic residues, positions e and g commonly contain charged residues, whereas positions b and c are random residues in the repeat [56], [57] (figure 2A). Interestingly, the H-NST heptadic repeat is composed of alanines and leucines occupying the a and d positions, whose side chains are less bulky compared to that of isoleucine and valine. Therefore, to assess the importance for oligomerization of having an alanine residue at position 16, we substituted Ala16 with Leu resulting in the mutant H-NST A16L, which potentially could restore H-NST function since the leucine does not harbor a β-branched chain, compared to that of valine and isoleucine.
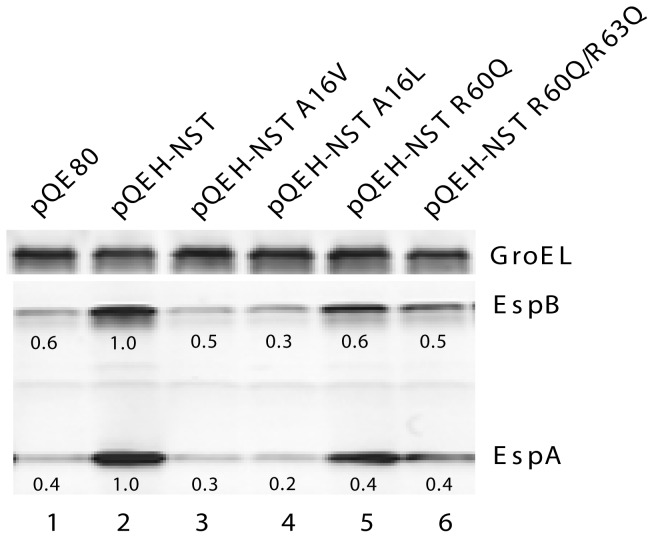
We tested the ability of H-NST mutants A16V, A16L, R60Q and R60Q/R63Q to affect levels of LEE-encoded proteins of EHEC in the exponential phase of growth. Results revealed a decreased ability of all H-NST mutants to induce the production of EspA and EspB compared to wild type H-NST (figure 3, lanes 3–6). Specifically, the H-NST mutants A16V and A16L exhibited decreased ability to induce LEE expression as reflected by a 2- to 6-fold decrease in EspA and EspB levels relative to wild type H-NST (figure 3, lanes 3–4). H-NST mutants at residues Arg60 and Arg63 also showed a decreased ability to affect LEE-encoded protein levels compared to the wild type H-NST with a 2- to 3-fold decrease in EspA and EspB levels (figure 3, lanes 5–6).
Figure 3. H-NST mutants exhibit decreased ability to induce the production of LEE-encoded proteins.
The effect of wild type H-NST and mutant derivatives on LEE-encoded protein levels was determined by western analysis as described in Materials and Methods . EHEC strain TUV93-0 containing the empty vector pQE80 (lane 1), TUV93-0 containing constructs producing wild type EPEC H-NST (lane 2) or the H-NST mutant derivatives H-NST A16V (lane 3), H-NST A16L (lane 4), H-NST R60Q (lane 5) and HNST R60Q/R63Q (lane 6) were grown in LB to a density of OD600∼0.5 and hnsT expression was induced by 0.5 mM IPTG for 60 min. Levels of EspA, EspB and GroEL were detected in total protein by western analysis using polyclonal antisera against the respective proteins as indicated. GroEL served a loading control for total protein. The relative levels of EspA and EspB normalized to that of GroEL are indicated by numbers below the protein bands. Data shown are representative of four independent experiments.
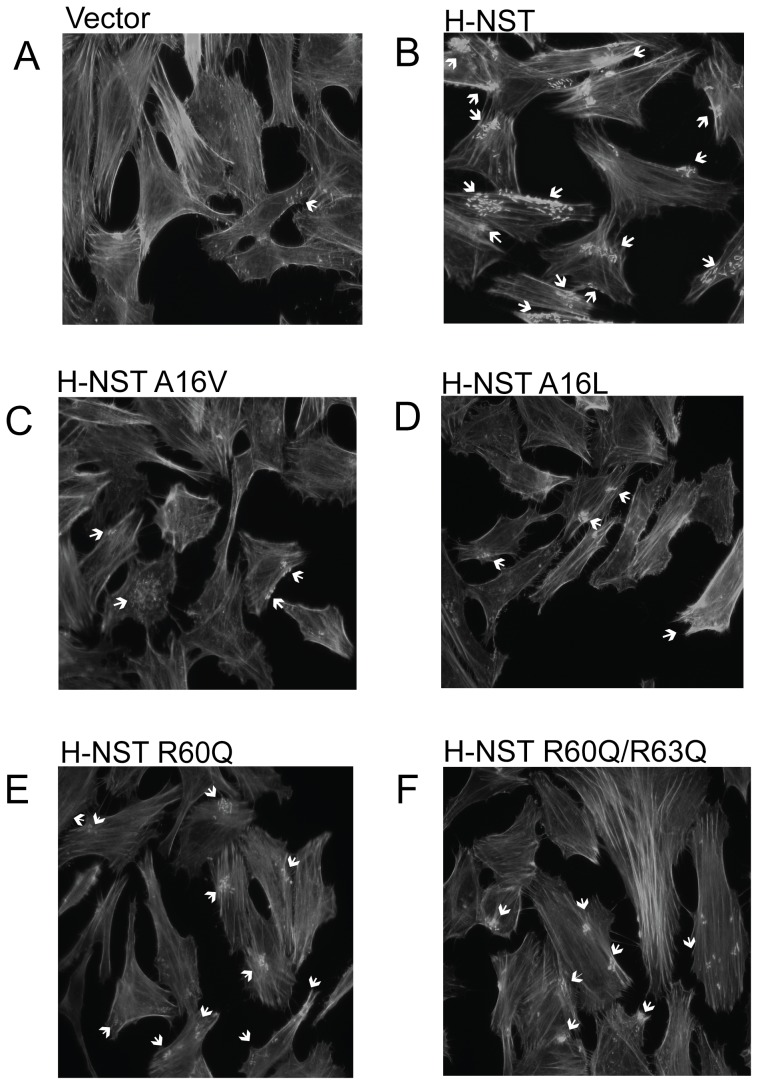
To determine whether the decreased ability of the H-NST mutants to induce accumulation of LEE-encoded proteins compared to wild type H-NST affected T3SS function, we evaluated the A/E lesion phenotype of EHEC producing wild type and H-NST mutant derivatives using the fluorescent actin staining assay (FAS). Actin filaments are stained by FITC-phalloidin in this assay to visualize condensed actin indicative of A/E lesions. FAS assays for EHEC typically involve a 5–6 h incubation time [48], [58]. We infected HeLa cells with EHEC producing wild type or mutant H-NST for 4 h, a point at which A/E lesion formation is minimal yet detectable [48]. Infection with EHEC containing the vector control demonstrated minimal actin pedestal formation as is expected with the co-infection time of 4 h (figure 4A). In contrast, EHEC producing wild type H-NST showed a high degree of A/E lesion formation (figure 4B), which correlates with increased EspA and EspB levels (figure 1B). H-NST mutants A16V and A16L both showed reduced A/E lesion formation compared to wild type H-NST (figure 4, panels C–D), indicating an important role of the Ala16 residue for H-NST to induce this virulence phenotype in EHEC. The H-NST R60Q and R60Q/R63Q mutants showed a slightly reduced degree of A/E lesion formation compared to wild type H-NST with the double arginine mutant showing a larger reduction than the single arginine mutant (figure 4, panels E–F). Neither of the arginine mutants exhibited impaired function to the extent seen with the H-NST A16V and A16L mutants, which correlates with the effect of these respective mutants on EspA and EspB levels (figure 3). Altogether, the H-NST Ala16 mutant exhibited the most severe effect on the ability of H-NST to affect the level of LEE-encoded proteins and induce pedestal formation, further supporting the functional importance of the Ala16 residue. The decreased activity of H-NST mutant at the Arg60 and Arg63 residues suggest functional importance of these residues, which potentially could be involved in DNA-binding.
