Abstract
A critical process during thymic development of the T cell repertoire is the induction of self-tolerance. Tolerance in developing T cells is highly dependent on medullary thymic epithelial cells (mTEC) and mTEC development in turn requires signals from mature single positive (SP) thymocytes, a bidirectional relationship termed thymus crosstalk. We show that CD28-CD80/86 and CD40-CD40L costimulatory interactions, which mediate negative selection and self-tolerance, upregulate expression of LTα, LTβ and RANK in the thymus and are necessary for medullary development. Combined absence of CD28-CD80/86 and CD40-CD40L results in profound deficiency in mTEC development comparable to that observed in the absence of SP thymocytes. This requirement for costimulatory signaling is maintained even in a TCR transgenic model of high affinity TCR-ligand interactions. CD4 thymocytes maturing in the altered thymic epithelial environment of CD40/CD80/86 KO mice are highly autoreactive in vitro and are lethal in congenic adoptive transfer in vivo, demonstrating a critical role for these costimulatory pathways in self-tolerance as well as thymic epithelial development. These findings demonstrate that cooperativity between CD28-CD80/86 and CD40-CD40L pathways is required for normal medullary epithelium and for maintenance of self-tolerance in thymocyte development.
Introduction
An important aspect of thymic T cell development is the generation of a functional T cell repertoire that is capable of responding to a broad universe of foreign antigens but that is not reactive to self. Self-tolerance is accomplished by a variety of mechanisms including deletion of thymocytes with high affinity for self and diversion of developing self-reactive thymocytes to a T regulatory cell fate. The thymic medulla plays a central role in this tolerization process, providing an environment in which developing thymocytes are exposed to a spectrum of self antigens during maturation and selection of CD4 and CD8 SP lineages. Medullary thymic epithelial cells (mTEC) are essential to successful induction of self-tolerance, expressing a wide array of peripheral antigens, at least in part due to expression of the autoimmune regulator (AIRE) gene (1, 2). The critical role of AIRE-expressing mTECs in tolerance induction is evidenced by the severe autoimmune disease that occurs when expression of AIRE protein is disrupted (2, 3). The dialogue between CD4 SP thymocytes and stromal cells, termed thymic crosstalk, is in turn important in supporting development of a normal mTEC compartment (4-7). There has therefore been considerable interest in defining the molecular interactions that mediate this critical cross-talk. We undertook studies to determine whether molecular interactions known to be important in the thymic tolerization process, by both promoting negative selection and supporting generation of regulatory T cells, might also have critical roles in maintaining a normal thymic medulla. Thymic T regulatory cell development is critically dependent on CD28-CD80/86 interactions while negative selection of self-reactive SP thymocytes is mediated by both CD28-CD80/86 (8, 9) and CD40-CD40L (10, 11) interactions. We therefore addressed the role of CD28-CD80/CD86 and CD40-CD40L costimulatory pathways in interactions between thymocytes and thymic stromal cells during thymic development.
We found that thymic epithelial cell development was only modestly perturbed by inactivation of the CD40-CD40L or CD28-CD80/86 pathway alone, while in striking contrast, the combined absence of CD28-CD80/86 and CD40-CD40L interactions in CD40/CD80/86 KO mice resulted in a decrease in mTEC numbers as profound as that observed in the complete absence of SP thymocytes. Examination of thymocyte development in the altered thymic environment of CD40/CD80/86 KO mice revealed that CD40/CD80/86 deficient SP thymocytes were highly autoreactive, responding strongly in vitro to syngeneic antigen presenting cells, in contrast to the minimal responses of either CD80/86 or CD40 deficient thymocytes, and causing accelerated death when transferred into congenic nude mice. These findings demonstrate that a strong cooperativity between CD28-CD80/86 and CD40-CD40L pathways is required for both normal epithelial and thymocyte development; in their absence, the tolerance-inducing thymic medullary compartment fails to properly develop and SP thymocytes are autoreactive.
Material and Methods
Mice
BALB/c (BALB) mice were obtained from the Frederick Cancer Research Facility (Frederick, MD) and maintained at Bioqual (Rockville, MD). BALB CD40 deficient mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained at Bioqual; and BALB CD80/86 deficient mice were a generous gift from Arlene Sharpe. BALB CD40/CD80/86 knockout (KO) mice were generated through crosses of CD40 KO and CD80/86 KO mice on a BALB background. BALB CD40L deficient mice were generated by backcrossing C57BL/6 (B6) CD40L KO mice from The Jackson Laboratory (Bar Harbor, ME) for 5 generations onto the BALB background. BALB CD28/CD40L KO mice were generated by crossing the BALB CD40L KO mice with BALB CD28 KO mice obtained from The Jackson Laboratory. LTβR KO (12) mice have been previously described and were crossed to either B6 CD40 KO or B6 CD28 KO (Jackson Laboratory) mice to generate LTβR/CD40 KO or LTβR/CD28 KO mice, respectively. DO11 TCR transgenic mice (13)were crossed to BALB CD40/CD80/86 KO or to B6 CD40/CD80/86 KO mice to generate DO11 tg+ mice lacking expression of CD40, CD80/86 or CD40/CD80/86 on H2d or H2d×b backgrounds, respectively. TCRα KO mice have been previously described (14). Athymic BALB nu/nu mice were obtained from Frederick National Laboratory for Cancer Research.
