Abstract
Background
The decline in cellular immunity with ageing is of considerable public health importance. Recent studies suggest that the redox equilibrium of dendritic cells (DC) is a key factor in maintaining protective cellular immunity and that a disturbance of this homeostatic mechanism could contribute to immune senescence.
Objectives
(i) To elucidate the role of DC redox equilibrium in the decline of contact hypersensitivity (CHS) and Th1 immunity during ageing; (ii) To determine how restoration of glutathione (GSH) levels by the Nrf2-mediated antioxidant defense pathway impacts this decline.
Methods
We assessed the effect of Nrf2 deficiency and boosting of GSH levels by the Nrf2 agonist, sulforaphane (SFN), or the thiol precursor, N-acetyl cysteine (NAC), on the CHS response to contact antigens in old mice. We studied the effect of SFN and NAC on restoring Th1 immunity by treating DC ex vivo before adoptive transfer and in vivo challenge.
Results
Ageing was associated with a decreased CHS response that was accentuated by Nrf2 deficiency. Systemic SFN treatment reversed this decline through Nrf2-mediated antioxidant enzyme expression and GSH synthesis. Adoptive transfer of DC from old animals induced a weakened CHS response in recipient animals. Treatment of DC from old animals with SFN or NAC ex vivo restored the in vivo challenge response.
Conclusion
SFN and NAC up-regulate Th1 immunity in ageing through a restoration of redox equilibrium.
Clinical Significance
Restoration of the redox equilibrium in the immune system could restore or delay the decline of cellular immunity with ageing.
Keywords: Ageing, redox equilibrium, cellular immunity, dendritic cells, Nrf2, glutathione, NAC, sulforaphane
INTRODUCTION
Immune senescence is an important topic from the perspective of ageing demographics and the associated increase in infectious disease episodes. While functional changes in cellular immunity such as a decline of naïve T-cells, aberrant signal transduction by lymphocyte antigen receptors, and a change in cytokine profiles have been described,(1) an overarching molecular hypothesis to explain these findings is lacking. Harman’s original free radical theory suggested that ageing could be attributed to the deleterious effects of reactive oxygen species (ROS).(2) Although it is known that ROS can damage structural cellular components and may induce a state of oxidative stress by GSH depletion, it is not intuitive how disrupting redox equilibrium could induce immune effects. We are beginning to understand, however, that oxidative stress is not just confined to oxidant injury but also has to consider antioxidant defense mechanisms that could determine whether ROS will induce oxidant injury. In fact, the coordinated antioxidant defense that is initiated by the Nrf2 pathway is the most sensitive oxidative stress response.(3) Our hierarchical oxidative stress hypothesis posits that lower levels of oxidative stress induce a protective and adaptive anti-oxidant defense that allows oxidant injury to become manifest only when this defense is overcome by high levels of ROS production.(4)
Nrf2 regulates the transcriptional activation of > 200 antioxidant and protective genes that constitute the so-called phase II response. Examples of phase II enzymes (p2E) include the rate-limiting enzyme in the GSH synthesis pathway, γ-glutamylcysteine ligase (γ-GCL), as well as glutathione peroxidase (GPx), heme oxygenase 1 (HO-1), superoxide dismutase (SOD), glutathione S-transferase (GST), and NADPH-quinone oxidoreductase (NQO1).(3) We propose that the dynamic equilibrium between the Nrf2 pathway and injurious oxidant stress responses could determine the impact of ageing in the immune system. This is compatible with the tendency towards a generalized decline in GSH levels and γ-GCL expression with ageing.(5) Ageing also leads to a decrease in Nrf2 activity and p2E expression in parallel with increased markers of oxidative stress.(6) While the exact reason for decreased Nrf2 activity is unknown, ageing leads to decreased binding of this transcription factor to the antioxidant response element (ARE), which regulates the transcriptional activation of p2E gene promoters.(5) Moreover, the decline in antioxidant activity is exaggerated during ageing of female nrf2 knockout mice.(7) In spite of this decline in Nrf2 activity, it is noteworthy that p2E expression and GSH production in old rats is correctable by the Nrf2 agonist, α-lipoic acid.(5) Thus, the fact that the Nrf2 pathway remains responsive in old animals could also be of benefit to elderly human subjects. This could include the use of even more potent agonists such as the broccoli chemical, sulforaphane (SFN).(8)
Nrf2 protects memory T-cells from aged-related oxidant injury, including protection against the decline in mitochondrial function and phenotypic changes in the T-cell compartment with ageing.(9) There is increasing evidence that Nrf2 also regulates the function of the innate immune system. Knockout of this gene leads to exaggerated cytokine production by innate cellular elements.(10) This includes our own demonstration that Nrf2 is important in regulating the APC activity of DC. Thus, exposure of myeloid DC to exogenous oxidative stress stimuli (e.g., pro-oxidative chemicals) have been shown to interfere in IL-12 production and Th1 immunity.(11) There is also growing evidence that the opposite may be true, namely that boosting of GSH levels at APC level may favor Th1 skewing of the immune response.(12;13)
We hypothesized that Nrf2 plays a critical role in the decline of Th1 immunity and contact hypersensitivity (CHS) during ageing. Moreover, we propose that this effect is, in part, explicable by the impact of the Nrf2 pathway on DC function. We assessed the effect of Nrf2 deficiency and boosting of GSH levels by SFN on the CHS response to contact sensitizing chemicals in old mice. We also made use of adoptive transfer of antigen-pulsed DC, which were treated of ex vivo with SFN to test CHS responses in vivo. We demonstrate that Nrf2 activation by in vivo or ex vivo SFN administration reverses the decline of Th1 immunity in aged mice.
