Abstract
Objective
We investigated the effects of antipsychotics on immune-challenged peripheral blood mononuclear cell (PBMC) cultures.
Methods
Blood samples were collected from twelve patients with first-episode schizophrenia. The PBMCs were separated and cultures were prepared and stimulated with lipopolysaccharide (LPS) or polyinosinic:polycytidylic acid (poly[I:C]), and then separately treated with a typical antipsychotic (haloperidol) or atypical antipsychotic (clozapine, quetiapine, or risperidone). Pro-inflammatory (interferon gamma [IFN-γ]) and anti-inflammatory (interleukin [IL]-4 and IL-10) cytokine levels were measured in the LPS- or poly(I:C)-stimulated PBMC cultures treated with antipsychotics.
Results
Haloperidol and quetiapine significantly increased the IL-4 levels (p<0.05) in LPS-stimulated PBMC cultures, while clozapine and quetiapine significantly enhanced the IL-4 levels (p<0.05) in poly(I:C)-stimulated PBMC cultures. Only treatment with haloperidol resulted in a significant increase in IL-10 production (p<0.05) in LPS-stimulated PBMC cultures, whereas clozapine, quetiapine, and risperidone treatment significantly increased IL-10 production (p<0.05) in poly(I:C)-stimulated PBMC cultures. All of the antipsychotics reduced the IFN-γ level significantly (p<0.05) in LPS- and poly(I:C)-stimulated PBMC cultures.
Conclusion
Antipsychotic treatment altered immune function by raising the levels of anti-inflammatory cytokines (IL-4 and IL-10) and suppressing the levels of pro-inflammatory cytokines (IFN-γ).
Keywords: Schizophrenia, Cytokines, Antipsychotic, Lipopolysaccharides
INTRODUCTION
The interaction of schizophrenia with the immune system has been investigated by various means. Previous research has associated schizophrenia with polymorphisms in genes that support immune function,1) prenatal infectious mechanisms,2) disrupted cytokine networks in adults,3,4) and changes in circulating peripheral immune cells.5) In patients with schizophrenia, there are increased numbers of immune cells, which travel through the bloodstream and migrate into peripheral tissues.5) For example, monocyte counts are higher in schizophrenic patients (SCP),6,7) and studies of leukocytes have reported unchanged, 8,9) increased,10,11) and decreased total T cell counts12) in similar populations. In addition to T cells, elevated numbers7) and the hyperfunction13) of B cells have been reported.
In SCP, alterations in cytokine concentrations,14) the number of cytokine receptors,15) and the amount of cytokine activity modifiers in peripheral blood and cerebrospinal fluid (CSF)15) have been reported. Cytokines and their receptors predominately control communication and cross-talk between the immune and central nervous systems, but are not well-regulated in SCP compared to healthy people, particularly in first-episode cases.16) Similarly, an imbalance in T-helper 1 (Th1) and T-helper 2 (Th2) immune responses in first-episode SCP has been reported.17) A decreased Th1 immune response results in the diminished release of interferon gamma (IFN-γ), while an enhanced Th2 response leads to increases in interleukin (IL)-4 and IL-10 release.18) In 2013, García-Bueno et al.19) collected blood samples from 117 first-episode SCP and stimulated peripheral blood mononuclear cell (PBMC) cultures to investigate pro- and anti-inflammatory cytokine dysregulation. They found increased levels of pro-inflammatory cytokines and decreased levels of anti-inflammatory cytokines in the cultures and suggested that the physiological balance of inflammatory pathways was disturbed in first-episode SCP. The authors emphasized the role of nuclear factor κB (NFκB) in this process and demonstrated that the activation of this factor leads to the release of pro-inflammatory components. In addition to cytokines, there are decreased concentrations of neopterin and increased concentrations of CD3+ and CD4+ cells in first-episode SCP. Moreover, there is an altered ratio of CD4+/CD8+ cells in SCP, which is more pronounced in first-episode cases.
