Abstract
Objective
We aimed to assess the effects of age, sex, body mass index (BMI), and anatomical site on skin thickness in children and adults with diabetes.
Methods
We studied 103 otherwise healthy children and adolescents with type 1 diabetes aged 5–19 years, and 140 adults with type 1 and type 2 diabetes aged 20–85 years. The thicknesses of both the dermis and subcutis were assessed using ultrasound with a linear array transducer, on abdominal and thigh skin.
Results
There was an age-related thickening of both dermis (p<0.0001) and subcutis (p = 0.013) in children and adolescents. Girls displayed a substantial pubertal increase in subcutis of the thigh (+54%; p = 0.048) and abdomen (+68%; p = 0.009). Adults showed an age-related decrease in dermal (p = 0.021) and subcutis (p = 0.009) thicknesses. Pubertal girls had a thicker subcutis than pubertal boys in both thigh (16.7 vs 7.5 mm; p<0.0001) and abdomen (16.7 vs 8.8 mm; p<0.0001). Men had greater thigh dermal thickness than women (1.89 vs 1.65 mm; p = 0.003), while the subcutis was thicker in women in thigh (21.3 vs 17.9 mm; p = 0.012) and abdomen (17.7 vs 9.8 mm; p<0.0001). In boys, men, and women, both dermis and subcutis were thicker on the abdomen compared to thigh; in girls this was only so for dermal thickness. In both children and adults, the skin (dermis and subcutis) became steadily thicker with increasing BMI (p<0.0001).
Conclusions
Skin thickness is affected by age, pubertal status, gender, BMI, and anatomical site. Such differences may be important when considering appropriate sites for dermal/subcutaneous injections and other transdermal delivery systems.
Introduction
Skin thickness is affected by a number of factors, including age, gender and body mass index (BMI). Such data may be of importance when determining ideal techniques and sites for intradermal/subcutaneous injections and transdermal delivery systems. This is a particular issue in children with diabetes, among whom subcutaneous insulin injections may be inadvertently delivered to muscle tissue, leading to altered insulin absorption and increased risk of hypoglycaemia [1], [2]. Thus, in this study we assessed the effects of age, sex, BMI, and anatomical site on skin thickness in children and adults with diabetes.
Methods
Ethics statement
The study was approved by the Auckland District Health Board Research Review Committee. Written informed consent was obtained from all adult participants. For younger participants, written informed consent was obtained from parents or guardians, as well as verbal or written consent from each child as was appropriate to their age.
Participants
This cohort was recruited as part of a study evaluating injection techniques in children [3]. Otherwise healthy children and adolescents with type 1 diabetes aged 5–19 years were recruited from the diabetes clinic at Starship Children's Hospital, Auckland, New Zealand. Adults with type 1 and type 2 diabetes aged between 20–85 years were recruited from the Auckland Diabetes Centre, Greenlane Clinical Centre, Auckland. Exclusion criteria included moderate-to-severe lipohypertrophy, other medical conditions such as coeliac disease or autoimmune thyroid disease, associated syndromes (e.g. Down's syndrome), and other secondary causes of diabetes (e.g. cystic fibrosis). Lipohypertrophy was assessed clinically, and, in those subjects who demonstrated mild lipohypertrophy, no measurements were made in areas where any adipose thickening was noted. Participants had weight and height measured, and pubertal status in children were assessed by a pediatric endocrinologist. BMI was calculated for adults, and BMI standard deviation score (BMI SDS) was calculated for each child according to British 1990 standards [4].