METHODS
Mice
Young (2–4 months) and old (19–22) female C57BL/6 (B6) mice were obtained from the Jackson Laboratory and the National Institute of Aging colony (Bethesda, MD), respectively. Nrf2+/+ and nrf2−/− mice, which were initially obtained from Dr. Y. Kan,(14) were backcrossed onto a C57BL/6 background for 7 generations.
Reagents
See the Online Repository.
CHS testing with contact sensitizing agents
Oxazalone (OXA; 3%), dissolved in 100% ethanol, was applied on the shaved mouse abdomen on day 0. Control animals were exposed to vehicle alone. Six days after sensitization, mice were challenged on both sides of both ears by epicutaneous application of 20 μl of a 1% OXA solution.(15) 2,4-dinitro-1-fluorobenzene (DNFB) sensitization was accomplished by the application of 0.5% of the chemical dissolved in 4:1 acetone/olive oil onto the shaved abdomen (days 0 and 1). On day 5, mice were challenged by epicutaneous application of 0.2 % DNFB on both ears.(15) Ear thickness was measured prior to and 24 and 48 hr after challenge, using a dial thickness gauge (Mitutoyo, Japan). Mice were sacrificed 48 hr after challenge and ear tissues were removed for RNA extraction and cytokine message expression as well as for H&E staining.
SFN oral administration
SFN (9 μmol/day/mouse), made up in 0.2 ml corn oil, was administered by gavage on consecutive days. The control group received corn oil alone. Pretreatment with SFN or corn oil commenced 5 days prior to and was carried through until the performance of the antigen challenge (i.e., 11 days total).
RNA isolation and Real-time RT-PCR
See Online Repository.
Generation of BM-derived DC
BM-DC were prepared as previously described.(13) See Online Repository for more details.
Surface staining, MBB staining and flow cytometry
Cells were surface stained with PE-labeled anti-CD11c. Monobromobimane (MBB) was used to stain intracellular thiol, followed by conducting flow cytometry as previously described.(13) See Online Repository for more details.
Magnetic bead separation of CD11c+ cells
Magnetic cell sorting was performed by using microbead-labeled anti-CD11c (Miltenyi) as described.(9;11) See Online Repository for more details.
Eliciting CHS responses by adoptive DC transfer
CHS was induced by in vivo inoculation of antigen-pulsed DC.(13) Cultured BM-DC were incubated with or without NAC (20 mM for 1 hr) or SFN (5 μM for 24 hr), then washed and resuspended in PBS containing 100 μg/ml 2,4-dinitrobenzene sulfonic acid (DNBS) for 30 minutes. For sensitization (day 0), 0.5×106 DNBS-treated DC were injected subcutaneously in 100 μl saline into the flank of recipient mice. Five days later, mice were challenged by DNFB application to the ear. Mice injected with the same number of unmodified DC or mock-treated and challenged with vehicle alone, served as negative controls.
H&E staining
See Online Repository.
Statistical analysis
Results were expressed as mean ± SD and analyzed by Student’s t-test. Differences of p<0.05 were considered as significant.
RESULTS
SFN restores the age-related decline in the contact hypersensitivity and Th1 immunity
We have previously shown that ageing leads to a decline of the CHS to contact antigens placed on the skin.(13) While a number of mechanisms may explain the increase in oxidant stress during ageing, it is important to consider the role of the Nrf2 pathway in the response outcome. Recent studies indicate that SFN significantly activates Nrf2-mediated p2E gene expression that is absent in Nrf2-deficient animals.(8) SFN administration can therefore be used to study the effect of the Nrf2 pathway on the decline of Th1 immunity in ageing. To determine whether SFN gavage affects the CHS response, a previously determined effective dose (9 μmol/day/mouse) of the nutraceutical was delivered to 20–22 month old mice before performance of the ear swelling response.(8) Non-treated animals of similar age or 2–3 month old mice were used as comparative controls. Indeed, the ear swelling response to DNFB challenge was significantly reduced in old compared to young animals. However, prior treatment of the old animals by daily SFN gavage prior to and during sensitization prevented the response decline and could restore the CHS response to the levels seen in young animals (Fig. 1A). These response differences were maintained after 48 hr and were also reflected by histological changes in the ear, which showed that the decline in lymphocyte infiltration and intercellular edema in old animals could be reversed by SFN administration (Fig. 1B). SFN had no effect on non-sensitized (control, CON) animals.