Various cytokines have been studied in connection with schizophrenia, but the findings are inconsistent. For example, studies have shown increased levels of IL-6 and tumor necrosis factor (TNF)-α8,20-23) but decreased levels of IL-224,25) in SCP, while others have found no change26,27) in cytokine levels. Moreover, there is contradictory evidence concerning anti-inflammatory cytokines such as IFN-γ, IL-4, and IL-10. Cell cultures from healthy individuals have been found to have decreased levels of IL-427) or to exhibit no difference.28) In 1994, Inglot and co-workers29) suggested a connection between IFN-γ concentrations and the psychopathology of schizophrenia. They found patients with positive symptoms exhibit elevated production of IFN-γ and negative symptoms are associated with decreased IFN-γ production. In contrast, other studies have found increased levels of IFN-γ in the serum and plasma of SCP27,28,30) but decreased levels of IFN-γ in whole blood cell cultures.9,31) Non-paranoid SCP exhibit increased levels of IL-10 relative to healthy controls32) but other evidence does not support this finding.33) However, a strong positive correlation between the severity of negative symptoms and the concentration of IL-10 in CSF has been demonstrated. A shift from Th1 immunity to Th2 immunity has been proposed as a pathophysiological mechanism underlying schizophrenia34,35) and would be indicated by a lower IFN-γ/IL-4 ratio.28) However, other studies have found a higher ratio in SCP. This suggests that the underlying pathology of schizophrenia involves disturbances in the balance between proand anti-inflammatory cytokines rather than a directional shift in the number of Th1 and Th2 cells. Alternatively, this shift could result from treatment with antipsychotics.36) Accordingly, it has recently been demonstrated that both typical and atypical antipsychotic drugs influence the production of cytokines.20)
Haloperidol is a typical antipsychotic. In PBMC cultures, haloperidol has been shown to normalize increased IL-2 levels37) and inhibit mitogen-stimulated IL-2 production,38,39) but inconsistent results have been found concerning the production of IFN-γ. Haloperidol may stimulate40) or inhibit38,39) IFN-γ in in vitro cell cultures. Moreover, haloperidol administration was shown to increase the level of IL-10 in PBMC cultures,39) but no changes were found in lipopolysaccharide (LPS)-treated mice.41)
Clozapine, risperidone, and quetiapine are common atypical antipsychotics. In 1994, the influence of clozapine on IL-2 was shown to be modulated by increases in the concentration of soluble IL-2 receptors;42) this finding was later confirmed by several other studies.43,44) No consistent results have been found regarding the immunomodulatory effects of risperidone, but a recent study investigated the beneficial effects of risperidone on IFN-γ-stimulated microglia.45) However, risperidone has also been shown to inhibit the production of pro-inflammatory cytokines such as TNF-α and IL-646) and to increase anti-inflammatory cytokines such as IL-1041) but not affect the plasma IL-4 concentration47) in SCP. There is no clear evidence regarding the effects of quetiapine on immunomodulation in SCP, but one study reported that the drug reduced IL-2 production.48)
The present study analyzed pro- and anti-inflammatory cytokine levels in cultured PBMCs from untreated first-episode SCP because this population typically produces high concentrations of cytokines. Moreover, the stimulation of PBMC cultures by LPS or polyinosinic:polycytidylic acid (poly[I:C]) leads to the release of various inflammatory cytokines and chemokines. Therefore, the immunomodulatory effects of typical (haloperidol) and atypical (clozapine, risperidone, and quetiapine) antipsychotic drugs could be observed in a situation that closely mimics natural circumstances. Typical and atypical antipsychotics were selected due to their negligible side effects and popularity for the treatment of schizophrenia. The concentrations of IFN-γ (a pro-inflammatory cytokine) and IL-4 and IL-10 (anti-inflammatory cytokines) were measured because these cytokines are consistently associated with schizophrenia.
LPS is a bacterial endotoxin found in the outer membrane of Gram-negative bacteria. It is primarily detected by its specific receptor, toll-like receptor 4 (TLR-4), leading to the production of several cytokines and chemokines. Poly(I:C) is a synthetic analog of double-stranded RNA, which is produced during the replication of RNA and DNA viruses.49) It is mainly detected by endosomally localized TLR-3 when added to culture medium,50) but the poly(I:C)-induced immune response is non-specific, meaning that it stimulates the production of inflammatory cytokines rather than particular anti-viral antibodies. LPS and poly(I:C) are a cost-effective means of inducing the short-term stimulation of PBMC cultures, which can be used to analyze the cytokine profile of schizophrenics. The effects of poly(I:C) last for approximately 48 hours.
METHODS
Subjects
Blood samples were collected from 12 first-episode SCP (6 females, 6 males; age range, 19-62 years; mean age, 34.08±13.39 years) to analyze the production of cytokines. All subjects provided written consent and the experimental procedure was previously approved by the ethics committee of the Department of Pharmacy at North South University in Dhaka, Bangladesh. Subjects were excluded on the basis of the following criteria: a) a past or present history of psychiatric disorders, b) use of major psychotropic medications such as antidepressants and antipsychotics, c) drug and/or alcohol abuse or dependence, d) any medical (e.g., endocrine, immune, or metabolic) disorder such as diabetes, autoimmune disorders, inflammatory bowel disease, or acquired immunodeficiency syndrome, or e) current (2 weeks prior to the first blood sample) diagnosis of an infectious, allergic, or inflammatory response. The subjects abstained from caffeine, alcohol, and nicotine for at least 8 hours prior to blood sampling and were asked to fast overnight.