Cell proliferation assays
CD8-depleted thymocytes (>80% CD4+ SP thymocytes) and T-depleted splenocytes were prepared using CD8-specific, or CD4- and CD8-specfic magnetic beads (Miltenyi), respectively. T-depleted splenocytes were irradiated at 500 rads, then 2 × 105 CD8- depleted thymocytes were added to titrated numbers of irradiated splenocyte APCs in 96 well round bottom plates. Cultures were incubated for 3-4 days prior to addition of 3H-thymidine for 16 hr.
Preparation of thymic stromal cells for flow cytometric analysis and sorting
Thymic stromal cell preparations were made using methods modified from those reported by Gray et al. (15). Following release of thymocytes by gentle teasing of the thymus, thymic fragments were digested with Collagenase/Dispase at 0.25% w/v plus DNase 1 at 0.125% w/v (Roche) in 4 sequential 15 minute incubations at 37°C. Reactions were stopped by addition of FCS to 20%. For TEC analysis, single cell suspensions were stained with anti-CD45.2-Fitc (104; BD), anti-Ly51-PE (BP-1; BD), anti-MHC class II-APC (M5-114; Ebiosciences) and UEA-1 biotin (Vector). Dead cells were excluded with propidium iodide staining. Intracellular staining for AIRE was performed using FoxP3 fix/permeabilization (eBioscience) and rat anti-AIRE (clone 5H12), a generous gift from H. Scott, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia (16) and anti-rat IgG2c-PE (clone 2C-8F1; Southern Biotechnology Associates, Inc.). For mTEC sorting, enriched TEC preparations were made by discontinuous density gradient fractionation (17). Enriched TECs were stained with anti-CD45.2, anti-MHC class II, and UEA-1. CD45.2- negative, MHC II+ UEA+ cells (mTEC) were collected using a FACSAria flow cytometer (BD) and analyzed using FloJo (TreeStar, San Carlos, CA) FACS analysis software. Gating strategies are included in figure legends.
Immunohistology
Sections (6 μm) of OCT-embedded frozen tissue were air-dried for 15 min and then incubated 2 h with optimal dilutions of the primary rabbit polyclonal anti-keratin 14 (Covance Research Products) and rat anti-keratin 8 (Troma-I; DHSB, Iowa University) or anti-Ly51-Pe (BD) and UEA-1 FITC (Vector). Tissues were washed, and after an amplification step (anti-rabbit-Alexa 546 for K14, anti-rat-Alexa 488 for K8, or biotinylated anti-PE followed by streptavidin Alexa 546 for Ly51), were mounted on microscope slides and imaged using a Zeiss axiovert 200M inverted epifluorescence microscope equipped with appropriate fluorescence filters (Chroma Technologies Inc.), a motorized scanning stage, a 10× plan-apochromat (N.A. 0.45) objective lens and a Photometrics CoolsnapES CCD camera (Roper Scientific, Inc.). Sequential images were acquired using the multi-dimensional mosaix algorithm in AxioVision software (Carl Zeiss MicroImaging Inc.). The resultant matrix of images, covering the entire area of the thymus section, was aligned and stitched using AxioVision software. The stitched image was converted to a single large TIFF file. In preparing figures for display, a copy of each TIFF file was rescaled and compiled using Adobe Photoshop.
qPCR
Total cellular RNA was DNaseI-treated and reverse-transcribed with oligo (dT) primer and SuperScript III reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed using SYBR Premix Ex Taq (TaKaRa) and Light Cycler DX400 (Roche). Amplified products were confirmed to be single bands by gel electrophoresis. The primer sequences are described previously (6, 18).
Adoptive transfer to athymic nude recipients
Thymocytes were prepared from wildtype (WT), CD40 KO, CD80/86 KO and CD40/CD80/86 KO mice on a BALB background and injected intravenously into BALB nu/nu recipient mice. Mice were weighed weekly and monitored daily for signs of morbidity.
Histopathology
For histological analysis, organs (liver, small intestine, colon, stomach, heart, spleen, lymph nodes, and lung) were fixed in 10% neutral buffered formalin (Sigma), embedded in paraffin, sectioned and stained with hematoxylin and eosin.
Statistical analysis
Student's t test (unpaired, 2-tailed) was used to determine p values.