Fig. 1.

SFN reverses the age-related decline in the CHS response. (A) Ear swelling response (mean±SD). (B) H&E staining of ear tissue. Real-time PCR for mRNA levels of genes in the ear (C) and liver (D). Results represent the fold-increase (mean±SD) compared to the CON-Young group. n=6, *p<0.05, **p<0.01, ***p<0.001. CON, vehicle-treated; SFN, SFN-treated; DFNB, DNFB-sensitized/challenged.
To determine whether the induction of the CHS response is accompanied by polarized T-cell differentiation, IFNγ and IL-4 message levels were measured in the ear tissues that were taken 48 hr after challenge. Quantitative RT-PCR showed that DNFB challenge induced the expression of the Th1 cytokine, IFNγ, which was significantly suppressed in old compared to young animals; SFN treatment significantly increased IFNγ expression (Fig. 1C). In contrast, the message level of a representative Th2 cytokine, IL-4, was not significantly affected by ageing or SFN administration (Fig. 1C). In addition to the cytokine changes, message levels for T-bet, a Th1-specific transcription factor,(16) were significantly decreased in old versus young sensitized animals upon DNFB challenge. However, SFN administration could prevent this decline to a lesser but significant degree (Fig. 1C). By contrast, ageing or SFN treatment did not affect the expression of GATA-3, a Th2-specific transcription factor (not shown).(16)
In order to show that SFN affects p2E expression in vivo, the mRNA levels of NQO1, GST, γ-GCLS, and GPx were determined by quantitative PCR (Fig. 1D). Compared to the expression levels in the liver of control animals, message levels for three of the four genes were increased by SFN administration (Fig. 1D).
Nrf2 deficiency accentuates the CHS response decline in old mice
Nrf2 deficiency impacts the immune function of old mice.(7;17) In order to see whether this includes an impact on Th1 immunity and CHS, we compared the ear swelling response of 22-month old nrf2−/− mice with littermate controls (nrf2+/+) during OXA sensitization and challenge. Nrf2-deficient mice showed a significant decrease in their ear swelling response compared to wild-type controls (Fig. 2A). The same effect was observed when mice were sensitized and challenged with DNFB (Fig. E1), thereby indicating that effect is not just limited to a single contact antigen. This response reduction was accompanied by decreased IFN-γ mRNA expression while IL-4 levels remain unaffected (Fig. 2B). Interestingly, when this experiment was repeated in younger (6 mo) animals, there was no response reduction in Nrf2-deficient mice (not shown). These results suggest that cumulative oxidative stress during ageing accentuates the impact of Nrf2 deficiency in the immune system. This is in accordance with previously published data.(7) All considered, above data suggest that through its ability to maintain redox equilibrium in the immune system, Nrf2 plays an important role in regulating Th1 immunity; this effect becomes particularly obvious under age-related oxidative stress conditions.
Fig. 2.
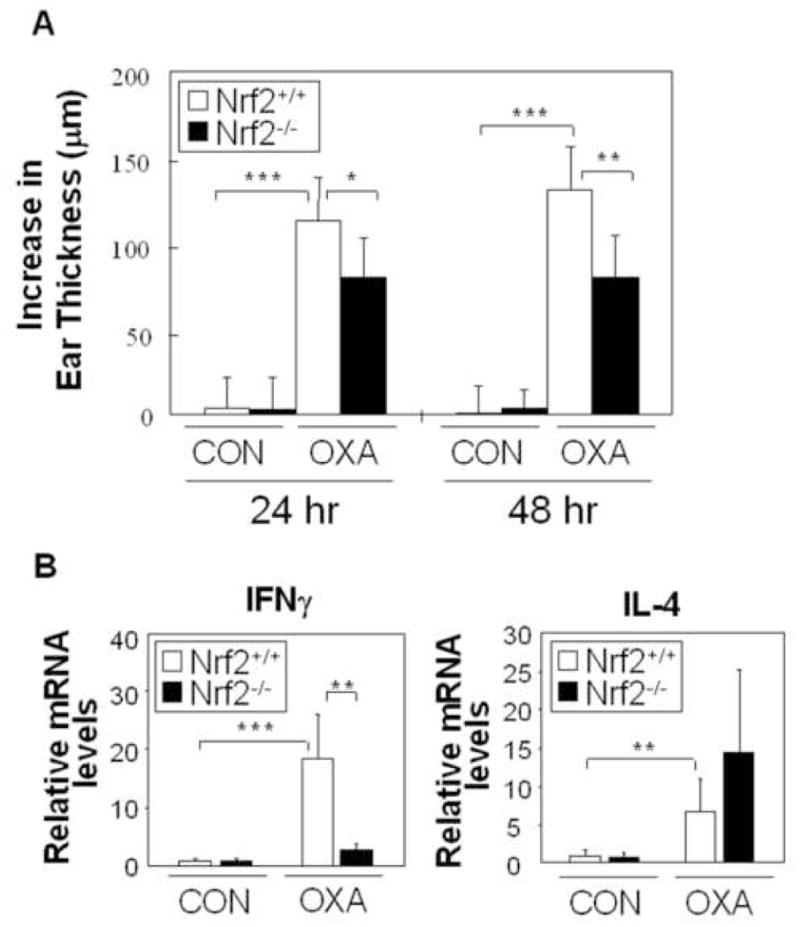
Nrf2 deficiency suppresses the CHS response. (A) Ear swelling response (mean±SD). (B) IFNγ and IL-4 mRNA was measure by real-time PCR. Results represent the fold-increase (mean±SD) compared to the CON-Nrf2+/+ group. n=6, *p<0.05, **p<0.01, ***p<0.001. CON, vehicle-treated; OXA, OXA-sensitized/challenged.