Blood Collection, PBMC Separation, and Culture Preparation
Venous blood (18 ml) from first-episode SCP was collected in heparinized tubes at approximately 8:00 AM. The samples were diluted (1:1) with sterile phosphate-buffered saline, layered over Ficoll-Hypaque (GE Healthcare, Little Chalfont, UK), and centrifuged at 1,500 rpm for 30 minutes at room temperature. The interphase layer was withdrawn and the isolated PBMCs were incubated in RPMI medium-1640 (R-8005; Sigma, St. Louis, MO, USA) containing 1% penicillin (Sigma) with L-glutamine and Phenol Red in microtitration plates at a concentration of 106 cells per well. The samples were incubated for 72 hours in a humidified atmosphere at 37℃ with 5% CO2 to obtain peak cumulative responses for most cytokines. The plates were centrifuged at 1,500 rpm for 8 minutes following incubation. The supernatants were carefully removed under sterile conditions, divided into Eppendorf tubes (Eppendorf India Ltd., Dattawadi, India), and immediately frozen at -70℃ until thawed for assay.
Immune Challenge and the Addition of Antipsychotic Medications
LPS and poly(I:C) (1 mg/ml) were added to PBMC cultures for bacterial and viral stimulation, respectively. In the untreated antipsychotic condition, 75µl of PBMC culture was combined with 225µl of stimulant medium (LPS or poly[I:C]) for a final volume of 300µl. The final volume was placed in 24-well sterile plates. In the antipsychotic treatment condition, 10µl of antipsychotic drug (haloperidol, clozapine, quetiapine, or risperidone) and 75µl of PBMC culture was combined with 215µl of stimulant medium (LPS or poly[I:C]) for a final volume 300µl. All antipsychotic drugs were obtained from a local pharmaceutical company in Bangladesh and given in concentrations of 1 mg/ml. The cytokine (IL-4, IL-10, and IFN-γ) concentrations in the PBMC culture supernatants were quantified using enzyme-linked immunosorbent assays. The intra-assay coefficient of variation was less than 8%, and the limits of detection were as follows: IL-10, 10 pg/ml; IFN-γ, 1.03 pg/ml; and IL-4, 0.39 pg/ml.
Statistical Analysis
To investigate the effects of antipsychotic drugs on cytokine (IL-4, IL-10, and IFN-γ) levels in immune-stimulated PBMC cultures, a repeated measure analysis of variance (ANOVA) was performed. The ANOVA was used to analyze: 1) within-subject variability associated with the effects of antipsychotic drugs and/or the effects of LPS or poly(I:C) treatment as a temporal condition, and 2) between-subject variability with sex as a factor. Differences were considered significant at p<0.05. All data are presented as mean±standard error of the mean. SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA) was used in all analyses.
RESULTS
Effect of Antipsychotics on the IL-4 Level in LPS- and Poly(I:C)-stimulated PBMC Cultures
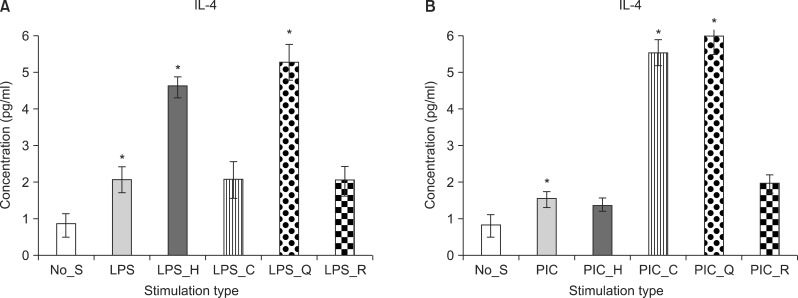
Both haloperidol (F[1, 9]=7.98, p<0.05) and quetiapine (F[1, 9]=7.86, p<0.05) significantly increased the level of IL-4 in LPS-stimulated PBMC cultures (Fig. 1A). In the poly(I:C)-stimulated PBMC cultures, clozapine (F[1, 9]=13.89, p<0.05) and quetiapine (F[1, 9]=6.73, p<0.05) significantly enhanced the level of IL-4 (Fig. 1B).
Fig. 1.
