Abstract
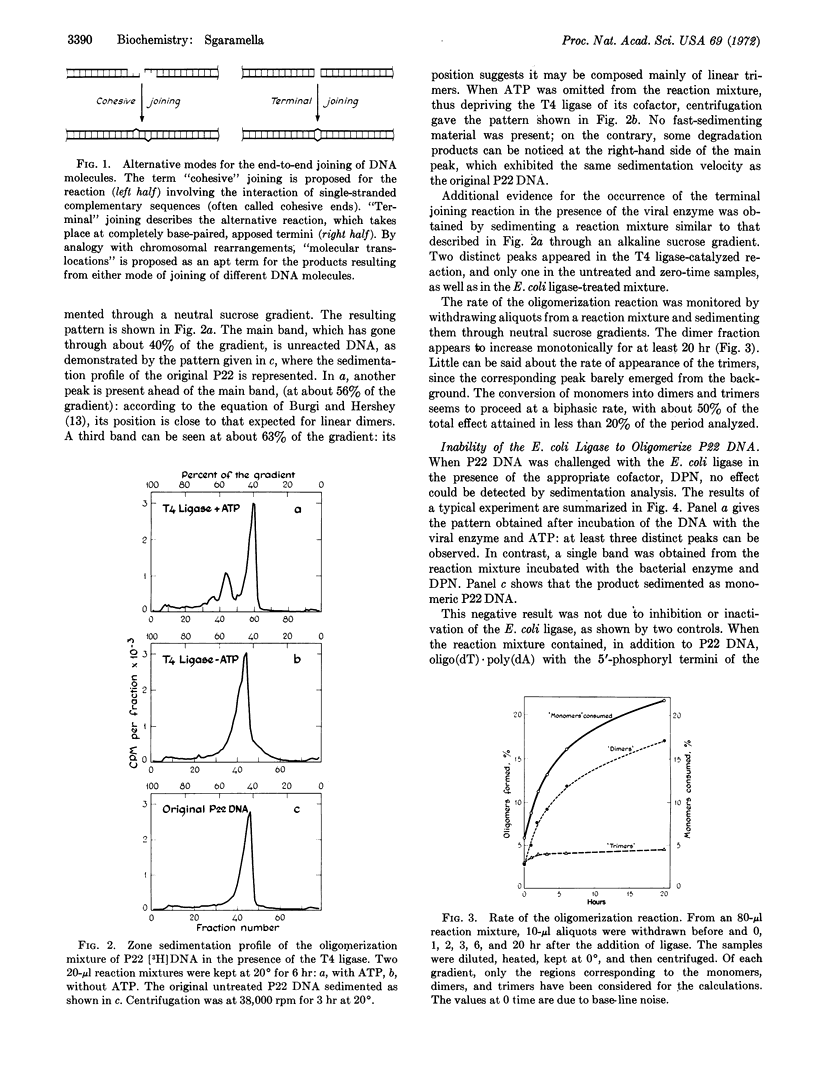
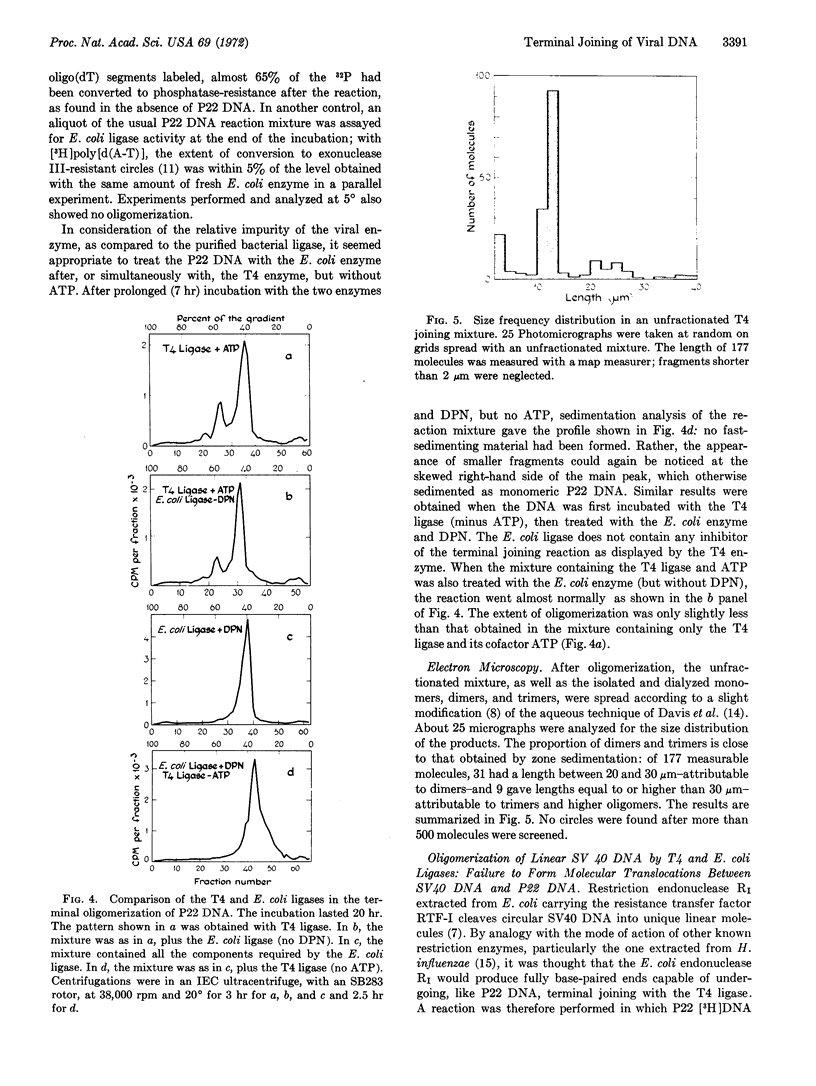
Linear double-stranded molecules of the circularly permuted and terminally redundant DNA of Salmonella bacteriophage P22 have been converted to oligomeric products in the presence of polynucleotide ligase coded for by the coliphage T4. The reaction has been monitored by sucrose density-gradient centrifugation and electron microscopy. It goes slowly and gives yields of 30-40%. The products are mainly dimers and trimers, but higher oligomers are also present.
