Abstract
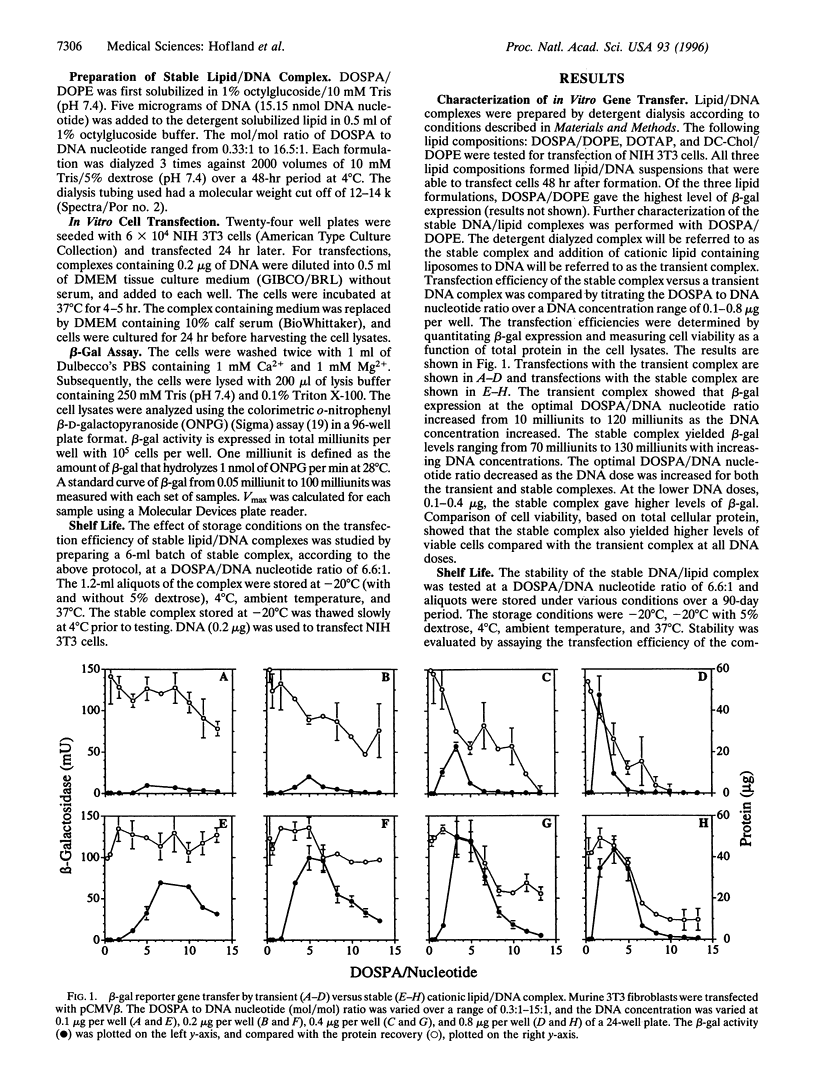
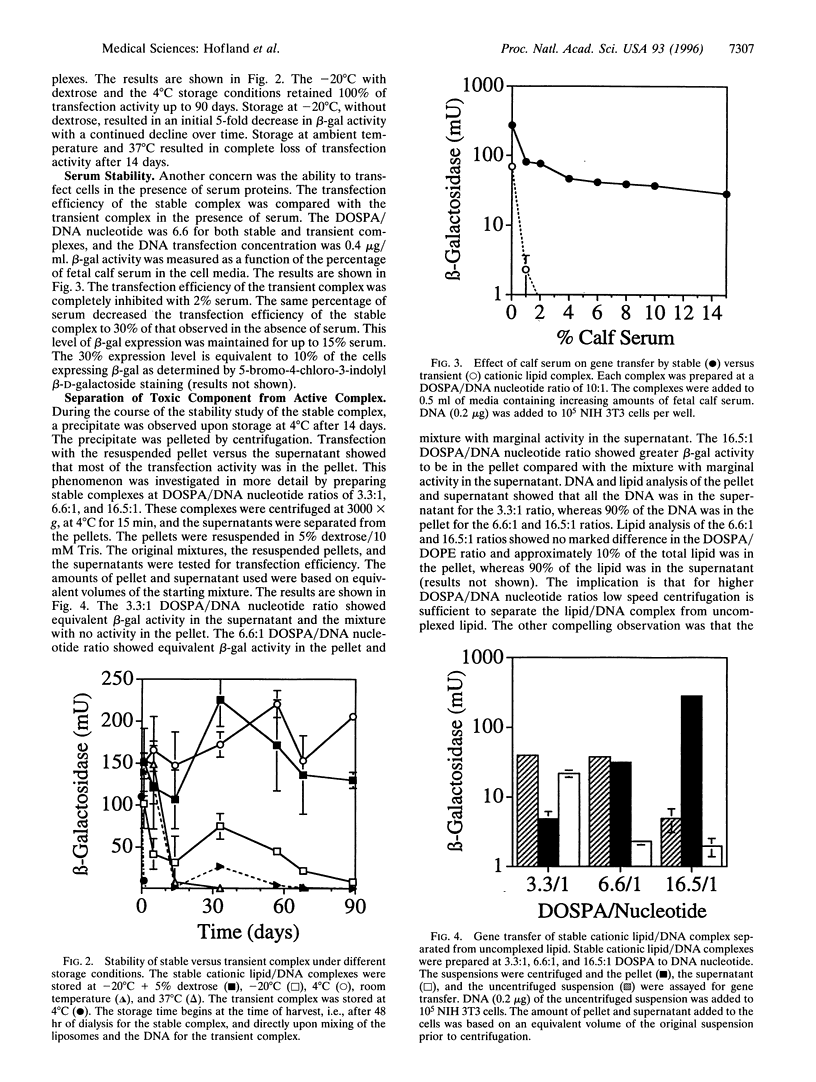
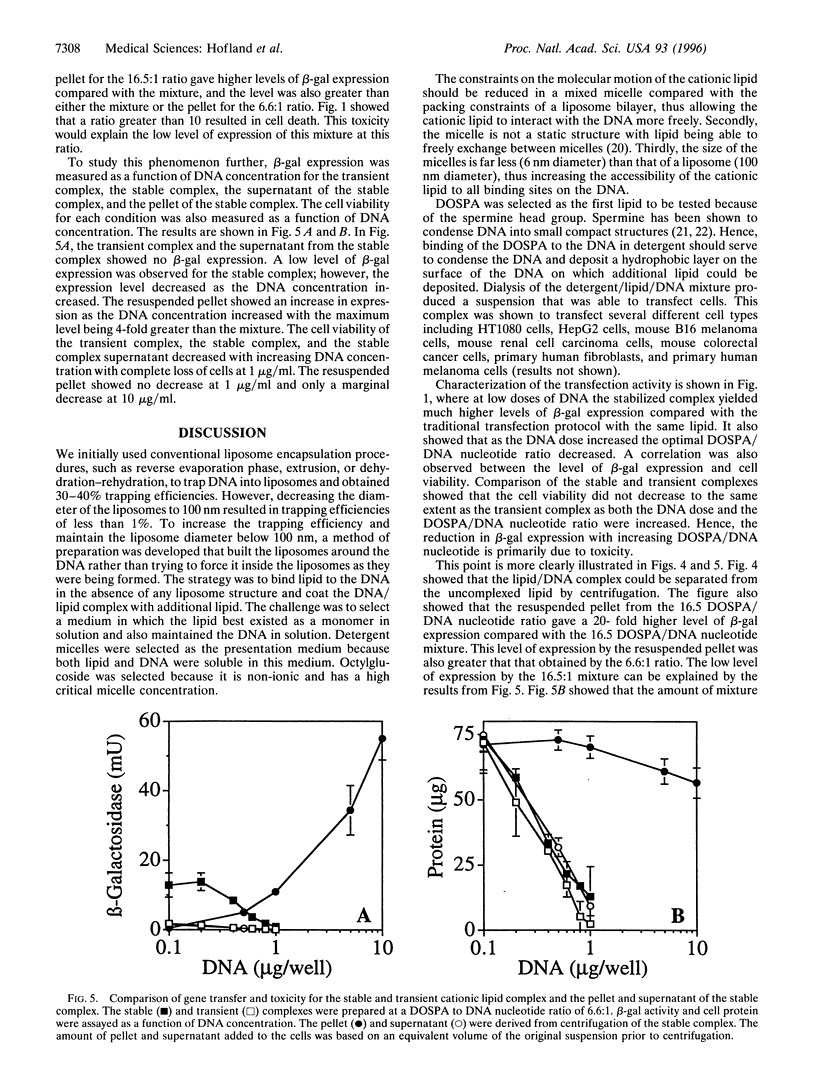
Stable cationic lipid/DNA complexes were formed by solubilizing cationic liposomes with 1% octylglucoside and complexing a DNA plasmid with the lipid in the presence of detergent. Removal of the detergent by dialysis yielded a lipid/DNA suspension that was able to transfect tissue culture cells up to 90 days after formation with no loss in activity. Similar levels of gene transfer were obtained by mixing the cationic lipid in a liposome form with DNA just prior to cell addition. However, expression was completely lost 24 hr after mixing. The transfection efficiency of the stable complex in 15% fetal calf serum was 30% of that obtained in the absence of serum, whereas the transient complex was completely inactivated with 2% fetal calf serum. A 90-day stability study comparing various storage conditions showed that the stable complex could be stored frozen or as a suspension at 4 degrees C with no loss in transfection efficiency. Centrifugation of the stable complex produced a pellet that contained approximately 90% of the DNA and 10% of the lipid. Transfection of cells with the resuspended pellet and the supernatant showed that the majority of the transfection activity was in the pellet and all the toxicity was in the supernatant. Formation of a stable cationic lipid/DNA complex has produced a transfection vehicle that can be stored indefinitely, can be concentrated with no loss in transfection efficiency, and the toxicity levels can be greatly reduced when the active complex is isolated from the uncomplexed lipid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behr J. P., Demeneix B., Loeffler J. P., Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O., Lezoualc'h F., Zanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J. P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Farhood