Mean value of interleukin 4 (IL-4) level in the peripheral blood mononuclear cell culture. Stimulation by LPS (A) and stimulation by PIC (B). No_S, no stimulation; LPS, lipopolysaccaride; PIC, polyinosinic:polycytidylic acid; H, haloperidol; C, clozapine; Q, quetiaine; R, risperidone. *p<0.05; mean±standard error of mean.
Effect of Antipsychotics on the IL-10 Level in LPS- and Poly(I:C)-stimulated PBMC Cultures
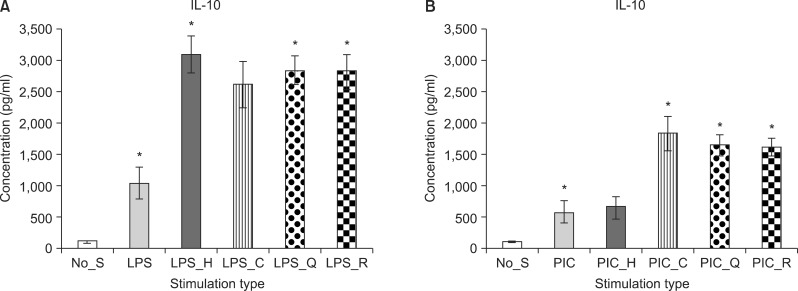
Haloperidol (F[1, 9]=5.37, p<0.05), quetiapine (F[1, 9]=5.32, p<0.05), and risperidone (F[1, 9]=4.68, p<0.05) treatment significantly increased the production of IL-10 in the LPS-stimulated PBMC cultures (Fig. 2A). In the poly(I:C)-stimulated PBMC cultures, clozapine (F[1, 9]=12.68, p<0.05), quetiapine (F[1, 9]=11.55, p<0.05), and risperidone (F[1, 9]=54.9, p<0.05) treatment resulted in significantly enhanced IL-10 production (Fig. 2B).
Fig. 2.
Mean value of interleukin 10 (IL-10) level in the peripheral blood mononuclear cell culture. Stimulation by LPS (A) and stimulation by PIC (B). No_S, no stimulation; LPS, lipopolysaccaride; PIC, polyinosinic:polycytidylic acid; H, haloperidol; C, clozapine; Q, quetiaine; R, risperidone. *p<0.05; mean±standard error of mean.
Effect of Antipsychotics on the IFN-γ Level in LPS- and poly(I:C)-stimulated PBMC Cultures
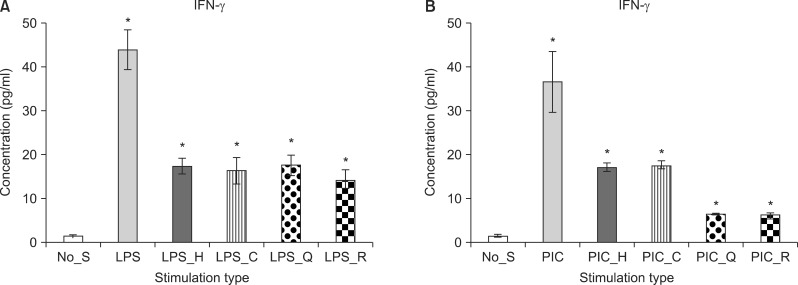
Haloperidol (F[1, 9]=20.06, p=0.002), clozapine (F[1, 9]=29.40, p<0.05), quetiapine (F[1, 9]=14.98, p<0.05), and risperidone (F[1, 9]=23.33, p<0.05) significantly reduced IFN-γ production in the LPS-stimulated PBMC cultures. Likewise, in the poly(I:C)-stimulated PBMC cultures, haloperidol (F[1, 9]=44.45, p<0.05), clozapine (F[1, 9]=16.93, p<0.05), quetiapine (F[1, 9]=65.04, p<0.05), and risperidone (F[1, 9]=10.28, p<0.05) significantly reduced the production of IFN-γ (Fig. 3).
Fig. 3.
Mean value of interferon gamma (IFN-γ) level in the peripheral blood mononuclear cell culture. Stimulation by LPS (A) and stimulation by PIC (B). No_S, no stimulation; LPS, lipopolysaccaride; PIC, polyinosinic:polycytidylic acid; H, haloperidol; C, clozapine; Q, quetiaine; R, risperidone. *p<0.05; mean±standard error of mean.