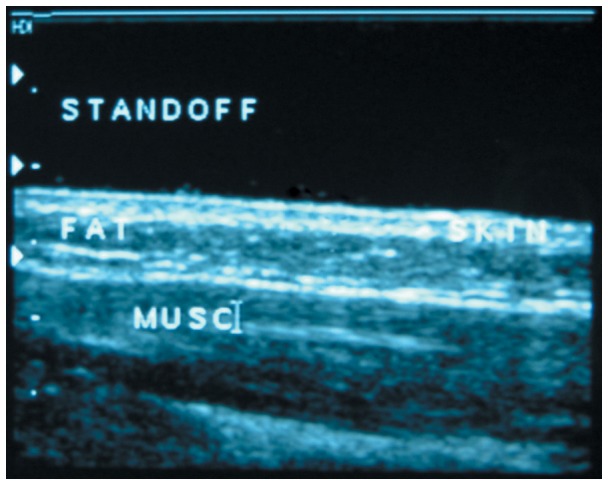
Dermis and subcutis thicknesses were assessed using ultrasound at each injection site, in the anterior abdomen 3–4 cm lateral to the umbilicus and at the lateral mid-thigh. Dermal thickness was defined as the distance between the air-skin surface interface and the proximal aspect of the subcutaneous tissue layer, and included the small contribution of the epidermis. Subcutis thickness was measured from the proximal subcutaneous fat boundary to the underlying muscle fascia. Assessments were performed using a Phillips IU-22 ultrasound machine (Phillips Healthcare, Best, Netherlands) and a 17 MHz linear array transducer, having an axial resolution of 0.08 mm [5]. The exact site of needle insertion was marked prior to injection, and the transducer centered over this point. A single measurement was obtained mid-transducer, with cursors centered at the air-skin interface, the skin-subcutaneous fat interface, and the fat-muscle fascia interface. Note that a standoff was used to optimize image quality by increasing the distance between the transducer and the skin (Figure 1). This method of assessing depth of skin layers at injection sites has been well-validated previously [1], [6].
Figure 1. Ultrasound image showing a cross-sectional view of the standoff, dermis (skin), subcutis (fat), and muscle tissue (musc).
Statistical analysis
Descriptive data were obtained in Minitab v.16 (Pennsylvania State University, State College, PA, USA). All multivariate analyses were performed in SAS v.9.3 (SAS Institute Inc. Cary, NC, USA). Random effect mixed models with repeated measures were used to assess the association of a number of parameters of interest with dermal and subcutis thickness. Models included age, BMI, anatomical site, and gender as independent variables. For children, BMI SDS rather than BMI was used. Models for children were also run without age, but with the inclusion of pubertal status as a categorical factor. All statistical tests were maintained at a 5% significance level. Age data are presented as means ± standard deviations, while outcome data are presented as model-adjusted means (estimated marginal means adjusted for the confounding factors in the models), with associated 95% confidence intervals.
Results
Participants
One hundred and three children and adolescents (54 boys) aged 12.3±3.2 years (range 6.0–19.0 years) were studied. The mean BMI SDS was 0.88±1.08 (range 1.79–3.48). A total of 140 adults (61 men) aged 44.2±14.3 years (range 20.0–81.0 years) were also studied; mean BMI was 28.1±5.8 kg/m2 (range: 17.7–45.3 kg/m2). A summary of the study's data is provided in Table 1.
Table 1. Summary of study results.
| Boys | Girls | Men | Women | ||
| n | 54 | 49 | 61 | 79 | |
| Age (years) | 12.2±3.1 | 12.3±3.3 | 43.5±14.3 | 44.8±19.0 | |
| [6.0–18.0] | [6.0–19.0] | [20.0–72.0] | [20.0–81.0] | ||
| Dermis (mm) | Abdomen | 1.89 (1.75–2.03) | 1.83 (1.68–1.97) | 2.10 (1.99–2.21) | 1.99 (1.89–2.09) |
| [1.00–5.00] | [1.00–3.40] | [0.80–3.00] | [0.90–3.60] | ||
| Thigh | 1.60 (1.50–1.70) | 1.57 (1.47–1.68) | 1.89 (1.78–2.01) | 1.65 (1.55–1.76) | |
| [0.80–2.90] | [0.10–3.00] | [0.90–3.00] | [0.09–3.20] | ||
| Subcutis (mm) | Abdomen | 9.13 (7.75–10.51) | 13.06 (11.70–14.42) | 17.88 (15.93–19.83) | 21.26 (19.54–22.99) |
| [2.30–26.0] | [2.80–41.0] | [4.0–50.6] | [3.00–58.0] | ||
| Thigh | 7.68 (6.25–6.12) | 13.39 (11.97–14.82) | 9.84 (8.21–11.48) | 17.68 (16.23–19.12) | |
| [2.70–18.4] | [4.00–35.8] | [2.30–23.6] | [6.20–60.4] |
Age data are means ± standard deviations; all other data are means and 95% confidence intervals (in brackets), adjusted for other confounding factors in the multivariate models. Data ranges are provided in square brackets for each parameter.