Figure 4. H-NST induces A/E formation.
FAS assays were used to determine the effect of H-NST on A/E lesion formation of EHEC as described in Materials and Methods . HeLa cell monolayers were co-cultured for four hours with EHEC strain TUV93-0 containing the empty vector pQE80 (A), constructs producing wild type EPEC H-NST (B) or the H-NST mutant derivatives H-NST A16V (C), H-NST A16L (D), H-NST R60Q (E) and HNST R60Q/R63Q (F). The images of FITC phalloidin-stained actin of infected HeLa cells are representative of three independent experiments. Arrows indicate examples of A/E lesions.
H-NST binds to the regulatory regions of LEE1 and LEE3
H-NS and Ler both modulate LEE operon expression through binding to DNA. The LEE1-encoded ler is expressed from two promoters named the distal (P1)- and proximal (P2) promoters with the distal promoter being the major promoter for LEE1 expression [59]. The molecular mechanism of Ler and H-NS-mediated regulation of the LEE1 and LEE2/LEE3 operons has been well studied [10], [30]. Specifically the curvature of DNA and the oligomeric state of the regulator is essential for the ability of these H-NS family proteins to bind LEE DNA [32], [36], [60]. We analyzed the ability of H-NST to bind to the regulatory regions of LEE1 and LEE3 DNA targets contained in EHEC using electrophoretic mobility shift assays (EMSA) (figure 5). We purified a C-terminal hexahistidine-tagged H-NST protein to about 95% purity and determined the ability of H-NST to bind to fluorescently-labeled LEE1 and LEE3 DNA fragments. We demonstrated that increasing concentrations of H-NST shifted the LEE1 P2 DNA fragment, which contains the proximal LEE1 P2 promoter and this 10 bp sequence, whereas the mobility of the DNA fragment in the absence of H-NST was unchanged (figure 5A, lanes 2–4). The DNA-binding specificity of H-NST to LEE1 P2 DNA was tested by adding unlabeled specific LEE1 P2 DNA and nonspecific DNA encoding rssB in the respective ratios of 6∶1 and 12∶1 along with the labeled DNA target. The binding of H-NST to the labeled LEE1 P2 DNA fragment was outcompeted in the presence of the unlabeled specific probe as reflected by a partial downshift of the labeled LEE1 P2 DNA fragment, whereas the DNA fragment shifted by H-NST remained unchanged in the presence of unlabeled rssB DNA (figure 5A, lanes 5 and 6), indicating that binding of H-NST to LEE1 P2 DNA is specific. The DNA sequence amplified from EHEC contained in the LEE1 P2 DNA fragment shares 97% identity to that in EPEC LEE1 P2 promoter [59]. We tested whether H-NST binds to the LEE1 P2 DNA fragment from EPEC, and as expected H-NST also bound to the LEE1 regulatory region from EPEC (unpublished data).
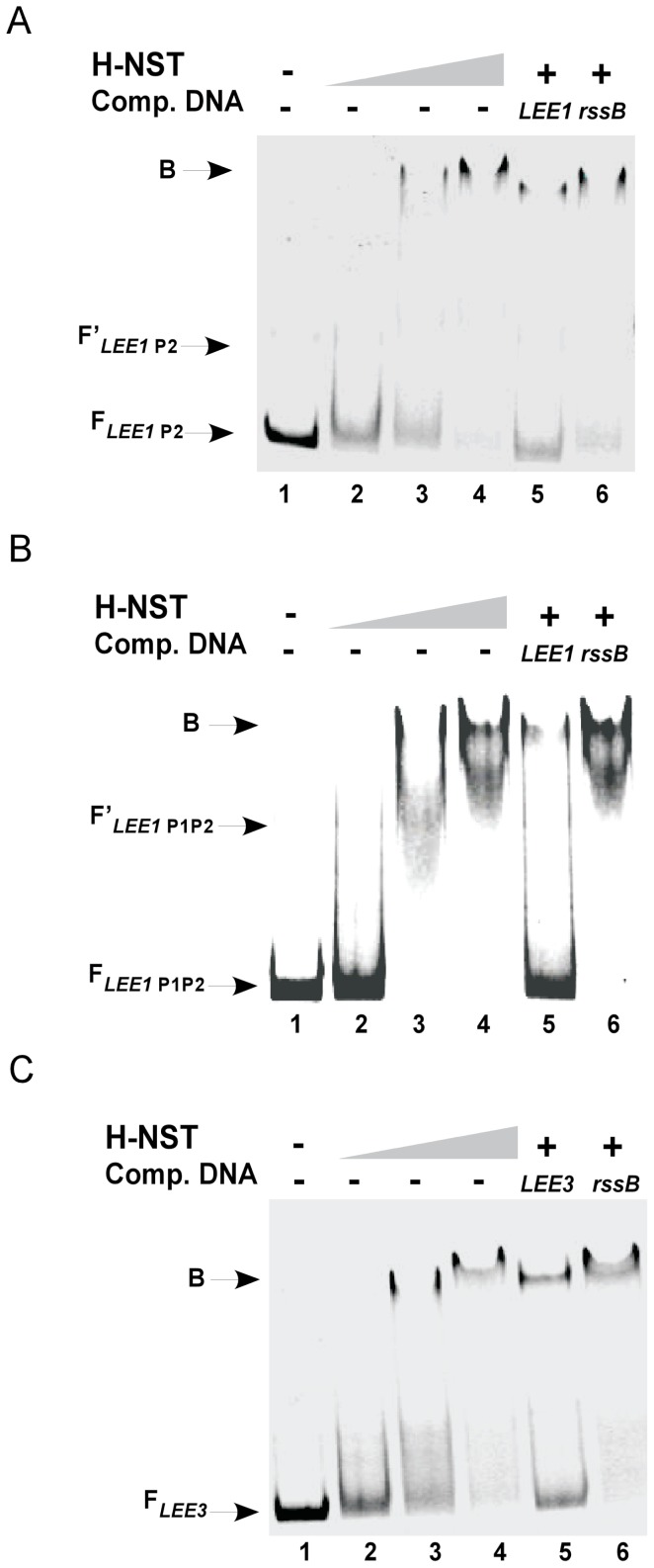
Figure 5. H-NST binds to regulatory regions of LEE1 and LEE3.