DC from old mice contain lower levels of phase II enzymes and a decreased thiol content
The contact hypersensitivity response involves several cell types in the skin, including helper T-cells, cytotoxic T-lymphocytes and langerhans cells (LC). While T-cell function is clearly impacted by the oxidative stress events during ageing,(9) increased ROS production also targets DC.(11;13;18) Because it is not possible to obtain a sufficient number of LC to study the impact of changes in redox status, we compared thiol levels from CD11c+ cells that were purified from the spleens of young and old mice. This showed a significant decrease in MBB MFI in the CD11c+ populations from old animals (Fig. 3A). Please note that this decline of 24 % is highly significant from a homeostatic perspective since a small decline in GSH content leads to a big decrease in the GSH/GSSG ratio. This aspect is further discussed in the Online Repository. Similar observations were made when CD11c+ BM-DC were compared in young and old mice (see later). RNA was also isolated from purified CD11c+ cells to perform quantitative PCR analysis to assess p2E message expression. Not only did we observe decreased mRNA expression of p2E in cells from old animals, but also demonstrated a decline in nrf2 message (Fig. 3B). All considered, these data indicate that ageing leads to altered redox equilibrium in DC. This could impact their APC function.
Fig. 3.
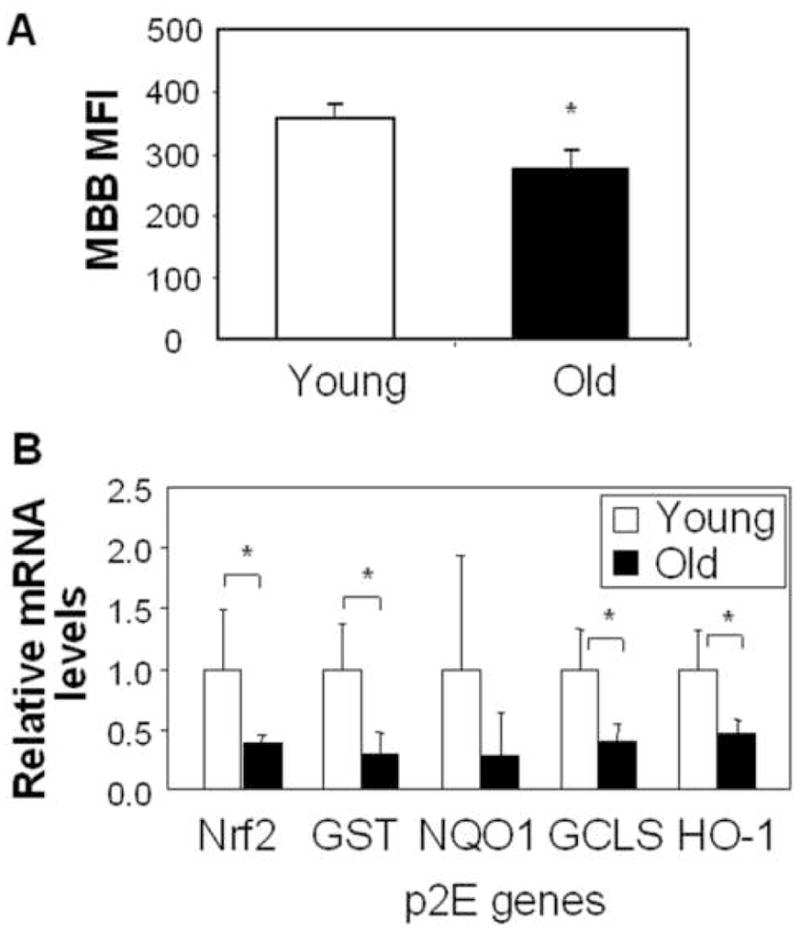
DC from old mice has lower thiol level and phase II mRNA expression. (A) MFI for MBB staining in CD11c+ splenocytes from young and old mice (mean±SD). (B) phase II mRNA expression in CD11c+ splenocytes from young and old mice. Results represent the fold-increase (mean ± SD) of old compared to young mice. n=4, *p<0.05.