Effects of age, puberty, and gender
Pubertal status had a strong effect on dermal thickness (p<0.0001), which was greater in pubertal than pre-pubertal children. Not surprisingly therefore, increasing age among children and adolescents was associated with a thicker dermis (+52 µm/year; p<0.0001) (Supplementary Figure S1). There was also a positive mild association between age and thickness of subcutis (p = 0.013; Supplementary Figure S1).
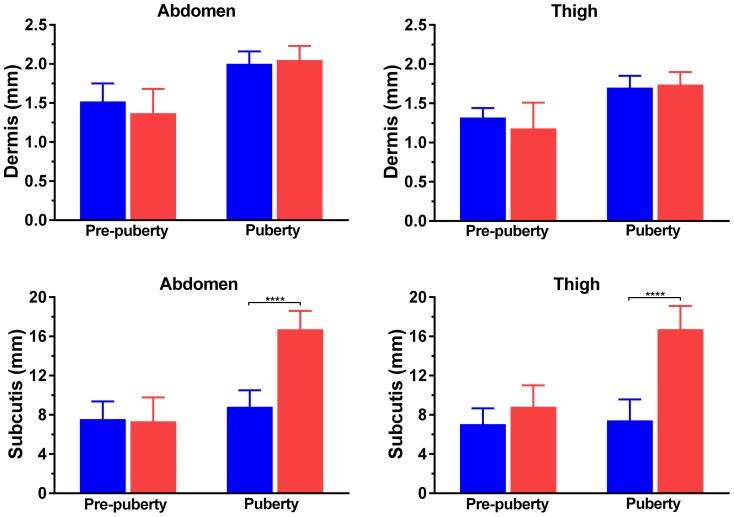
Prior to puberty, there were no differences in thickness of either the dermis or subcutis between boys and girls (Figure 2). The dermal thickness in both anatomical sites remained similar among boys and girls in puberty (Figure 2). However, girls displayed a substantial pubertal thickening of the subcutis in thigh (+54%; p = 0.048) and abdomen (+68%; p = 0.009), while there was little change among boys (Figure 2). As a result, the subcutis was considerably thicker in pubertal girls than in boys in both thigh (16.7 vs 7.5 mm; p<0.0001) and abdomen (16.7 vs 8.8 mm; p<0.0001) (Figure 2).
Figure 2. Skin thickness in boys (blue bars) and girls (red bars), according to pubertal status.
****p<0.0001.
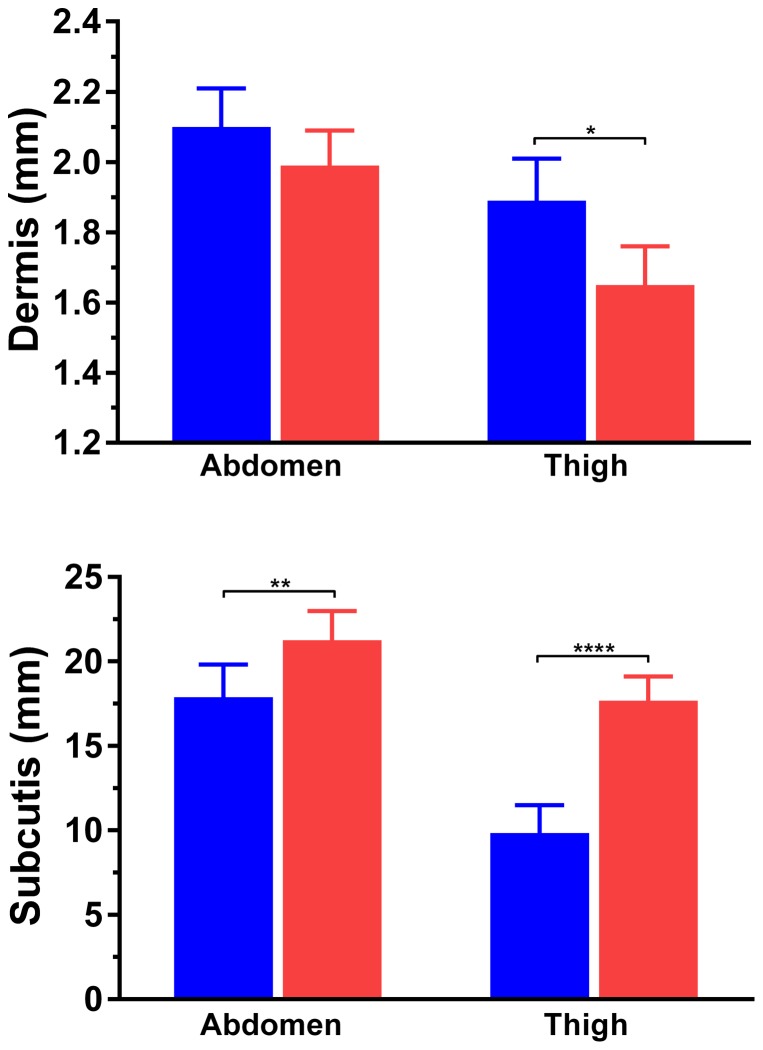
In contrast to the pattern observed among children, increasing age in adults was associated with a slight decrease in both dermis (−6 µm/year; p = 0.021) and subcutis (−82 µm/year; p = 0.009) thicknesses (Supplementary Figure S1). Abdominal dermal