The binding of H-NST to fluorescently-labeled LEE DNA targets was determined using electrophoretic mobility shift assays as described in Materials and Methods . Fluorescently-labeled DNA fragments containing the proximal promoter region of LEE1 (LEE1 P2) (A), the distal and proximal promoter regions of LEE1 (LEE1 P1P2) (B), and a regulatory region of LEE3 (LEE3) (C) were incubated in the absence of H-NST (lane 1) and with increasing concentrations of H-NST (lane 2: 25 nM; lane 3: 50 nM; and lane 4: 100 nM). To determine the binding specificity of H-NST, fluorescently-labeled LEE DNA targets were incubated with 100 nM H-NST in the presence of unlabeled competitor DNA fragments (Comp. DNA) containing specific (LEE1 P2, LEE1 P1P2 or LEE3) (lane 5) or non-specific (rssB) (lane 6) DNA targets in the ratios 1∶6 and 1∶12 respectively. Bound and unbound DNA fragments were separated by PAGE on a 4–20% TBE gel. Arrows labeled F indicate unbound DNA, while F′ arrows indicate an unbound DNA subpopulation. The arrows labeled B indicate DNA fragments with H-NST bound. Data shown are representative of three independent experiments.
To gain insight into the binding of H-NST to the complete regulatory region of LEE1 containing both P1 and P2 promoters, we evaluated the binding of H-NST to the LEE1 P1P2 DNA target. H-NST bound specifically to LEE1 P1P2 DNA as demonstrated by a downshift of the bound labeled fragment in the presence of specific unlabeled LEE1 P1P2 DNA as opposed to the presence of unlabeled nonspecific rrsB DNA (figure 5B, lanes 5–6). The presence of increasing H-NST concentrations caused a greater degree of shift for the LEE1 P1P2 DNA fragment compared to that of the LEE1 P1 DNA fragment, suggesting that the binding affinity of H-NST for the LEE1 P1P2 DNA fragment harboring the entire LEE1 regulatory region might be higher than that of the LEE1 P1 fragment that contains only parts of the LEE1 regulatory region (figure 5A–B, lanes 3–4). Since Ler regulation of the LEE2/LEE3 is well characterized and that the molecular mechanism of Ler binding to the LEE3 regulatory region recently was elucidated [30], [36], we wanted to determine whether H-NST binds to this region. Indeed, H-NST bound at increasing concentrations to the LEE3 DNA fragment containing the LEE3 regulatory region containing a 10 bp Ler target sequence identified by Codiero et al 2011 [36] (figure 5C, lanes 2–4). As for the LEE1 regulatory region, H-NST exhibited specific binding to the LEE3 DNA fragment as reflected by a partial downshift of the labeled LEE3 DNA fragment with H-NST bound in the presence of the specific unlabeled LEE3 DNA fragment, whereas non-specific unlabeled rrsB DNA had no effect on the LEE3 DNA fragment shifted by H-NST (figure 5C, lanes 5–6). Altogether, we demonstrated that H-NST binds in a specific manner to the regulatory regions of LEE1 and LEE3, which to our knowledge represents the first demonstration that H-NST binds to DNA.
The H-NST Arg60 and Arg63 residues of the C-terminal region are important for DNA-binding
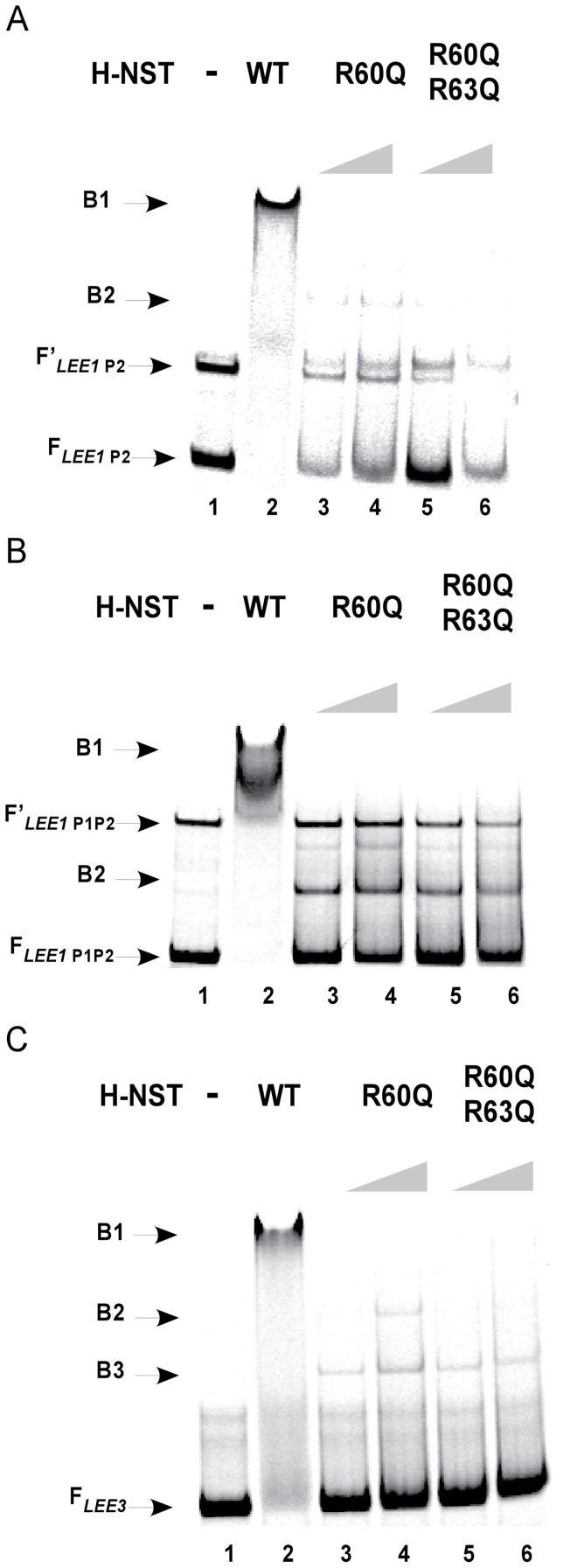