DISCUSSION
The stimulation of cultured PBMCs from first-episode SCP by LPS and poly(I:C) resulted in the altered production of pro- and anti-inflammatory cytokines. Additionally, cytokine levels were affected by treatment with antipsychotics. The use of cultured PBMCs may better reflect the inflammatory process than the assessment of serum cytokine levels. The production of IFN-γ, IL-4, and IL-10 have not been reported by previous studies of PBMC cultures from first-episode SCP. LPS and poly(I:C) are agents that stimulate immune cells. The stimulation of PBMCs by LPS leads to binding with TLR-4 and the subsequent release of cytokines and other inflammatory mediators, whereas stimulation by poly(I:C) leads to binding with TLR-3 and cytokine release. LPS and poly(I:C) both initiate nuclear localization of the transcription factor NFκB and the subsequent activation of genes in pro-inflammatory pathways. Two important conclusions may be drawn from this study. First, antipsychotics enhance the production of anti-inflammatory cytokines (IL-4 and IL-10) in LPS- and poly(I:C)-stimulated PBMC cultures, and, second, antipsychotics reduce pro-inflammatory cytokines (IFN-γ) in LPS- and poly(I:C)-stimulated PBMC cultures.
The findings of the present study are consistent with those of previous studies. For example, increased levels of IL-10 in cultured PBMCs have been found following haloperidol treatment,39) and a robust increase in IL-10 serum levels induced by clozapine has been reported.41) There are no consistent findings regarding the immunomodulatory effects of risperidone. Some studies found that risperidone decreased plasma levels of IFN-γ,37,46,47) while other studies reported no differences.37,47) A recent study investigating the beneficial effects of risperidone on IFN-γ-stimulated microglia found that it inhibits the production of nitric oxide (NO) and other pro-inflammatory cytokines, including IFN-γ and IL-6.45) Furthermore, increased levels of IL-10 and unchanged levels of IL-4 have been observed in the plasma of SCP.47) The treatment of in vitro cell cultures has been shown to either stimulate40) or inhibit38,39) IFN-γ, and a recent study reported a weak effect on the inhibition of harmful NO by IFN-γ-activated microglia.45) Haloperidol seems to normalize increased IL-2 plasma and serum levels37) and inhibit mitogen-stimulated IL-2 production in PBMCs.38,39) The effects of haloperidol are particularly evident in patients with positive symptoms.51) Haloperidol lowers TNF-α production in LPS-stimulated monocytes,52) but does not affect the IL-6 concentration in serum, plasma,37,53,54) or CSF55) from SCP.
In this study, only one concentration was used for all antipsychotic drugs. This concentration was identified by a review of previous literature56) and was chosen on the basis of bioavailability following oral administration. Different concentrations of antipsychotic drugs should be employed in future trials to better evaluate changes in the levels of anti-inflammatory cytokines in stimulated PBMC cultures.
An important consideration of this study was to not include healthy volunteers because this population is not as vulnerable to changes in the cytokine system following LPS and poly(I:C) stimulation as are first-episode SCP. However, we previously conducted an experiment in which PBMC cultures were prepared from the blood of healthy volunteers and the same cytokines (IFN-γ, IL-4, and IL-10) were measured.57) Several major differences were observed between the PBMC cultures of healthy subjects and first-episode SCP. First, LPS and poly(I:C) caused an increase in pro-inflammatory cytokine levels and a decrease in anti-inflammatory cytokine levels (IL-4 and IL-10) in first-episode SCP compared to healthy volunteers, consistent with previous studies. Second, both typical and atypical antipsychotics alter immune function via the suppression of pro-inflammatory cytokine levels (IFN-γ) and the elevation of anti-inflammatory cytokines (IL-10).57) Third, antipsychotics do not influence IL-4 concentrations in healthy volunteers. In first-episode SCP, however, antipsychotics cause an increase in IL-4 release.
There are a number of limitations to this study. First, the present study utilized a small sample size. A larger number of patient samples would provide more opportunity to analyze the parameters of this group. Second, only three inflammatory cytokines were considered because this study was backed by personal finance. TNF-α, IL-2, IL-6, and other important inflammatory cytokines should also be analyzed to better explore the consequences of first-episode schizophrenia. Third, the limited resources and facilities of research laboratories in our country make it very challenging to conduct this type of cell culture research. Finally, in some instances, the IL-4 levels were not similarly increased in LPS- and poly(I:C)-treated cultures. This may be due to the short half-life of cytokines. In conjunction with previous findings, the present results suggest that antipsychotics have a positive influence on proand anti-inflammatory cytokines in first-episode SCP.
The role of the cytokine system in the pathogenesis of schizophrenia is well-established. Here, we demonstrated that antipsychotic agents might provide benefits in terms of the imbalance of peripheral pro- and anti-inflammatory cytokine levels in schizophrenic subjects. These data should be confirmed through an analysis of a large number of first-episode SCP and the measurement of additional cytokines.