DNA ligase extracted from uninfected Escherichia coli seems unable to perform a similar reaction, which is concluded to involve the fully base-paired termini.
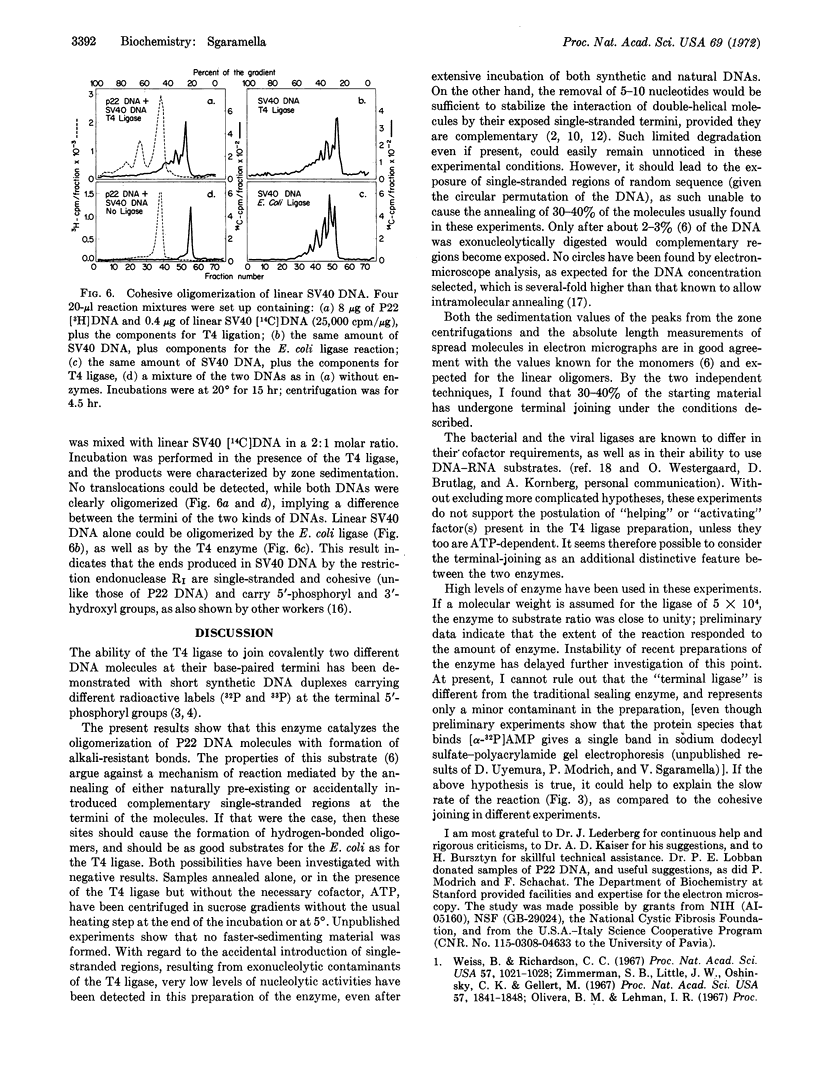
Linear double-stranded molecules of simian virus(SV) 40 DNA, produced by the action of the bacterial restriction endonuclease R1, are oligomerized by either ligase; therefore, this reaction seems to involve single-stranded cohesive ends. No mixed products could be found when P22 DNA and linear SV 40 DNA were exposed together to the T4 ligase.