We demonstrated that the single mutant H-NST R60Q and the double mutant H-NST R60Q/R63Q exhibit a decreased ability to positively affect the levels of LEE-encoded proteins and induce A/E lesion formation (figures 3 and 4E–F). This finding highlights the functional importance of these arginine residues located in the C-terminal region of H-NST. Since H-NST residues Arg60 and Arg63 aligned with arginine residues of Ler known to be important for DNA-binding (figure 2B), we tested whether these residues played a role in DNA-binding by H-NST. To this end, we purified H-NST mutants R60Q and R60Q/R63Q to determine their ability to bind LEE DNA (figure 6). The H-NST R60Q and R60Q/R63Q mutants both showed diminished ability to bind to the LEE1 P2, LEE1 P1P2 and LEE3 DNA targets compared to wild type H-NST (figure 6A–C, lanes 3–6), indicating an importance of these arginines in DNA-binding by H-NST. Further, the H-NST R60Q/R63Q double mutant was less capable of binding the LEE1 P2 and LEE3 DNA fragments as reflected by decreased amounts of the complex designated as B2 when increasing concentrations of the H-NST double mutant rather than the R60Q single mutant were present (figure 6A and 6C, compare lanes 5–6 with lanes 3–4), suggesting that H-NST Arg63 like the corresponding Ler Arg93 residue positively affects DNA-binding. Notably, the H-NST R60Q and R60Q/R63Q mutants did not exhibit a differential effect on binding to the LEE1 P1P2 fragment (figure 6B, lanes 3–6), which could be due to the possibility that H-NST binds more strongly to the LEE1 P1P2 DNA fragment than to the shorter LEE1 P2 DNA fragment. The EMSA analyses involving H-NST mutated at the Arg60 and Arg63 residues correlated with the decreased ability of the mutants to induce levels of LEE-encoded proteins and A/E lesion formation, and revealed an important role of these arginine residues in DNA-binding by H-NST.
Figure 6. The H-NST C-terminal Arg60 and Arg63 residues positively affect DNA-binding.
Electrophoretic mobility shift assays were used to assess the binding of wild type H-NST and H-NST mutants containing the substitutions R60Q and R60Q/R63Q to the fluorescently-labeled LEE DNA targets: LEE1 P2 (A), LEE1 P1P2 (B) and LEE3 (C). The DNA fragments were incubated in the absence of H-NST (lane 1), with 100 nM wild type H-NST (lane 2), H-NST R60Q (lane 3: 50 nM and lane 4: 100 nM), and with H-NST R60Q/R63Q (lane 5: 50 nM and lane 6: 100 nM). Arrows labeled F indicate unbound DNA, while F′ arrows indicate an unbound DNA subpopulation. The arrows labeled B1 indicate fully shifted DNA fragments, while B2 and B3 indicate partially shifted DNA fragments. Data shown are representative of two independent experiments.
H-NST Ala16 is important for DNA-binding
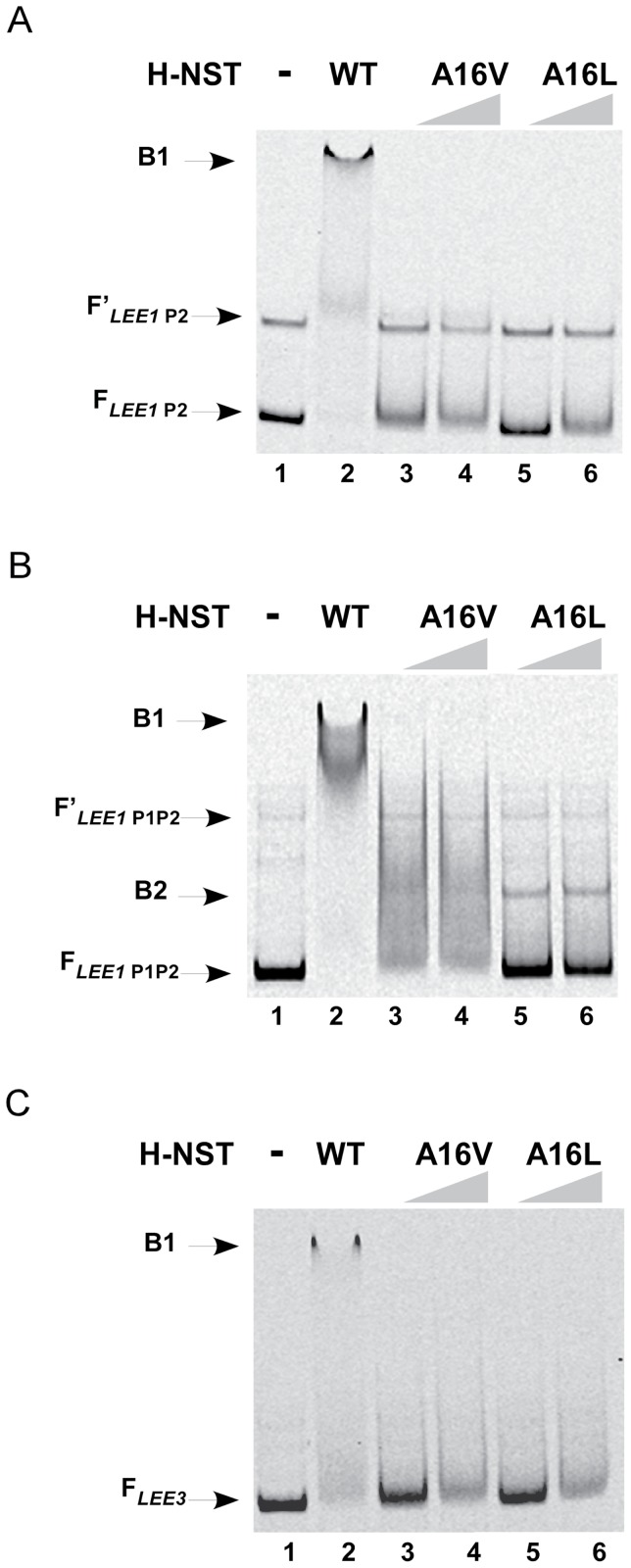
We demonstrated that the H-NST Ala16 residue that is required for H-NST oligomerization with H-NS [37] is also required for H-NST to cause an increase in levels of LEE-encoded proteins and induce pedestal formation on epithelial cell monolayers (figures 3 and 4C–D). In addition to playing a role in oligomerization, we determined whether Ala16 is also involved in DNA-binding by H-NST. We purified the H-NST mutants A16V and A16L and tested their ability to bind LEE DNA targets (figure 7). EMSA analyses revealed that neither of these H-NST mutants bound to LEE1 P2 and LEE3 DNA fragments as reflected by the lack of a change in mobility of these DNA fragments (figure 7A and C, lanes 3–4). Nevertheless, the H-NST A16V and A16L mutants exhibited weak binding to the LEE1 P1P2 DNA as reflected by the appearance of a defined weak partially shifted band, which in particular appeared in the presence of the H-NST A16L mutant (indicated as B2 in