References
- 1.Hänninen K, Katila H, Saarela M, Rontu R, Mattila KM, Fan M, et al. Interleukin-1 beta gene polymorphism and its interactions with neuregulin-1 gene polymorphism are associated with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258:10–15. doi: 10.1007/s00406-007-0756-9. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G, Maes M. Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:5–19. doi: 10.1016/j.pnpbp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun. 2006;20:532–545. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Karanikas EP. Psycho-immunological mechanisms in schizophrenia. Psychiatrike. 2011;22:43–52. [PubMed] [Google Scholar]
- 5.Gardiner EJ, Cairns MJ, Liu B, Beveridge NJ, Carr V, Kelly B, et al. Gene expression analysis reveals schizophrenia-associated dysregulation of immune pathways in peripheral blood mononuclear cells. J Psychiatr Res. 2013;47:425–437. doi: 10.1016/j.jpsychires.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothermundt M, Arolt V, Weitzsch C, Eckhoff D, Kirchner H. Immunological dysfunction in schizophrenia: a systematic approach. Neuropsychobiology. 1998;37:186–193. doi: 10.1159/000026501. [DOI] [PubMed] [Google Scholar]
- 7.Zorrilla EP, Cannon TD, Gur RE, Kessler J. Leukocytes and organ-nonspecific autoantibodies in schizophrenics and their siblings: markers of vulnerability or disease? Biol Psychiatry. 1996;40:825–833. doi: 10.1016/0006-3223(95)00598-6. [DOI] [PubMed] [Google Scholar]
- 8.Theodoropoulou S, Spanakos G, Baxevanis CN, Economou M, Gritzapis AD, Papamichail MP, et al. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res. 2001;47:13–25. doi: 10.1016/s0920-9964(00)00007-4. [DOI] [PubMed] [Google Scholar]
- 9.Wilke I, Arolt V, Rothermundt M, Weitzsch C, Hornberg M, Kirchner H. Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1996;246:279–284. doi: 10.1007/BF02190280. [DOI] [PubMed] [Google Scholar]
- 10.Maino K, Gruber R, Riedel M, Seitz N, Schwarz M, Müller N. T- and B-lymphocytes in patients with schizophrenia in acute psychotic episode and the course of the treatment. Psychiatry Res. 2007;152:173–180. doi: 10.1016/j.psychres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Craddock RM, Lockstone HE, Rider DA, Wayland MT, Harris LJ, McKenna PJ, et al. Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS One. 2007;2:e692. doi: 10.1371/journal.pone.0000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiner J, Jacobs R, Panteli B, Brauner M, Schiltz K, Bahn S, et al. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260:509–518. doi: 10.1007/s00406-010-0098-x. [DOI] [PubMed] [Google Scholar]
- 13.Mach DM, Schütt C, Börner I. Schizophrenia and B-lymphocyte alteration-a hypothesis. Psychiatr Neurol Med Psychol (Leipz) 1983;35:390–397. [PubMed] [Google Scholar]
- 14.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barak V, Barak Y, Levine J, Nisman B, Roisman I. Changes in interleukin-1 beta and soluble interleukin-2 receptor levels in CSF and serum of schizophrenic patients. J Basic Clin Physiol Pharmacol. 1995;6:61–69. doi: 10.1515/jbcpp.1995.6.1.61. [DOI] [PubMed] [Google Scholar]
- 16.Di Nicola M, Cattaneo A, Hepgul N, Di Forti M, Aitchison KJ, Janiri L, et al. Serum and gene expression profile of cytokines in first-episode psychosis. Brain Behav Immun. 2013;31:90–95. doi: 10.1016/j.bbi.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller M, Schwarz MJ. Immune system and schizophrenia. Curr Immunol Rev. 2010;6:213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang SSW, Riedel M, Gruber R, Müller N, Schwarz M. In vivo type II T-helper cells shift in schizophrenia compared to sex- and age-matched healthy controls. Eur J Psychiat. 2011;25:192–204. [Google Scholar]
- 19.García-Bueno B, Bioque M, Mac-Dowell KS, Barcones MF, Martínez-Cengotitabengoa M, Pina-Camacho L, et al. Pro-/Anti-inflammatory dysregulation in patients with first episode of psychosis toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maes M, Bosmans E, Calabrese J, Smith R, Meltzer HY. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J Psychiatr Res. 1995;29:141–152. doi: 10.1016/0022-3956(94)00049-w. [DOI] [PubMed] [Google Scholar]