thickness was not different between men and women (2.10 vs 1.99 mm; p = 0.16), but men had a thicker dermis on thighs compared to women (1.89 vs 1.65 mm; p = 0.003) (Figure 3). The subcutis was thicker in women's abdomens than in men's (21.3 vs 17.9 mm; p = 0.012), and even more so on the thigh (17.7 vs 9.8 mm, respectively; p<0.0001) (Figure 3).
Figure 3. Skin thickness in men (blue bars) and women (red bars).
*p<0.05, **p<0.01, and ****p<0.0001.
Anatomical site
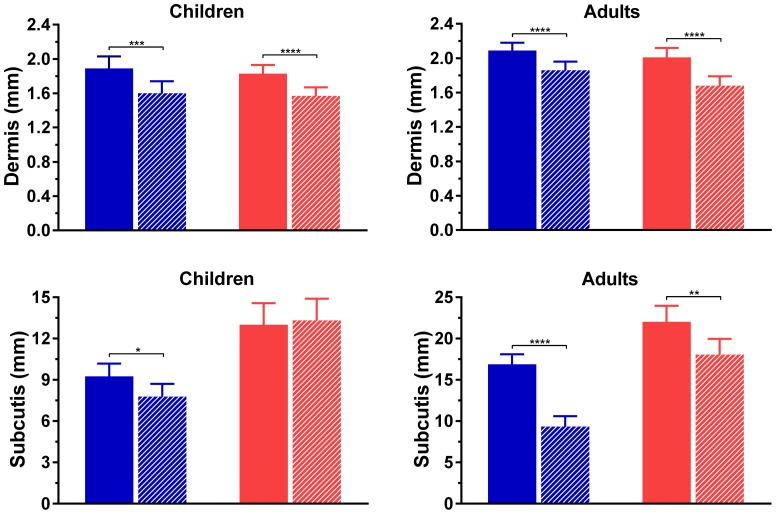
Boys had abdominal skin that was thicker than their thigh skin (dermis: 1.89 vs 1.60 mm, p = 0.0003; subcutis: 9.25 vs 7.78 mm, p = 0.012) (Figure 4). Girls also had a thicker abdominal dermis compared to their thighs (1.83 vs 1.57 mm; p<0.0001), but there was no difference in subcutis thickness between anatomical sites (13.0 vs 13.3 mm; p = 0.76) (Figure 4).
Figure 4. Thickness of skin layers in abdomen (full bars) and thigh (striped bars) in males (blue) and females (red).
*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 for comparisons between abdominal and thigh measurements.
Among adults, the dermis of men was greater in the abdomen than thigh (2.09 vs 1.86 mm; p<0.0001), with an 80% difference observed in subcutis thickness (16.9 vs 9.3 mm, respectively; p<0.0001) (Figure 4). Women displayed a similar pattern (dermis: 2.01 vs 1.68 mm, respectively; p<0.0001), but had less of a difference in the subcutis (22.0 vs 18.1 mm; p = 0.007) (Figure 4).