figure 7B, compare lanes 3–4 and 4–5 with lane 1). The smeared appearance of the LEE1 P1P2 DNA fragment incubated with the H-NST A16V mutant could reflect a weak and/or indiscriminant binding to this DNA fragment containing the complete LEE1 regulatory region (figure 7B, lanes 3–4). In all, our results indicate that the H-NST Ala16 residue is important for DNA-binding activity of H-NST. The importance of having the conserved Ala16 residue for H-NST function was demonstrated by the inability of the H-NST A16L mutant to restore H-NST function. It remains to be demonstrated whether oligomerization of H-NST is a prerequisite for DNA-binding or if the Ala16 residue directly affects the binding of H-NST to DNA, an issue which is beyond the scope of this study.
Figure 7. The H-NST Ala16 residue is important for DNA-binding.
The binding of wild type H-NST and mutant H-NST to LEE DNA targets was determined by electrophoretic mobility shift assays. Fluorescently-labeled DNA fragments containing LEE1 P2 (A), LEE1 P1P2 (B) and LEE3 (C) regulatory regions were incubated in the absence of H-NST (lane 1), with 100 nM H-NST (lane 2); and with the H-NST mutants H-NST A16V (lane 3: 50 nM and lane 4: 100 nM H-NST) and H-NST A16L (lane 5: 50 nM and lane 6: 100 nM). Bound and unbound DNA fragments were resolved by PAGE on a 4–20% TBE gel. Arrows labeled F indicate unbound DNA, while F′ arrows indicate an unbound DNA subpopulation. The arrows labeled B1 indicate fully shifted DNA fragments, while B2 indicates partially shifted DNA fragments. Data shown are representative of three independent experiments.
H-NST positively affects the binding of Ler to LEE3 DNA pre-bound by H-NS
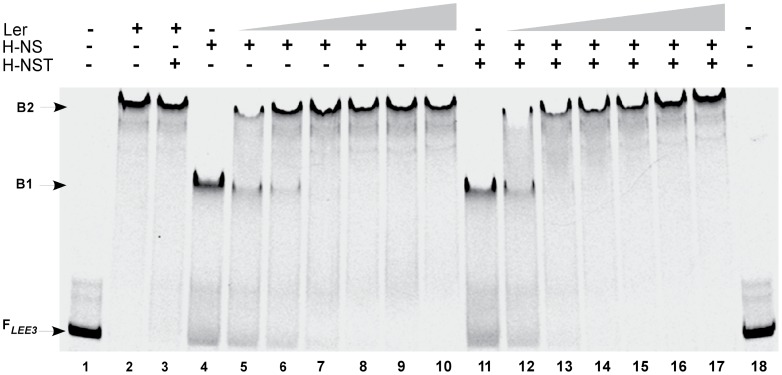
The present mechanism for H-NST function suggests that by forming dominant-negative-acting oligomers with H-NS, H-NST prevents H-NS from modulating H-NS regulon expression [37]. We demonstrated that H-NST functions independently of ler (figure 1B). To obtain additional insight into the role of H-NST-mediated regulation of the LEE, we used EMSA analyses to determine whether H-NST promotes the binding of Ler to LEE DNA that already has H-NS bound. To allow protein-protein interactions to occur we added H-NST and/or H-NS prior to incubation with the LEE3 DNA target, and then added increasing amounts of Ler to determine whether the presence of H-NST helped binding of Ler to LEE3 DNA pre-bound by H-NS. Binding of H-NST and/or H-NS to LEE3 DNA was visualized as a shifted band (indicated as B1), which migrated at the same position regardless of whether one or both proteins were present (figure 8, lanes 4 and 11). The presence of H-NST did not affect Ler binding to the LEE3 DNA fragment in the absence of H-NS as reflected by the similar shift observed (indicated as B2 in figure 8, lanes 2–3). Ler bound at increasing concentrations to LEE3 DNA pre-bound with either H-NS (figure 8, lanes 5–10) or both H-NS and H-NST (figure 8, lanes 12–17) resulted in the shifted band indicated B2. Interestingly, Ler at a 100 nM concentration caused a complete shift of DNA pre-bound to H-NS (indicated as B1) only in the presence of H-NST (figure 8, compare lanes 6 and 13). This result suggests that H-NST could help Ler outcompete H-NS bound to LEE DNA, which correlates with our finding that H-NST positively affects the LEE.
Figure 8. H-NST promotes the binding of Ler to LEE3 DNA bound by H-NS.
