Abstract
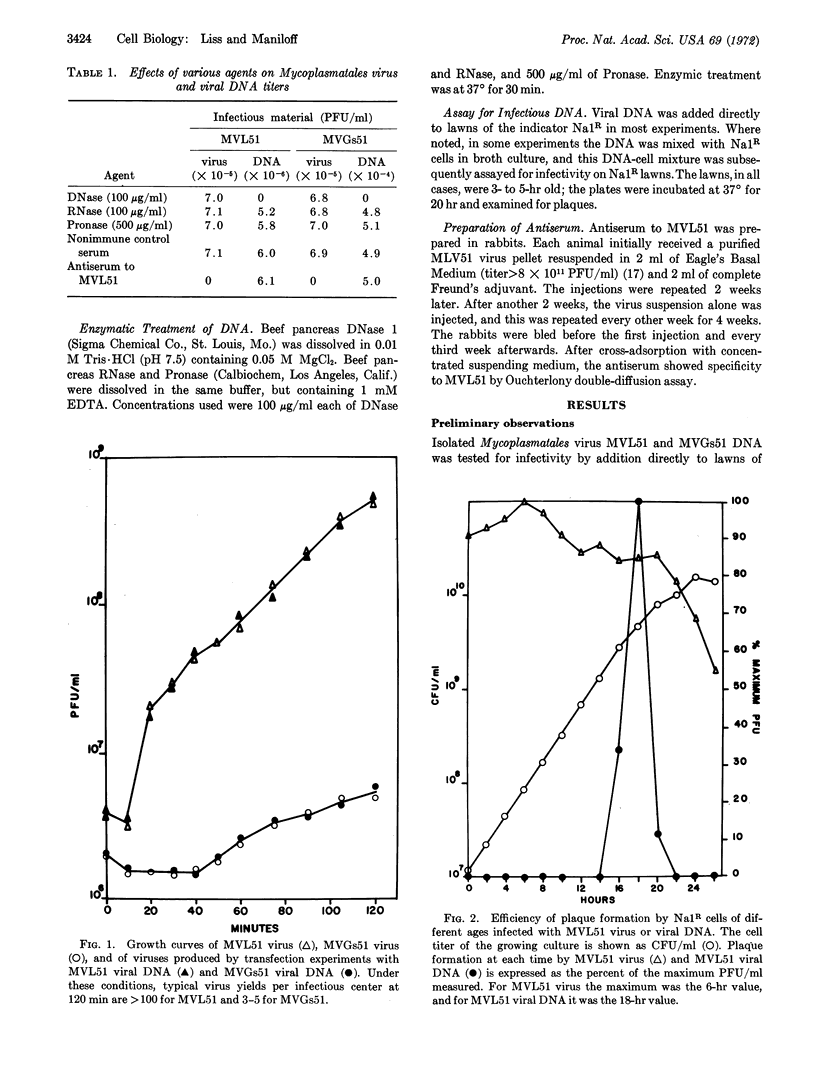
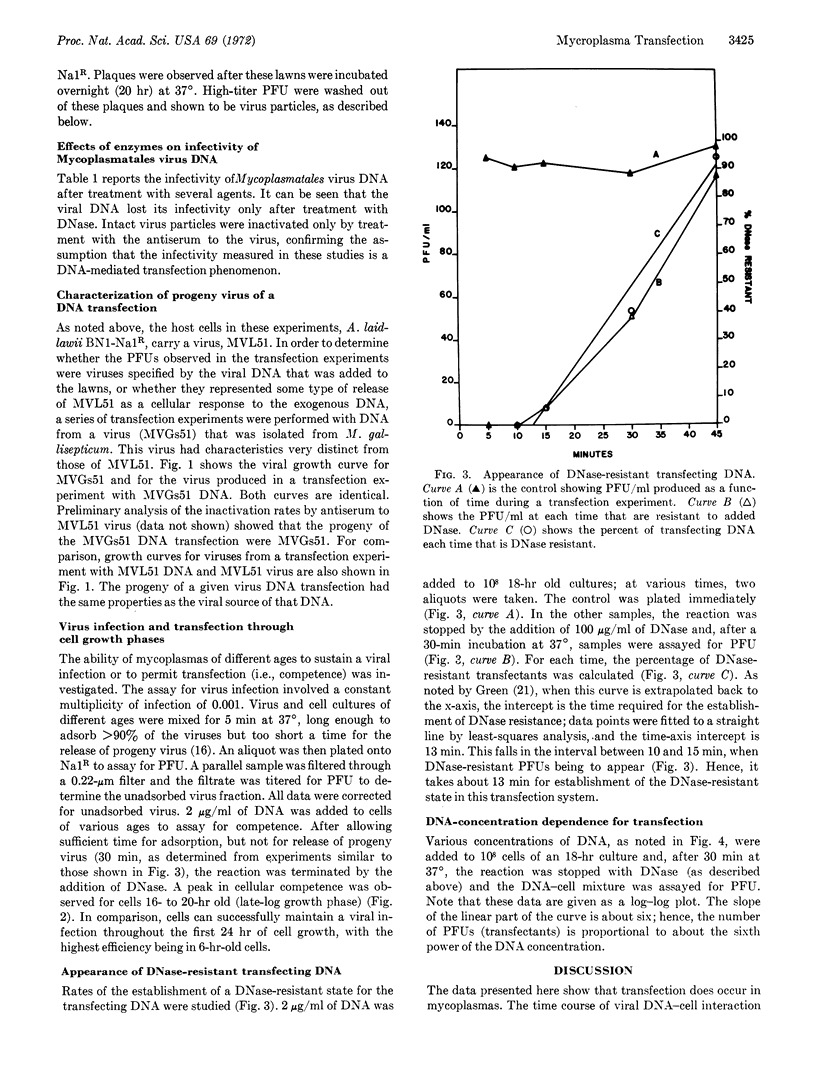
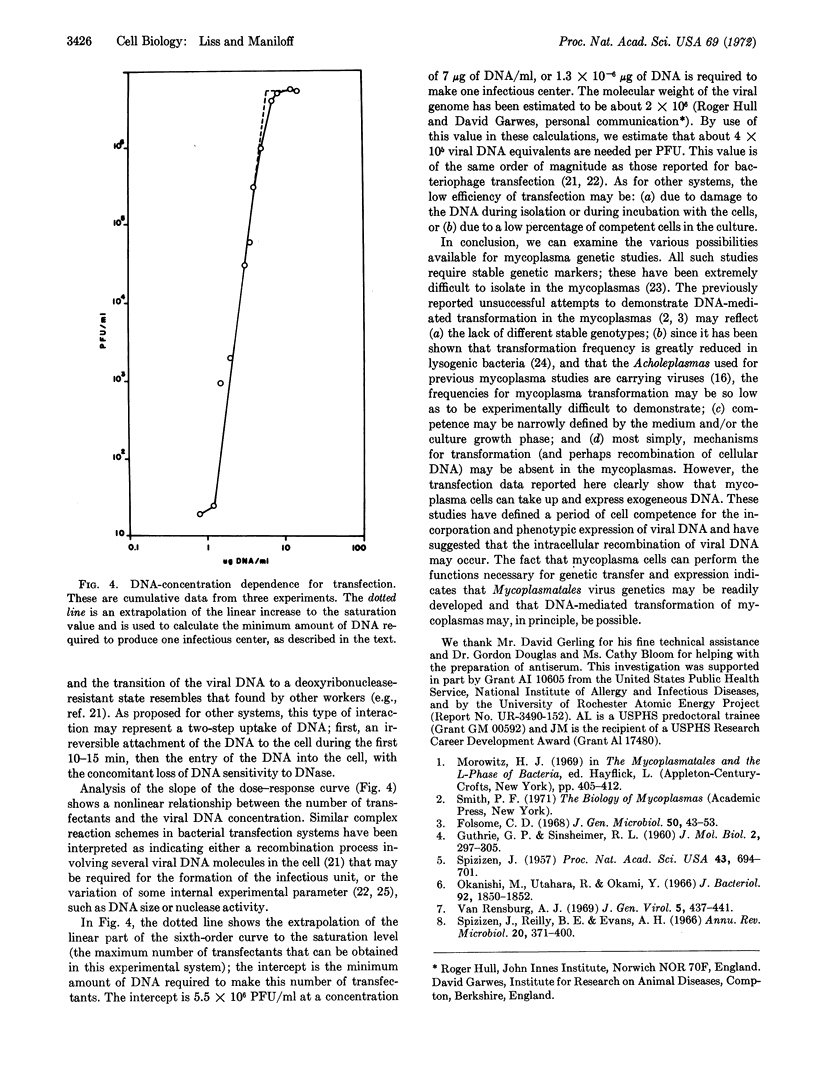
DNA isolated from Mycoplasmatales viruses MVL51 and MVGs51 was infectious when mixed with Acholeplasma laidlawii BN1-Na1R cells. Infectivity was destroyed by deoxyribonuclease but not by ribonuclease, Pronase, or specific antiserum to the virus. Host mycoplasma cells were only competent for transfection during late-log growth phase. The rates of the establishment of DNase insensitivity of viral DNA transfectants were similar to those of bacteriophage systems. The dose-response curve for transfection suggested that an average of six molecules of DNA must interact with a cell in order to produce one infectious center. Mycoplasmatales virus DNA exhibited a low efficiency of infection; one infectious center required 4 × 105 virus equivalents of DNA.