DC redox disequilibrium interferes in the CHS response that can be elicited by adoptive transfer of myeloid DC, while the restoration of DC thiol levels can reverse this effect
Through the use of an adoptive transfer protocol, we have previously demonstrated that it is possible to elicit a CHS response in recipient animals receiving antigen-pulsed BM-DC from another donor.(13) Moreover, we have demonstrated that GSH depletion of these DC at the time of antigen processing leads to a reduced ear swelling response in vivo.(13) We hypothesized that a similar disturbance of the DC redox equilibrium by ageing could impact the adoptive CHS. Thus, BM-DC from young and old mice were used for ex vivo pulsing with the water-soluble DNFB analogue, DNBS, before subcutaneous injection into recipient young naïve mice.(13) Five days later, a CHS response was elicited by DNFB application to the ears of the recipient. This demonstrated a significant decrease in the ear swelling response when DC from old compared to DC from young animals were used (Fig. 4A). Their differences were also reflected by the reduced inflammatory infiltrates in the ear tissue of old animals (not shown). No response was obtained in animals receiving naïve DC (CON) (Fig. 4A). Since the data suggest that altered redox equilibrium could be responsible for the decline in DC function, we also performed MBB staining to look at BM-DC thiol levels (Fig. E2A). The small but significant decline (14%, p<0.05) of total thiol levels in DC from old animals could be responsible for a significant change in the GSH/GSSG ratio.
Fig. 4.

DC redox disequilibrium interferes in the DTH response upon adoptive transfer. Ear swelling response in recipient mice (3 mo old) receiving DNBS-pulsed DC from young vs. old mice (A) or nrf2+/+ vs nrf2−/− mice (B), followed by DNFB challenge (n=6). *p<0.05, **p<0.01, ***p<0.001. CON, vehicle-treated; DNBS, DNBS-pulsed.
The same experiment was performed with DC from old nrf2+/+ and nrf2−/− mice. Fig. 4C shows that there is a significant decrease in the ear swelling response in mice receiving DNBS-pulsed DC from nrf2−/− compared to nrf2+/+ mice. This was accompanied by a significant reduction (13%, p<0.05) in the thiol content of BM-DC from nrf2−/− compared to nrf2+/+ mice (Fig. E2B).
In order to confirm that the age-related changes in DC redox equilibrium is important for the maintenance of Th1 immunity, we used the adoptive transfer approach to determine whether ex vivo thiol repletion could restore the CHS response. First, we confirmed that the ear swelling response of recipient mice injected with DNBS-pulsed DC from old animals was significantly decreased compared to DC from young animals. Second, we showed that ex vivo treatment with NAC restored the CHS response in the recipients (Fig. 5A). MBB staining showed a 25% increase (p<0.05) in cellular thiol content by NAC exposure (Fig. E3). These data demonstrate that a perturbation of DC redox equilibrium as a result of ageing, thiol repletion or nrf2 knockout impacts APC activity in vivo.
Fig. 5.
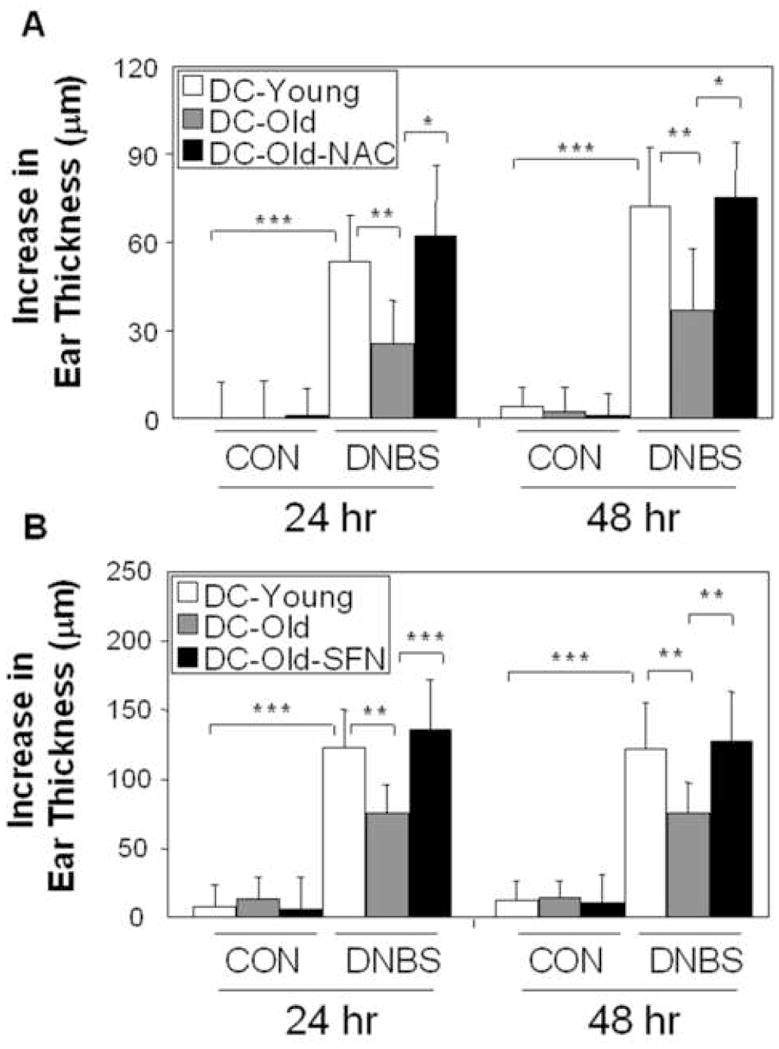
NAC or SFN treatment of DC reverses the age-related decline in the CHS response upon adoptive transfer. NAC (A) or SFN (B)-treated DC were exposed to DNBS ex vivo and adoptively transferred into mice that were challenged with DNFB. Ear swelling response. (mean ± SD, n=6). *p<0.05, **p<0.01, ***p<0.001. CON, vehicle-treated; DNBS, DNBS-pulsed.