Fluorescently-labeled DNA fragments containing the LEE3 regulatory region were incubated alone (lanes 1 and 18), with 175 nM Ler (lane 2), with 175 nM Ler and 50 nM H-NST (lane 3), with 50 nM H-NS (lane 4), with 50 nM HNS in the presence of increasing Ler concentrations (50, 100, 125, 150, 175 and 250 nM Ler; lanes 5–10 respectively), with 50 nM H-NST and 50 nM H-NS (lane 11), with 50 nM HNS and 50 nM H-NST along with increasing Ler concentrations (50, 100, 125, 150, 175 and 250 nM Ler; lanes 12–17). Bound and unbound DNA fragments were separated by PAGE on a 4–20% TBE gel. The arrow labeled F indicates unbound DNA. DNA fragments shifted by H-NS and/or H-NST are indicated as B1, while DNA fragments shifted by Ler are labeled B2. Data shown are representative of three independent experiments.
Discussion
The expression of virulence factors including those of A/E pathogens encoded in the LEE are subject to extensive regulation involving many environmental signals to ensure that virulence-associated factors are produced under conditions optimal for infection. Under such conditions, silencing of virulence gene expression by the global modulator H-NS is counteracted by the major activator of virulence gene expression in A/E pathogens, Ler. H-NST present in EPEC, which is defined as a truncated H-NS derivative lacking the DNA-binding domain, was previously shown to positively affect the relief of H-NS-mediated repression in E. coli K-12 by interacting with H-NS to prevent H-NS oligomerization [37]. Since H-NST could provide an additional mechanism that promotes the relief from H-NS-mediated repression of virulence gene expression in A/E pathogens and in other pathogens encoding H-NST (figure 2A), we evaluated the role of H-NST on the production of the virulence-associated LEE-encoded factors. To determine whether H-NST is required for the production of LEE-encoded proteins in EPEC we deleted hnsT and tested the effect on EspA and EspB levels, which appeared unaffected in the absence of H-NST (unpublished data), suggesting that H-NST is dispensable for LEE expression under the growth in DMEM. However, H-NST when expressed from an inducible promoter positively affected LEE-encoded protein levels and subsequently A/E lesion formation of EHEC (figures 3 and 4A–B), indicating that H-NST can positively impact the expression of virulence genes in response to a yet-to-be identified environmental signal(s).
Arginine residues are commonly involved in DNA-binding by proteins [61] including those of the H-NS family such as Ler. Here, we demonstrated that the arginine residues important for DNA-binding by H-NS (Arg114) and Ler (Arg90 and Arg93) are present in H-NST as Arg60 and Arg63 (figure 2B). This observation propelled us to explore the DNA-binding potential of H-NST by testing the ability of H-NST to bind to LEE target DNA in vitro. We demonstrated that H-NST binds to DNA fragments containing the regulatory regions of LEE1 and LEE3 (figure 5), which is the first demonstration of DNA-binding by H-NST. Further, our data revealed that residues Arg60 and Arg63 are important for H-NST DNA-binding activity, which correlates with a positive effect of these residues on the induction of LEE-encoded protein levels and A/E lesion formation (figures 3 and 4E–F). Though DNA-binding by H-NST R60Q and R60Q/R63Q mutants was diminished compared to that of wild type H-NST, these mutants still exhibited residual DNA-binding (figure 6), suggesting that the positively charged nature of the H-NST C-terminal region, due to the high prevalence of lysine and arginine residues, positively influences H-NST DNA-binding in addition to the functionally important Arg60 and Arg63 residues. Altogether, we demonstrate that H-NST binds to DNA and that this DNA-binding activity is required for H-NST to affect the expression of the LEE contained in A/E pathogens.
We demonstrated that the Ala16 residue, which is known to be functionally important for H-NST to positively affect the expression of the H-NS-repressed genes proU and bgl in E. coli K-12 [37], is also required for H-NST to control the LEE and A/E lesion formation (figures 3 and 4C-D), further indicating that H-NST affects the expression of horizontally-acquired virulence-associated genes. H-NST Ala16 was reported to be functionally important for H-NST to counteract H-NS-mediated repression based on the finding that a H-NST A16V mutant exhibited a diminished ability to derepress proU expression, which was suggested to be due to the inability of the A16V mutant to form functional oligomers [37]. The Ala16 residue located in the proposed second α-helix of the N-terminal coiled-coil region of the oligomerization domain occupies the first position in the predicted heptadic repeat (figure 2A). In case of the inactive H-NST A16V mutant, having a valine at position 16 as the first residue in the heptadic repeat could be associated with steric constraints since valine harbors a β-branched side-chain absent from alanine, which could negatively affect coiled-coil packing [62]–[64], and thereby could prevent oligomerization of H-NST itself and with H-NS. We therefore expected that the introduction of leucine that contains an unbranched β chain at position 16 in H-NST would result in a functional H-NST mutant. However, our data revealed that a H-NST A16L mutant like the A16V mutant was incapable of inducing LEE-encoded protein levels and A/E lesion formation (figure 3, 4C–D), suggesting that the presence of a residue containing a short β chain at position 16 is required for H-NST functionality. We tested the DNA-binding capacity of H-NST mutants A16V and A16L, and found that these mutants exhibited diminished DNA-binding activity (figure 7), suggesting that the ability to oligomerize could be important for H-NST to bind DNA. However, we cannot exclude the possibility that Ala16 also directly affects DNA-binding by H-NST.
Interestingly, our data indicated that H-NST positively affects the binding of Ler to the LEE3 regulatory region pre-bound by H-NS (figure 8), suggesting that H-NST helps Ler-binding to DNA perhaps by promoting the dissociation of H-NS from the LEE3 regulatory region. It is possible that H-NST when bound to DNA can change DNA topology to a conformation that is more suitable for DNA-binding by Ler than H-NS. The finding that H-NST affects LEE expression independently of ler further supports a model in which H-NST positively regulates expression through H-NS. Whether H-NST does so by modulating the DNA curvature by binding to DNA and/or prevents the binding of H-NS to DNA by forming H-NST/H-NS oligomers as previously suggested [37], [41], is unresolved. Further investigation, beyond the scope of this current study, is required to elucidate the molecular basis for the H-NST function, in particular with regard to how H-NST promotes the binding of Ler to DNA pre-bound by H-NS.