BMI
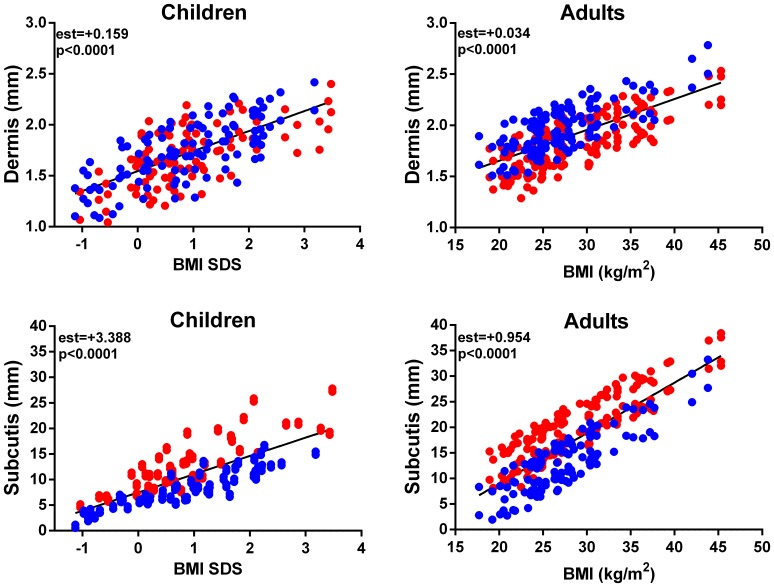
Skin thickness (both dermal and subcutis) was strongly associated with BMI over the entire life span (p<0.0001; Figure 5). In both children and adults, skin layers became progressively thicker with increasing BMI (Figure 5).
Figure 5. The association of BMI SDS and BMI with thickness of skin layers in children (n = 103) and adults (n = 140), respectively.
Data for females are in red and for males in blue.
Discussion
This study on diabetic patients shows that skin thickness is affected by age, pubertal status, gender, BMI, and anatomical site. Similar to our results, Hofman et al. showed no gender differences in the subcutis prior to puberty [2], but pubertal girls had thicker subcutis than boys [1], [2], [7]. Our findings also confirm the existing evidence that adult men have thinner subcutis and thicker dermis than women [8]–[17].
There are relatively limited data on skin thickness throughout childhood, but three other studies have noted an age-related increase in dermis among children [18]–[20]. Considerably more data exist in adulthood, and several studies have shown a thinning of the dermis with increasing age [10], [12], [13], [18], [20]–[24]. In particular, our data are in accordance with the findings of Tan et al. showing a linear increase in dermis until the age of 20, with a subsequent decline thereafter [20]. Although Shuster et al. also found this pattern of decreasing dermal thickness among men, they observed that it was relatively unchanged in women until their 50 s after which it began to decline [25] (probably due to decreasing oestrogen levels after menopause). In regards to the subcutis, at least one other study has observed a decrease associated with ageing [24].
Variations in the thickness of the dermis at different anatomical sites have been shown in numerous studies [7], [8], [10], [13]–[16], [19], [20], [24], [26]. Similar to our findings, one study observed a thicker dermis in abdominal skin compared to thigh skin in adults [8], and a non-significant difference of 0.2 mm in dermal thickness was noted between abdomen and thigh in diabetic children [2].
While an increase in subcutaneous tissue thickness with increasing BMI is obviously expected, other investigations have also found increasing BMI to be associated with a thicker dermis in both children and adults [2], [8], [12], [26]. Conversely, Smalls et al. showed a negative association between BMI and skin thickness on the shoulder of healthy females [27].
Our study shows that skin thickness is affected by age, pubertal status, gender, BMI, and anatomical site in patients with diabetes. This may be of importance when considering appropriate sites for dermal/subcutaneous injections and other transdermal delivery systems, especially given the increasing use of shorter and finer needles. Clearly, skin thickness is an important factor in the selection of needle length for intradermal or subcutaneous injection, particularly for auto-injector devices (e.g. insulin, adrenaline, and biologic response modifiers [28]). Mathematical modeling of transdermal delivery systems however, seldom include the thickness of the dermis and/or subcutis in their models, generally concentrating only on the barrier role of the stratum corneum [29], [30]. For lipophilic drugs, such as testosterone, this may lead to unexpected results [31]. Although this study was conducted in diabetic patients, who have been shown to have slightly thicker skin [32], we believe our data may be extrapolated to the general population.