Keywords: T4 and E. coli ligase, terminal and cohesive joining, zone sedimentation, electron microscopy
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Büchi H., Caruthers M. H., Gupta N., Khorana H. G., Kleppe K., Kumar A., Ohtsuka E., Rajbhandary U. L., Van de Sande J. H. Total synthesis of the gene for an alanine transfer ribonucleic acid from yeast. Nature. 1970 Jul 4;227(5253):27–34. doi: 10.1038/227027a0. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Melechen N. E., Jovin T. M., Kornberg A. Polynucleotide cellulose as a substrate for a polynucleotide ligase induced by phage T4. Biochem Biophys Res Commun. 1967 Aug 23;28(4):578–586. doi: 10.1016/0006-291x(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Wilt E. M., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. 8. Hybrids of ribo- and deoxyribonucleotide homopolymers as substrates for polynucleotide ligase of bacteriophage T4. J Biol Chem. 1971 Feb 25;246(4):925–932. [PubMed] [Google Scholar]
- Gefter M. L., Becker A., Hurwitz J. The enzymatic repair of DNA. I. Formation of circular lambda-DNA. Proc Natl Acad Sci U S A. 1967 Jul;58(1):240–247. doi: 10.1073/pnas.58.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N. K., Ohtsuka E., Weber H., Chang S. H., Khorana H. G. Studies on polynucleotides. LXXXVII. The joining of short deoxyribopolynucleotides by DNA-joining enzymes. Proc Natl Acad Sci U S A. 1968 May;60(1):285–292. doi: 10.1073/pnas.60.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Symons R. H., Berg P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2904–2909. doi: 10.1073/pnas.69.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Kleppe K., Van de Sande J. H., Khorana H. G. Polynucleotide ligase-catalyzed joining of deoxyribo-oligonucleotides on ribopolynucleotide templates and of ribo-oligonucleotides on deoxyribopolynucleotide templates. Proc Natl Acad Sci U S A. 1970 Sep;67(1):68–73. doi: 10.1073/pnas.67.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic joining of polynucleotides. IX. A simple and rapid assay of polynucleotide joining (ligase) activity by measurement of circle formation from linear deoxyadenylate-deoxythymidylate copolymer. J Biol Chem. 1970 Jul 25;245(14):3626–3631. [PubMed] [Google Scholar]
- Sgaramella V., Van de Sande J. H., Khorana H. G. Studies on polynucleotides, C. A novel joining reaction catalyzed by the T4-polynucleotide ligase. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1468–1475. doi: 10.1073/pnas.67.3.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. A., Jr, Kelly T. J., Jr, Rhoades M. The intracellular forms of T7 and P22 DNA molecules. Cold Spring Harb Symp Quant Biol. 1968;33:417–424. doi: 10.1101/sqb.1968.033.01.048. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Davidson N. Cyclization of phage DNAs. Cold Spring Harb Symp Quant Biol. 1968;33:409–415. doi: 10.1101/sqb.1968.033.01.047. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Little J. W., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands: a novel reaction of diphosphopyridine nucleotide. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1841–1848. doi: 10.1073/pnas.57.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]