Funding Statement
This work was supported by National Institutes of Health grants DK58957 and AI21657 to JBK (http://www.niaid.nih.gov). DAR and THH are funded in part by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under NIH grant number U19 AI090873 (http://www.niaid.nih.gov), as well as funds from the State of Maryland (http://www.maryland.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dorman CJ (2004) H-NS: a universal regulator for a dynamic genome. Nature Reviews Microbiology 2: 391–400. [DOI] [PubMed] [Google Scholar]
- 2. Dorman CJ (2010) Horizontally acquired homologues of the nucleoid-associated protein H-NS: implications for gene regulation. Molecular Microbiology 75: 264–267. [DOI] [PubMed] [Google Scholar]
- 3. Tupper AE, Owen-Hughes TA, Ussery DW, Santos DS, Ferguson DJ, et al. (1994) The chromatin-associated protein H-NS alters DNA topology in vitro . The EMBO Journal 13: 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorman CJ (2007) H-NS, the genome sentinel. Nature Reviews Microbiology 5: 157–161. [DOI] [PubMed] [Google Scholar]
- 5. Fang FC, Rimsky S (2008) New insights into transcriptional regulation by H-NS. Current Opinion in Microbiology 11: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lucht JM, Dersch P, Kempf B, Bremer E (1994) Interactions of the nucleoid-associated DNA-binding protein H-NS with the regulatory region of the osmotically controlled proU operon of Escherichia coli . The Journal of Biological Chemistry 269: 6578–6578. [PubMed] [Google Scholar]
- 8. Lucchini S, Rowley G, Goldberg MD, Hurd D, Harrison M, et al. (2006) H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathogens 2: e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caramel A, Schnetz K (1998) Lac and lambda repressors relieve silencing of the Escherichia coli bgl promoter. Activation by alteration of a repressing nucleoprotein complex. Journal of Molecular Biology 284: 875–883. [DOI] [PubMed] [Google Scholar]
- 10. Mellies JL, Barron AM, Carmona AM (2007) Enteropathogenic and enterohemorrhagic Escherichia coli virulence gene regulation. Infection and Immunity 75: 4199–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nataro JP, Kaper JB (1998) Diarrheagenic Escherichia coli . Clinical Microbiology Reviews 11: 142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bustamante VH, Santana FJ, Calva E, Puente JL (2001) Transcriptional regulation of type III secretion genes in enteropathogenic Escherichia coli: Ler antagonizes H-NS-dependent repression. Molecular Microbiology 39: 664–678. [DOI] [PubMed] [Google Scholar]
- 13. Haack KR, Robinson CL, Miller KJ, Fowlkes JW, Mellies JL (2003) Interaction of Ler at the LEE5 (tir) operon of enteropathogenic Escherichia coli . Infection and Immunity 71: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barba J, Bustamante VH, Flores-Valdez MA, Deng W, Finlay BB, et al. (2005) A positive regulatory loop controls expression of the locus of enterocyte effacement-encoded regulators Ler and GrlA. Journal of Bacteriology 187: 7918–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Umanski T, Rosenshine I, Friedberg D (2002) Thermoregulated expression of virulence genes in enteropathogenic Escherichia coli . Microbiology 148: 2735–2744. [DOI] [PubMed] [Google Scholar]
- 16. Laaberki MH, Janabi N, Oswald E, Repoila F (2006) Concert of regulators to switch on LEE expression in enterohemorrhagic Escherichia coli O157:H7: interplay between Ler, GrlA, HNS and RpoS. International Journal of Medical Microbiology 296: 197–210. [DOI] [PubMed] [Google Scholar]
- 17. Sanchez-SanMartin C, Bustamante VH, Calva E, Puente JL (2001) Transcriptional regulation of the orf19 gene and the tir-cesT-eae operon of enteropathogenic Escherichia coli . Journal of Bacteriology 183: 2823–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torres AG, Slater TM, Patel SD, Popov VL, Arenas-Hernandez MM (2008) Contribution of the Ler- and H-NS-regulated long polar fimbriae of Escherichia coli O157:H7 during binding to tissue-cultured cells. Infection and Immunity 76: 5062–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogers MT, Zimmerman R, Scott ME (2009) Histone-like nucleoid-structuring protein represses transcription of the ehx operon carried by locus of enterocyte effacement-negative Shiga toxin-expressing Escherichia coli . Microbial Pathogenesis 47: 202–211. [DOI] [PubMed] [Google Scholar]
- 20. McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB (1995) A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proceedings of the National Academy of Sciences of the United States of America 92: 1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarvis KG, Giron JA, Jerse AE, McDaniel TK, Donnenberg MS, et al. (1995) Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proceedings of the National Academy of Sciences of the United States of America 92: 7996–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perna NT, Mayhew GF, Posfai G, Elliott S, Donnenberg MS, et al. (1998) Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infection and Immunity 66: 3810–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elliott SJ, Wainwright LA, McDaniel TK, Jarvis KG, Deng YK, et al. (1998) The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Molecular Microbiology 28: 1–4. [DOI] [PubMed] [Google Scholar]
- 24. Lai Y, Rosenshine I, Leong JM, Frankel G (2013) Intimate host attachment: enteropathogenic and enterohaemorrhagic Escherichia coli . Cellular Microbiology 15: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tree JJ, Wolfson EB, Wang D, Roe AJ, Gally DL (2009) Controlling injection: regulation of type III secretion in enterohaemorrhagic Escherichia coli . Trends in Microbiology 17: 361–370. [DOI] [PubMed] [Google Scholar]
- 26. Hansen AM, Jin DJ (2012) SspA up-regulates gene expression of the LEE pathogenicity island by decreasing H-NS levels in enterohemorrhagic Escherichia coli . BMC Microbiology 12: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torres AG, Lopez-Sanchez GN, Milflores-Flores L, Patel SD, Rojas-Lopez M, et al. (2007) Ler and H-NS, regulators controlling expression of the long polar fimbriae of Escherichia coli O157:H7. Journal of Bacteriology 189: 5916–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mellies JL, Elliott SJ, Sperandio V, Donnenberg MS, Kaper JB (1999) The Per regulon of enteropathogenic Escherichia coli : identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Molecular Microbiology 33: 296–306. [DOI] [PubMed] [Google Scholar]
- 29. Elliott SJ, Sperandio V, Giron JA, Shin S, Mellies JL, et al. (2000) The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli . Infection and Immunity 68: 6115–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sperandio V, Mellies JL, Delahay RM, Frankel G, Crawford JA, et al. (2000) Activation of enteropathogenic Escherichia coli (EPEC) LEE2 and LEE3 operons by Ler. Molecular Microbiology 38: 781–793. [DOI] [PubMed] [Google Scholar]