SFN treatment in DC reverses the age-related decline of CHS response upon adoptive transfer
To determine whether SFN could exert similar effects in the DC adoptive transfer model as during oral administration (Fig. 1), BM-DC from old mice were prior treated with SFN before DNBS pulsing and injection into young recipient mice. Again, the CHS response with antigen-pulsed DC from old animals was reduced compared to cells from young animals. Second, the data demonstrate that ex vivo SFN exposure could restore the CHS response elicited by DC from old animals (Fig. 5B). These data clearly indicate that activation of the Nrf2 pathway in DC can reverse the age-related decline in Th1 immunity. To confirm up-regulation of antioxidant enzyme expression in SFN-treated DC, real-time PCR showed significant up-regulation of the mRNA levels for NOQ1 (p<0.001), γ-GCLS (p<0.05), and HO-1 (p<0.05) (Fig. E4A). This was accompanied by an 15% increase (p<0.05) in cellular thiol levels (Fig. E4B).
DISCUSSION
In this study, we demonstrate that manipulation of the Nrf2 pathway impacts CHS and Th1-mediated immune responses in old mice. Similar observations were made using an adoptive transfer approach that uses ex vivo modification of DC redox status to study CHS responses in vivo. This is compatible with the growing recognition of the importance of the Nrf2 pathway on innate immunity.(10) We demonstrate that the oral administration of a potent Nrf2 agonist, SFN, reverses the decline of CHS responses in old mice. This effect can be reproduced when antigen-pulsed DC from old mice are treated with SFN ex vivo before adoptive transfer. This finding is compatible with decreased nrf2 expression, decreased p2E expression and lower thiol levels in DC from old animals. GSH repletion by SFN and NAC restored the DC redox equilibrium, allowing DC from old animals to function normally in vivo. Taken together, these data show that the state of redox equilibrium of DC is important in the decline of Th1 immunity with ageing.
A decline in Th1 immunity with ageing is of particular importance in defense against viral and mycobacterial pathogens as well as for immune surveillance against cancer. While a host of specific molecular and cellular events have been described in senescent immune cells,(1) it is not clear whether ageing is responsible for a common mechanism of immune decline. We propose, however, that a disturbance of the redox equilibrium of cells of the immune system may provide an overarching mechanism by which to dissect the functional decline of the immune system during ageing. This view shifts the free radical theory of ageing to an adaptive multi-factorial process that is determined by a dynamic interplay between pro- and antioxidant forces.(19) Such an evolution also allows us to postulate that people with decreased antioxidant protection may be more prone to develop immune senescence and that dietary or therapeutic intervention to strengthen the effects of the antioxidant pathway could reverse the injurious effects of oxidative stress in the immune system. Our data clearly show that it is possible to reverse the age-related decline in Th1 immunity in old mice within days of restoring redox equilibrium in the immune system.
We have previously shown that the oxidative stress effects of ageing may be involved in the loss of naïve T-cells and a decline in Th1 immunity.(9;13) Our focus on DC originates from the demonstration that knockout of the nrf2 gene has prominent effects on the innate immune system, including the function of macrophages and DC.(10) Changes in DC function may contribute to effects on T-cell differentiation. Although a lot needs to be learned about the role of DC lineage and biology in determining the outcome of T-cell differentiation, a number of recent studies have emphasized the role of the redox equilibrium in these events. This is illustrated by the finding that in vivo GSH augmentation in APC favors Th1 development, while thiol depletion shifts the immune response from Th1 to Th2 dominance.(12) Moreover, we have shown that GSH depletion of antigen-pulsed DC interfere in their ability to mount a CHS response upon adoptive transfer in vivo.(13) While there are a number of possible explanations of why redox equilibrium could determine DC function, one possible explanation is that oxidative stress decreases IL-12 and subsequent IFNγ production in T-cells.(20) This could involve a role for Nrf2 that, through its effects on GSH synthesis and p2E expression, could modify the signaling pathways that are required for DC maturation, cytokine production and co-stimulatory receptor expression.(13;14;21) Another possible explanation is that DC play an important role in neutralizing extracellular oxidative stress through the expression of surface thiol groups.(22;23) Not only does this allow the DC to survive in an oxidative stress environment(22) but also contributes to the maintenance of thiol levels and viability in bystander T-lymphocytes.(23) While there is evidence that the redox homeostasis of DC is important in shaping their activity under conditions of systemic oxidative stress, it has not been demonstrated previously that the redox equilibrium of DC from old animals is important in their immune decline. Here we report for the first time that DC from old animals exhibit lower thiol levels and decreased expression of nrf2 and phase II gene message levels than DC from young animals. Thus, the decrease in DC antioxidant capacity could contribute in a number of ways to the decline in the ear swelling response and Th1 immunity in old animals (Fig. 3).