Keywords: viral DNA isolation, genetic transfer, competence
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dimayorca G. A., Eddy B. E., Stewart S. E., Hunter W. S., Friend C., Bendich A. ISOLATION OF INFECTIOUS DEOXYRIBONUCLEIC ACID FROM SE POLYOMA-INFECTED TISSUE CULTURES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1805–1808. doi: 10.1073/pnas.45.12.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. T. Source of the nonlinear dependence of bacteriophage SP82 transfection on deoxyribonucleic acid concentration. J Virol. 1971 Jun;7(6):749–752. doi: 10.1128/jvi.7.6.749-752.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsome C. E. Deoxyribonucleate binding and transformation in Mycoplasma laidlawii. J Gen Microbiol. 1968 Jan;50(1):43–53. doi: 10.1099/00221287-50-1-43. [DOI] [PubMed] [Google Scholar]
- GERBER P. An infectious deoxyribonucleic acid derived from vacuolating virus (SV40). Virology. 1962 Jan;16:96–97. doi: 10.1016/0042-6822(62)90209-x. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Isolation of a virus infecting a strain of Mycoplasma laidlawii. Nature. 1970 Mar 21;225(5238):1165–1165. doi: 10.1038/2251165a0. [DOI] [PubMed] [Google Scholar]
- ITO Y. A tumor-producing factor extracted by phenol from papillomatous tissue (Shope) of cottontail rabbits. Virology. 1960 Dec;12:596–601. doi: 10.1016/0042-6822(60)90182-3. [DOI] [PubMed] [Google Scholar]
- Ito Y., Hsia S., Evans C. A. Rabbit kidney vacuolating virus: extraction of infectious DNA. Virology. 1966 May;29(1):26–31. doi: 10.1016/0042-6822(66)90192-9. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Isolation of Mycoplasmatales viruses and characterization of MVL1, MVL52, and MVG51. Science. 1971 Aug 20;173(3998):725–727. doi: 10.1126/science.173.3998.725. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Utahara R., Okami Y. Infection of the protoplasts of Streptomyces kanamyceticus with deoxyribonucleic acid preparation from actinophage PK-66. J Bacteriol. 1966 Dec;92(6):1850–1852. doi: 10.1128/jb.92.6.1850-1852.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. INFECTION OF PROTOPLASTS BY DISRUPTED T2 VIRUS. Proc Natl Acad Sci U S A. 1957 Aug 15;43(8):694–701. doi: 10.1073/pnas.43.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J., Reilly B. E., Evans A. H. Microbial transformation and transfection. Annu Rev Microbiol. 1966;20:371–400. doi: 10.1146/annurev.mi.20.100166.002103. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Young F. E. The influence of temperate bacteriophage phi105 on transformation and transfection in Bacillus subtilis. Biochem Biophys Res Commun. 1972 Apr 28;47(2):365–371. doi: 10.1016/0006-291x(72)90722-x. [DOI] [PubMed] [Google Scholar]