H., Bottega R., Epand R. M., Huang L. Effect of cationic cholesterol derivatives on gene transfer and protein kinase C activity. Biochim Biophys Acta. 1992 Nov 9;1111(2):239–246. doi: 10.1016/0005-2736(92)90316-e. [DOI] [PubMed] [Google Scholar]
- Farhood H., Serbina N., Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995 May 4;1235(2):289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- Felgner J. H., Kumar R., Sridhar C. N., Wheeler C. J., Tsai Y. J., Border R., Ramsey P., Martin M., Felgner P. L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994 Jan 28;269(4):2550–2561. [PubMed] [Google Scholar]
- Felgner P. L., Ringold G. M. Cationic liposome-mediated transfection. Nature. 1989 Jan 26;337(6205):387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- Ferkol T., Perales J. C., Eckman E., Kaetzel C. S., Hanson R. W., Davis P. B. Gene transfer into the airway epithelium of animals by targeting the polymeric immunoglobulin receptor. J Clin Invest. 1995 Feb;95(2):493–502. doi: 10.1172/JCI117690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. J., Wilson J. M. Biochemical and functional analysis of an adenovirus-based ligand complex for gene transfer. Biochem J. 1994 Apr 1;299(Pt 1):49–58. doi: 10.1042/bj2990049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensler J., Szoka F. C., Jr Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem. 1993 Sep-Oct;4(5):372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- Holmen S. L., Vanbrocklin M. W., Eversole R. R., Stapleton S. R., Ginsberg L. C. Efficient lipid-mediated transfection of DNA into primary rat hepatocytes. In Vitro Cell Dev Biol Anim. 1995 May;31(5):347–351. doi: 10.1007/BF02634283. [DOI] [PubMed] [Google Scholar]