Our focus on DC is fortuitous from the perspective that relatively little work has been done on the cell type in immune senescence. Moreover, previous studies have yielded conflicting results.(24–27) For instance, some studies did not observe a difference in DC surface marker expression(24) while others have shown decreased MHC class II and costimulatory receptor expression in aged individuals.(25) Other abnormalities reported in DC with ageing include abnormal recruitment to sites of immune pathology, decreased transportation of antigens to lymph node germinal centers, impaired capacity towards IFNγ production, and interference in APC activity due to a putative increase in IL-10 levels.(26)
While it would have been ideal to have access to LC to conduct this study, it was not logistically possible to purify enough LC to conduct these studies. Instead, we settled for the use of myeloid DC that are derived from the same precursors as LC. Minimally, this has allowed us to provide proof-of-principle that DC from old animals do not perform as well as DC from young animals in the adoptive transfer model. Not only is this approach highly reproducible but also allowed us to show that manipulation of the DC redox status influence APC activity in vivo. This allowed us to demonstrate that either an increase or a decrease in Nrf2 activity exerts major effects on APC function. This is directly relevant to the study of ageing in which a decline of Nrf2 levels has been reported previously.(5;6) While the mechanism for this decline is unknown, we know that Nrf2 function is regulated at multiple levels and that ageing may target one or more of these events. This aspect is further discussed in the online repository for the interested reader.
In spite of the decline in Nrf2 activity with ageing, we show that SFN can effectively restore redox equilibrium in old animals in parallel with an improvement in CHS and Th1 immunity. This finding could be of major significance in preventing or reversing the effects of immune senescence in elderly humans. Dietary antioxidants have been shown to have important effects on immune function, including improvement of CHS and vaccination responses in humans.(28) To this list we may now add broccoli and other cruciferous vegetables that are deserving of a human trail. In addition, the electrophilic chemistry that leads to Nrf2 release from its chaperone provides a platform for further drug discovery. Noteworthy, it has been demonstrated that treatment of old rats with α-lipoic acid can increase nuclear Nrf2 levels in parallel with increased γ-CLC expression and GSH production.(5) Finally, our study shows the potential for using DC to conduct vaccination therapy as a means of restoring in vivo cellular immune function during ageing.
Further studies are needed to delineate all the mechanisms and cell types that are involved in the improvement of Th1 immunity during treatment with Nrf2 agonists. While for the most part we focused on DC in this communication, it is also possible that other cell types might contribute. It is quite possible that enhanced Nrf2 activity could have equally important effects on T-cells and other cell types that participate in the contact hypersensitivity response. It is also important to mention that previous studies in mice have shown conflicting data with respect to the impact of NAC and N,N′-diacetyl-L-cystine on the CHS response to chemicals.(29) While there could be a number of experimental reasons for these differences, it is possible that the thiol group in these antioxidants could covalently bind to the contact sensitizing chemicals and interfere in their ability to generate the haptens that are required for antigen presentation.(30;31) This aspect is further discussed in the Online Repository.
In summary, intervention via the Nrf2 pathway provides a rational approach to improve cellular immune function during ageing. In addition to the beneficial effects on specific immunity, it is possible that many of the chronic inflammatory changes that develop in the elderly may originate in the innate immune system. It is possible that an age-related decline in Nrf2 activity could lead to oxidative stress mediated pro-inflammatory responses in cells from the innate immune system. It is possible that Nrf2 agonists could also intervene in this aspect of ageing.
Supplementary Material
Acknowledgments
This work was supported by US Public Health Science support from the National Institute of Ageing (RO1 AG14992), the UCLA Claude D. Pepper Older Americans Independence Center (5P30 AG028748), and the NIAID-funded UCLA Asthma and Allergic Disease Clinical Research Center (U19 AI070453).