- 21.Lin A, Kenis G, Bignotti S, Tura GJ, De Jong R, Bosmans E, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res. 1998;32:9–15. doi: 10.1016/s0920-9964(98)00034-6. [DOI] [PubMed] [Google Scholar]
- 22.Naudin J, Capo C, Giusano B, Mège JL, Azorin JM. A differential role for interleukin-6 and tumor necrosis factor-alpha in schizophrenia? Schizophr Res. 1997;26:227–233. doi: 10.1016/s0920-9964(97)00059-5. [DOI] [PubMed] [Google Scholar]
- 23. O'Brien SM, Scully P, Dinan TG. Increased tumor necrosis factor-alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res. 2008;160:256–262. doi: 10.1016/j.psychres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Arolt V, Rothermundt M, Wandinger KP, Kirchner H. Decreased in vitro production of interferon-gamma and interleukin-2 in whole blood of patients with schizophrenia during treatment. Mol Psychiatry. 2000;5:150–158. doi: 10.1038/sj.mp.4000650. [DOI] [PubMed] [Google Scholar]
- 25.Bessler H, Levental Z, Karp L, Modai I, Djaldetti M, Weizman A. Cytokine production in drug-free and neuroleptic-treated schizophrenic patients. Biol Psychiatry. 1995;38:297–302. doi: 10.1016/0006-3223(94)00299-I. [DOI] [PubMed] [Google Scholar]
- 26.Monteleone P, Fabrazzo M, Tortorella A, Maj M. Plasma levels of interleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment. Psychiatry Res. 1997;71:11–17. doi: 10.1016/s0165-1781(97)00036-x. [DOI] [PubMed] [Google Scholar]
- 27.Kamińska T, Wysocka A, Marmurowska-Michalowska H, Dubas-Slemp H, Kandefer-Szerszeń M. Investigation of serum cytokine levels and cytokine production in whole blood cultures of paranoid schizophrenic patients. Arch Immunol Ther Exp (Warsz) 2001;49:439–445. [PubMed] [Google Scholar]
- 28.Avgustin B, Wraber B, Tavcar R. Increased Th1 and Th2 immune reactivity with relative Th2 dominance in patients with acute exacerbation of schizophrenia. Croat Med J. 2005;46:268–274. [PubMed] [Google Scholar]
- 29.Inglot AD, Leszek J, Piasecki E, Sypula A. Interferon responses in schizophrenia and major depressive disorders. Biol Psychiatry. 1994;35:464–473. doi: 10.1016/0006-3223(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 30.Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ, et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1129–1134. doi: 10.1016/j.pnpbp.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 31.Rothermundt M, Arolt V, Weitzsch C, Eckhoff D, Kirchner H. Production of cytokines in acute schizophrenic psychosis. Biol Psychiatry. 1996;40:1294–1297. doi: 10.1016/S0006-3223(96)00360-5. [DOI] [PubMed] [Google Scholar]
- 32.Cazzullo CL, Scarone S, Grassi B, Vismara C, Trabattoni D, Clerici M, et al. Cytokines production in chronic schizophrenia patients with or without paranoid behaviour. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:947–957. doi: 10.1016/s0278-5846(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 33.Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol. 1997;159:2994–2999. [PubMed] [Google Scholar]
- 34.Schwarz MJ, Chiang S, Müller N, Ackenheil M. T-helper-1 and T-helper-2 responses in psychiatric disorders. Brain Behav Immun. 2001;15:340–370. doi: 10.1006/brbi.2001.0647. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz MJ, Müller N, Riedel M, Ackenheil M. The Th2-hypothesis of schizophrenia: a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses. 2001;56:483–486. doi: 10.1054/mehy.2000.1203. [DOI] [PubMed] [Google Scholar]
- 36.Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medication-naïve and medication-free schizophrenic patients. Neuropsychobiology. 2009;59:123–129. doi: 10.1159/000213565. [DOI] [PubMed] [Google Scholar]
- 37.Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry. 2004;65:940–947. doi: 10.4088/jcp.v65n0710. [DOI] [PubMed] [Google Scholar]
- 38.Leykin I, Mayer R, Shinitzky M. Short and long-term immunosuppressive effects of clozapine and haloperidol. Immunopharmacology. 1997;37:75–86. doi: 10.1016/s0162-3109(97)00037-4. [DOI] [PubMed] [Google Scholar]
- 39.Szuster-Ciesielska A, Słotwińska M, Stachura A, Marmurowska-Michałowska H, Kandefer-Szerszeń M. Neuroleptics modulate cytokine and reactive oxygen species production in blood leukocytes of healthy volunteers. Arch Immunol Ther Exp (Warsz) 2004;52:59–67. [PubMed] [Google Scholar]