- Jain S., Zon G., Sundaralingam M. Base only binding of spermine in the deep groove of the A-DNA octamer d(GTGTACAC). Biochemistry. 1989 Mar 21;28(6):2360–2364. doi: 10.1021/bi00432a002. [DOI] [PubMed] [Google Scholar]
- Leventis R., Silvius J. R. Interactions of mammalian cells with lipid dispersions containing novel metabolizable cationic amphiphiles. Biochim Biophys Acta. 1990 Mar 30;1023(1):124–132. doi: 10.1016/0005-2736(90)90017-i. [DOI] [PubMed] [Google Scholar]
- Perales J. C., Ferkol T., Beegen H., Ratnoff O. D., Hanson R. W. Gene transfer in vivo: sustained expression and regulation of genes introduced into the liver by receptor-targeted uptake. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4086–4090. doi: 10.1073/pnas.91.9.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy J. S., Sirlin C., Vierling P., Behr J. P. Gene transfer with a series of lipophilic DNA-binding molecules. Bioconjug Chem. 1994 Nov-Dec;5(6):647–654. doi: 10.1021/bc00030a021. [DOI] [PubMed] [Google Scholar]
- Stankovics J., Crane A. M., Andrews E., Wu C. H., Wu G. Y., Ledley F. D. Overexpression of human methylmalonyl CoA mutase in mice after in vivo gene transfer with asialoglycoprotein/polylysine/DNA complexes. Hum Gene Ther. 1994 Sep;5(9):1095–1104. doi: 10.1089/hum.1994.5.9-1095. [DOI] [PubMed] [Google Scholar]
- Strydom S., Van Jaarsveld P., Van Helden E., Ariatti M., Hawtrey A. Studies on the transfer of DNA into cells through use of avidin-polylysine conjugates complexed to biotinylated transferrin and DNA. J Drug Target. 1993;1(2):165–174. doi: 10.3109/10611869308996073. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Huang L. pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7851–7855. doi: 10.1073/pnas.84.22.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Zabner J., Fasbender A. J., Moninger T., Poellinger K. A., Welsh M. J. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995 Aug 11;270(32):18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]