Abbreviations
- APC
antigen-presenting cells
- BM-DC
bone-marrow derived DC
- CHS
contact hypersensitivity
- DC
dendritic cells
- DNBS
2,4-dinitrobenzene sulfonic acid
- DNFB
2,4-dinitro-1-fluorobenzene
- GSH
glutathione
- MBB
monobromobimane
- MFI
mean fluorescence intensity
- NAC
N-acetyl cysteine
- OXA
oxazolone
- p2E
phase II enzyme
- RT-PCR
reverse transcription-polymerase chain reaction
- SFN
sulforaphane
- Th1
T-helper type 1
- Th2
T-helper type 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fulop T, Larbi A, Dupuis G, Pawelec G. Ageing, autoimmunity and arthritis: Perturbations of TCR signal transduction pathways with ageing - a biochemical paradigm for the ageing immune system. Arthritis Res Ther. 2003;5(6):290–302. doi: 10.1186/ar1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harman D. Ageing: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 4.Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–90. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 5.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- 7.Hirayama A, Yoh K, Nagase S, Ueda A, Itoh K, Morito N, et al. EPR imaging of reducing activity in Nrf2 transcriptional factor-deficient mice. Free Radic Biol Med. 2003;34(10):1236–42. doi: 10.1016/s0891-5849(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 8.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 9.Kim HJ, Nel AE. The role of phase II antioxidant enzymes in protecting memory T cells from spontaneous apoptosis in young and old mice. J Immunol. 2005;175:2948–2959. doi: 10.4049/jimmunol.175.5.2948. [DOI] [PubMed] [Google Scholar]
- 10.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116(4):984–95. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan RC, Wang M, Li N, Yanagawa Y, Onoe K, Lee JJ, Nel AE. Pro-oxidative diesel exhaust particle chemicals inhibit lipopolysaccharide-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol. 2006;118:455–65. doi: 10.1016/j.jaci.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Barajas B, Chan RC, Nel AE. Glutathione Depletion Inhibits Dendritic Cell Maturation and Delayed-type Hypersensitivity: Implications for Systemic Disease and Immunosenescence. J Allergy Clin Immunol. 2007;119:1225–33. doi: 10.1016/j.jaci.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA. 1997;96:12731. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaspari AA, Katz SI. Contact Hypersensitivity. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons, Inc; NJ: 2003. [Google Scholar]
- 16.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23(3):147–61. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004;18(3):261–72. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 18.Kantengwa S, Jornot L, Devenoges C, Nicod LP. Superoxide anions induce the maturation of human dendritic cells. Am J Respir Crit Care Med. 2003;167:431–437. doi: 10.1164/rccm.200205-425OC. [DOI] [PubMed] [Google Scholar]
- 19.Lane N. A unifying view of ageing and disease: the double-agent theory. J Theor Biol. 2003;225:531–540. doi: 10.1016/s0022-5193(03)00304-7. [DOI] [PubMed] [Google Scholar]
- 20.D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma- production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3(3):166–75. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivollier A, Perrin-Cocon L, Luche S, Diemer H, Strub JM, Hanau D, et al. High expression of antioxidant proteins in dendritic cells: possible implications in atherosclerosis. Mol Cell Proteomics. 2006;5(4):726–36. doi: 10.1074/mcp.M500262-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Thoren FB, Betten A, Romero AI, Hellstrand K. Cutting edge: Antioxidative properties of myeloid dendritic cells: protection of T cells and NK cells from oxygen radical-induced inactivation and apoptosis. J Immunol. 2007;179(1):21–5. doi: 10.4049/jimmunol.179.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Lung TL, Saurwein-Teissl M, Parson W, Schonitzer D, Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18(16):1606–12. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 25.Miller C, Kelsoe G, Han S. Lack of B7–2 expression in the germinal centers of aged mice. Aging Immunol Infect Dis. 1994;5:249–257. [Google Scholar]
- 26.Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J Leukoc Biol. 1998;64(6):703–12. doi: 10.1002/jlb.64.6.703. [DOI] [PubMed] [Google Scholar]
- 27.Shurin MR, Shurin GV, Chatta GS. Aging and the dendritic cell system: Implication for cancer. 2007 doi: 10.1016/j.critrevonc.2007.03.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meydani M, Lipman RD, Han SN, Wu D, Beharka A, Martin KR, Bronson R, Cao G, Smith D, Meydani SN. The effect of long-term dietary supplementation with antioxidants. Ann N Y Acad Sci. 1998;854:352–60. doi: 10.1111/j.1749-6632.1998.tb09915.x. [DOI] [PubMed] [Google Scholar]
- 29.Särnstrand B, Jansson AH, Matuseviciene G, Scheynius A, Pierrou S, Bergstrand H. N,N′-Diacetyl-L-cystine-the disulfide dimer of N-acetylcysteine-is a potent modulator of contact sensitivity/delayed type hypersensitivity reactions in rodents. J Pharmacol Exp Ther. 1999;288(3):1174–84. [PubMed] [Google Scholar]
- 30.Bruchhausen S, Zahn S, Valk E, Knop J, Becker D. Thiol antioxidants block the activation of antigen-presenting cells by contact sensitizers. J Invest Dermatol. 2003;121(5):1039–44. doi: 10.1046/j.1523-1747.2003.12510.x. [DOI] [PubMed] [Google Scholar]
- 31.Becker D, Valk E, Zahn S, Brand P, Knop J. Coupling of contact sensitizers to thiol groups is a key event for the activation of monocytes and monocyte-derived dendritic cells. J Invest Dermatol. 2003;120(2):233–8. doi: 10.1046/j.1523-1747.2003.12026.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