Supporting Information
The association of age with thickness of skin layers in children (n = 103) and adults (n = 140). Data for females are in red and for males in blue.
(PDF)
Acknowledgments
We thank all participants for very kindly volunteering to take part in this study. We also thank the Auckland Radiology Group (in particular Julie Heaney), Caroline Adamson (Auckland District Health Board), and Natalie Billett (Liggins Institute, University of Auckland) for valuable assistance.
Funding Statement
This research was supported by an unrestricted grant from Novo Nordisk. The funders had any role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
References
- 1. Birkebaek NH, Johansen A, Solvig J (1998) Cutis/subcutis thickness at insulin injection sites and localization of simulated insulin boluses in children with type 1 diabetes mellitus: need for individualization of injection technique? Diabet Med 15: 965–971. [DOI] [PubMed] [Google Scholar]
- 2. Hofman PL, Lawton SA, Peart JM, Holt JA, Jefferies CA, et al. (2007) An angled insertion technique using 6-mm needles markedly reduces the risk of intramuscular injections in children and adolescents. Diabet Med 24: 1400–1405. [DOI] [PubMed] [Google Scholar]
- 3. Hofman PL, Derraik JGB, Pinto TE, Tregurtha S, Faherty A, et al. (2010) Defining the ideal injection techniques when using 5-mm needles in children and adults. Diabetes Care 33: 1940–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cole TJ, Freeman JV, Preece MA (1995) Body mass index reference curves for the UK, 1990. Archives of Disease in Childhood 73: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winter MD, Londono L, Berry CR, Hernandez JA (2013) Ultrasonographic evaluation of relative gastrointestinal layer thickness in cats without clinical evidence of gastrointestinal tract disease. J Feline Med Surg [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tubiana-Rufi N, Belarbi N, Du Pasquier-Fediaevsky L, Polak M, Kakou B, et al. (1999) Short needles (8 mm) reduce the risk of intramuscular injections in children with type 1 diabetes. Diabetes Care 22: 1621–1625. [DOI] [PubMed] [Google Scholar]
- 7. Smith CP, Sargent MA, Wilson BP, Price DA (1991) Subcutaneous or intramuscular insulin injections. Arch Dis Child 66: 879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibney MA, Arce CH, Byron KJ, Hirsch LJ (2010) Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin 26: 1519–1530. [DOI] [PubMed] [Google Scholar]
- 9. Kanehisa H, Miyatani M, Azuma K, Kuno S, Fukunaga T (2004) Influences of age and sex on abdominal muscle and subcutaneous fat thickness. Eur J Appl Physiol 91: 534–537. [DOI] [PubMed] [Google Scholar]
- 10. Lasagni C, Seidenari S (1995) Echographic assessment of age-dependent variations of skin thickness. Skin Res Technol 1: 81–85. [DOI] [PubMed] [Google Scholar]
- 11. Burbridge BE (2007) Computed tomographic measurement of gluteal subcutaneous fat thickness in reference to failure of gluteal intramuscular injections. Can Assoc Radiol J 58: 72–75. [PubMed] [Google Scholar]
- 12. Bliznak J, Staple TW (1975) Roentgenographic measurement of skin thickness in normal individuals. Radiology 116: 55–60. [DOI] [PubMed] [Google Scholar]
- 13. Laurent A, Mistretta F, Bottigioli D, Dahel K, Goujon C, et al. (2007) Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine 25: 6423–6430. [DOI] [PubMed] [Google Scholar]
- 14. Lee Y, Hwang K (2002) Skin thickness of Korean adults. Surg Radiol Anat 24: 183–189. [DOI] [PubMed] [Google Scholar]