- 40.Rudolf S, Peters M, Rothermundt M, Arolt V, Kirchner H. The influence of typical and atypical neuroleptic drugs in the production of interleukin-2 and interferon-gamma in vitro. Neuropsychobiology. 2002;46:180–185. doi: 10.1159/000067807. [DOI] [PubMed] [Google Scholar]
- 41.Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:303–307. doi: 10.1016/j.pnpbp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Maes M, Meltzer HY, Bosmans E. Immune-inflammatory markers in schizophrenia: comparison to normal controls and effects of clozapine. Acta Psychiatr Scand. 1994;89:346–351. doi: 10.1111/j.1600-0447.1994.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 43.Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kühn M, Schuld A, et al. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33:407–418. doi: 10.1016/s0022-3956(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 44.Pollmächer T, Hinze-Selch D, Mullington J, Holsboer F. Clozapine-induced increase in plasma levels of soluble interleukin-2 receptors. Arch Gen Psychiatry. 1995;52:877–878. doi: 10.1001/archpsyc.1995.03950220087016. [DOI] [PubMed] [Google Scholar]
- 45.Kato T, Monji A, Hashioka S, Kanba S. Risperidone significantly inhibits interferon-gamma-induced microglial activation in vitro. Schizophr Res. 2007;92:108–115. doi: 10.1016/j.schres.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Lü LX, Guo SQ, Chen W, Li Q, Cheng J, Guo JH. Effect of clozapine and risperidone on serum cytokine levels in patients with first-episode paranoid schizophrenia. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:1251–1254. [PubMed] [Google Scholar]
- 47.Cazzullo CL, Sacchetti E, Galluzzo A, Panariello A, Adorni A, Pegoraro M, et al. Cytokine profiles in schizophrenic patients treated with risperidone: a 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:33–39. doi: 10.1016/s0278-5846(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 48.Himmerich H, Schönherr J, Fulda S, Sheldrick AJ, Bauer K, Sack U. Impact of antipsychotics on cytokine production in-vitro. J Psychiatr Res. 2011;45:1358–1365. doi: 10.1016/j.jpsychires.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Reimer T, Brcic M, Schweizer M, Jungi TW. poly(I:C) and LPS induce distinct IRF3 and NF-kappaB signaling during type-I IFN and TNF responses in human macrophages. J Leukoc Biol. 2008;83:1249–1257. doi: 10.1189/jlb.0607412. [DOI] [PubMed] [Google Scholar]
- 50.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowalski J, Blada P, Kucia K, Lawniczek T, Madej A, Belowski D, et al. In-vitro immunomodulatory effects of haloperidol and perazine in schizophrenia. World J Biol Psychiatry. 2000;1:190–196. doi: 10.3109/15622970009150591. [DOI] [PubMed] [Google Scholar]
- 52.Kowalski J, Blada P, Kucia K, Madej A, Herman ZS. Neuroleptics normalize increased release of interleukin- 1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophr Res. 2001;50:169–175. doi: 10.1016/s0920-9964(00)00156-0. [DOI] [PubMed] [Google Scholar]
- 53.Kim YK, Kim L, Lee MS. Relationships between interleukins, neurotransmitters and psychopathology in drug-free male schizophrenics. Schizophr Res. 2000;44:165–175. doi: 10.1016/s0920-9964(99)00171-1. [DOI] [PubMed] [Google Scholar]
- 54.Pollmächer T, Hinze-Selch D, Fenzel T, Kraus T, Schuld A, Mullington J. Plasma levels of cytokines and soluble cytokine receptors during treatment with haloperidol. Am J Psychiatry. 1997;154:1763–1765. doi: 10.1176/ajp.154.12.1763. [DOI] [PubMed] [Google Scholar]
- 55.Yao JK, Sistilli CG, van Kammen DP. Membrane polyunsaturated fatty acids and CSF cytokines in patients with schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:429–436. doi: 10.1016/j.plefa.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 56.Song C, Lin Ah, Kenis G, Bosmans E, Maes M. Immunosuppressive effects of clozapine and haloperidol: enhanced production of the interleukin-1 receptor antagonist. Schizophr Res. 2000;42:157–164. doi: 10.1016/s0920-9964(99)00116-4. [DOI] [PubMed] [Google Scholar]
- 57.Al-Amin MM. Effect of different antipsychotics on cytokine production after immunologically stimulated pbmc culture. Int Neuropsychiatr Dis J. 2013;1:35–45. [Google Scholar]