- 31. Mellies JL, Larabee FJ, Zarr MA, Horback KL, Lorenzen E, et al. (2008) Ler interdomain linker is essential for anti-silencing activity in enteropathogenic Escherichia coli . Microbiology 154: 3624–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rojas-Lopez M, Arenas-Hernandez MM, Medrano-Lopez A, Martinez de la Pena CF, Puente JL, et al. (2011) Regulatory control of the Escherichia coli O157:H7 lpf1 operon by H-NS and Ler. Journal of Bacteriology 193: 1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mellies JL, Benison G, McNitt W, Mavor D, Boniface C, et al. (2011) Ler of pathogenic Escherichia coli forms toroidal protein-DNA complexes. Microbiology 157: 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yerushalmi G, Nadler C, Berdichevski T, Rosenshine I (2008) Mutational analysis of the locus of enterocyte effacement-encoded regulator (Ler) of enteropathogenic Escherichia coli . Journal of Bacteriology 190: 7808–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia J, Cordeiro TN, Prieto MJ, Pons M (2012) Oligomerization and DNA binding of Ler, a master regulator of pathogenicity of enterohemorrhagic and enteropathogenic Escherichia coli . Nucleic Acids Research 40: 10254–10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cordeiro TN, Schmidt H, Madrid C, Juarez A, Bernado P, et al. (2011) Indirect DNA readout by an H-NS related protein: structure of the DNA complex of the C-terminal domain of Ler. PLoS Pathogens 7: e1002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williamson HS, Free A (2005) A truncated H-NS-like protein from enteropathogenic Escherichia coli acts as an H-NS antagonist. Molecular Microbiology 55: 808–827. [DOI] [PubMed] [Google Scholar]
- 38. Liu Q, Richardson CC (1993) Gene 5.5 protein of bacteriophage T7 inhibits the nucleoid protein H-NS of Escherichia coli . Proceedings of the National Academy of Sciences of the United States of America 90: 1761–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ali SS, Beckett E, Bae SJ, Navarre WW (2011) The 5.5 protein of phage T7 inhibits H-NS through interactions with the central oligomerization domain. Journal of Bacteriology 193: 4881–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu B, Lee SJ, Tan M, Wang ED, Richardson CC (2012) Gene 5.5 protein of bacteriophage T7 in complex with Escherichia coli nucleoid protein H-NS and transfer RNA masks transfer RNA priming in T7 DNA replication. Proceedings of the National Academy of Sciences of the United States of America 109: 8050–8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banos RC, Pons JI, Madrid C, Juarez A (2008) A global modulatory role for the Yersinia enterocolitica H-NS protein. Microbiology 154: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 42. Banos RC, Aznar S, Madrid C, Juarez A (2011) Differential functional properties of chromosomal- and plasmid-encoded H-NS proteins. Research in Microbiology 162: 382–385. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J RD (2001) Molecular cloning: a laboratory manual Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory. [Google Scholar]
- 44. Hazen TH, Sahl JW, Fraser CM, Donnenberg MS, Scheutz F, et al. (2013) Refining the pathovar paradigm via phylogenomics of the attaching and effacing Escherichia coli . Proceedings of the National Academy of Sciences of the United States of America 110: 12810–12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 46. Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, et al. (2010) A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Research 38: W695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Donohue-Rolfe A, Kondova I, Oswald S, Hutto D, Tzipori S (2000) Escherichia coli O157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. The Journal of Infectious Diseases 181: 1825–1829. [DOI] [PubMed] [Google Scholar]
- 48. Hansen AM, Kaper JB (2009) Hfq affects the expression of the LEE pathogenicity island in enterohaemorrhagic Escherichia coli . Molecular Microbiology 73: 446–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knutton S, Baldwin T, Williams PH, McNeish AS (1989) Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli . Infection and Immunity 57: 1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cox WG, Singer VL (2004) Fluorescent DNA hybridization probe preparation using amine modification and reactive dye coupling. BioTechniques 36: 114–122. [DOI] [PubMed] [Google Scholar]
- 51. Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, et al. (2012) Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease. PLoS One 7: e41594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, et al. (2010) The global burden of nontyphoidal Salmonella gastroenteritis. Clinical Infectious Diseases 50: 882–889. [DOI] [PubMed] [Google Scholar]
- 53. De Baere T, Verhelst R, Labit C, Verschraegen G, Wauters G, et al. (2004) Bacteremic infection with Pantoea ananatis . Journal of Clinical Microbiology 42: 4393–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gordon BR, Li Y, Cote A, Weirauch MT, Ding P, et al. (2011) Structural basis for recognition of AT-rich DNA by unrelated xenogeneic silencing proteins. Proceedings of the National Academy of Sciences of the United States of America 108: 10690–10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ali SS, Whitney JC, Stevenson J, Robinson H, Howell PL, et al. (2013) Structural insights into the regulation of foreign genes in Salmonella by the Hha/H-NS complex. The Journal of Biological Chemistry 288: 13356–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lupas A (1996) Prediction and analysis of coiled-coil structures. Methods in Enzymology 266: 513–525. [DOI] [PubMed] [Google Scholar]
- 57. Stone DSJ, Johnson P, Smillie LB (1975) Tropomyosin: correlation of amino acid sequence and structure. FEBS Proceedings 31: 125–136. [Google Scholar]
- 58. Campellone KG, Roe AJ, Lobner-Olesen A, Murphy KC, Magoun L, et al. (2007) Increased adherence and actin pedestal formation by dam-deficient enterohaemorrhagic Escherichia coli O157:H7. Molecular Microbiology 63: 1468–1481. [DOI] [PubMed] [Google Scholar]
- 59. Russell RM, Sharp FC, Rasko DA, Sperandio V (2007) QseA and GrlR/GrlA regulation of the locus of enterocyte effacement genes in enterohemorrhagic Escherichia coli . Journal of Bacteriology 189: 5387–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ueguchi C, Suzuki T, Yoshida T, Tanaka K, Mizuno T (1996) Systematic mutational analysis revealing the functional domain organization of Escherichia coli nucleoid protein H-NS. Journal of Molecular Biology 263: 149–162. [DOI] [PubMed] [Google Scholar]
- 61. Seeman NC, Rosenberg JM, Rich A (1976) Sequence-specific recognition of double helical nucleic acids by proteins. Proceedings of the National Academy of Sciences of the United States of America 73: 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harbury PB, Zhang T, Kim PS, Alber T (1993) A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science 262: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 63. Lupas AN, Gruber M (2005) The structure of alpha-helical coiled coils. Advances in Protein Chemistry 70: 37–78. [DOI] [PubMed] [Google Scholar]
- 64. Ramos J, Lazaridis T (2006) Energetic determinants of oligomeric state specificity in coiled coils. Journal of the American Chemical Society 128: 15499–15510. [DOI] [PubMed] [Google Scholar]