- 15. Olsen LO, Takiwaki H, Serup J (1995) High-frequency ultrasound characterization of normal skin. Skin thickness and echographic density of 22 anatomical sites. Skin Res Technol 1: 74–80. [DOI] [PubMed] [Google Scholar]
- 16. Sandby-Moller J, Poulsen T, Wulf HC (2003) Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol 83: 410–413. [DOI] [PubMed] [Google Scholar]
- 17. Sjöström L, Smith U, Krotkiewski M, Björntorp P (1972) Cellularity in different regions of adipose tissue in young men and women. Metabolism 21: 1143–1153. [DOI] [PubMed] [Google Scholar]
- 18. Lo Presti D, Ingegnosi C, Strauss K (2012) Skin and subcutaneous thickness at injecting sites in children with diabetes: ultrasound findings and recommendations for giving injection. Pediatr Diabetes 13: 525–533. [DOI] [PubMed] [Google Scholar]
- 19. Seidenari S, Giusti G, Bertoni L, Magnoni C, Pellacani G (2000) Thickness and echogenicity of the skin in children as assessed by 20-MHz ultrasound. Dermatology 201: 218–222. [DOI] [PubMed] [Google Scholar]
- 20. Tan CY, Statham B, Marks R, Payne PA (1982) Skin thickness measurement by pulsed ultrasound: its reproducibility, validation and variability. Br J Dermatol 106: 657–667. [DOI] [PubMed] [Google Scholar]
- 21. Branchet MC, Boisnic S, Frances C, Robert AM (1990) Skin thickness changes in normal aging skin. Gerontology 36: 28–35. [DOI] [PubMed] [Google Scholar]
- 22. Levakov A, Vuckovic N, Dolai M, Kacanski MM, Bozanic S (2012) Age-related skin changes. Med Pregl 65: 191–195. [PubMed] [Google Scholar]
- 23. Petrofsky JS, McLellan K, Bains GS, Prowse M, Ethiraju G, et al. (2008) Skin heat dissipation: the influence of diabetes, skin thickness, and subcutaneous fat thickness. Diabetes Technol Ther 10: 487–493. [DOI] [PubMed] [Google Scholar]
- 24. Petrofsky JS, Prowse M, Lohman E (2008) The influence of ageing and diabetes on skin and subcutaneous fat thickness in different regions of the body. J Appl Res 8: 55. [Google Scholar]
- 25. Shuster SAM, Black MM, McVitie EVA (1975) The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol 93: 639–643. [DOI] [PubMed] [Google Scholar]
- 26. Ploin D, Schwarzenbach F, Dubray C, Nicolas JF, Goujon C, et al. (2011) Echographic measurement of skin thickness in sites suitable for intradermal vaccine injection in infants and children. Vaccine 29: 8438–8442. [DOI] [PubMed] [Google Scholar]
- 27. Smalls LK, Randall Wickett R, Visscher MO (2006) Effect of dermal thickness, tissue composition, and body site on skin biomechanical properties. Skin Res Technol 12: 43–49. [DOI] [PubMed] [Google Scholar]
- 28. Kivitz A, Segurado OG (2007) HUMIRA pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab. Expert Rev Med Devices 4: 109–116. [DOI] [PubMed] [Google Scholar]
- 29. Mitragotri S, Anissimov YG, Bunge AL, Frasch HF, Guy RH, et al. (2011) Mathematical models of skin permeability: an overview. Int J Pharm 418: 115–129. [DOI] [PubMed] [Google Scholar]
- 30. Kretsos K, Kasting GB, Nitsche JM (2004) Distributed diffusion-clearance model for transient drug distribution within the skin. J Pharm Sci 93: 2820–2835. [DOI] [PubMed] [Google Scholar]
- 31. Wilkinson SC, Maas WJ, Nielsen JB, Greaves LC, van de Sandt JJ, et al. (2006) Interactions of skin thickness and physicochemical properties of test compounds in percutaneous penetration studies. Int Arch Occup Environ Health 79: 405–413. [DOI] [PubMed] [Google Scholar]
- 32. Van Hattem S, Bootsma AH, Thio HB (2008) Skin manifestations of diabetes. Cleve Clin J Med 75: 772–787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The association of age with thickness of skin layers in children (n = 103) and adults (n = 140). Data for females are in red and for males in blue.
(PDF)