Results
Normal thymic medullary development requires CD40-CD40L and CD28-CD80/86 interactions
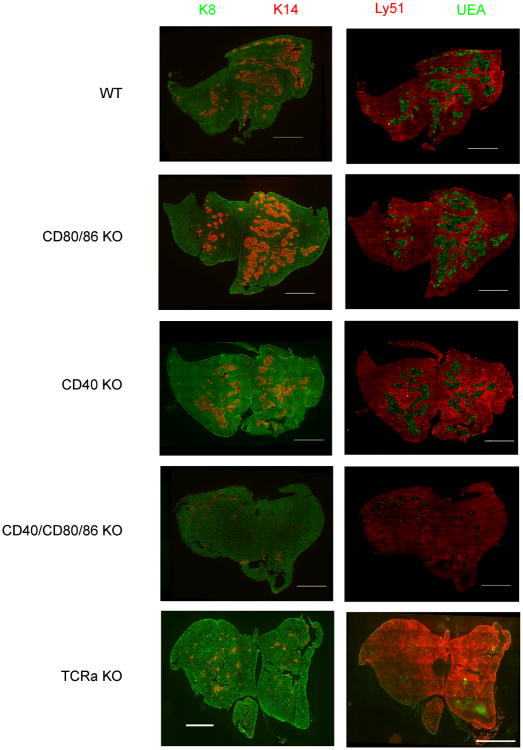
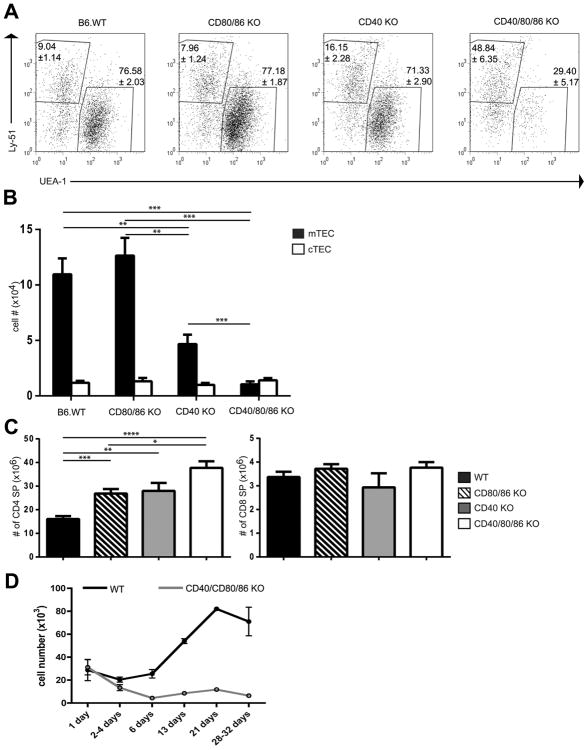
Previous work has documented the importance of thymocyte/stromal cell crosstalk in establishing normal thymic medullary structure (4, 5). Both CD28-CD80/86 and CD40-CD40L pathways are known to be important interactions for deletion of autoreactive thymocytes and mediation of self-tolerance in the thymus (8-11), which prompted us to examine the roles of these costimulatory pathways in the thymic crosstalk that results in development of the thymic medulla. To accomplish this, we analyzed thymic sections from BALB wild type (WT), CD80/86 KO, CD40 KO and CD40/CD80/86 KO mice. As can be seen in Fig 1, deletion of CD40 alone or of CD80/86 alone had little effect on cortico-medullary architecture as defined using either anti-keratin 14 or the lectin UEA-1 to stain thymic medulla, and Ly-51 (cortex specific ) or anti-keratin 8 (stains cortex and some medullary epithelium) to reveal the cortex. In contrast, the medullary epithelium was much reduced in the CD40/CD80/86 KO thymus relative to other genotypes, and was comparable to the profound reduction seen in TCRα KO thymus lacking SP thymocytes. This analysis was extended by flow cytometric characterization. Thymic epithelial cells were defined as CD45negMHCII+ and assigned further as mTEC and cTEC based on staining with UEA and Ly51 (mTEC=UEA+; cTEC=UEAnegLy51+) (Fig 2A). The CD40 KO had reduced numbers of mTEC relative to both WT and CD80/86 KO mice (Fig. 2B). Strikingly, however, the CD40/80/86 KO had a profound reduction in mTEC numbers relative to all other groups, including the CD40 KO. Notably, the number of CD4 or CD8 SP thymocytes in CD40/80/86 KO mice was not decreased, indicating that the defect in mTEC development does not result from a decrease in SP cells known to play a role in medullary development (Fig.2 C). CD4 SP cells were in fact increased in CD80/86 and CD40 KO thymuses, consistent with previous observations (19-21). A significant reduction in mTEC numbers was also observed in BALB CD40L/CD28 KO mice relative to wildtype, CD28 or CD40L single KOs, confirming that it is simultaneous disruption of the CD40-CD40L and CD28-CD80/86 pathways that results in a deficient mTEC compartment (Fig S1).
Figure 1.
Altered thymic microenvironment in CD40/CD80/86 KO mice. Thymic cryosections from WT, CD80/86 KO, CD40 KO, CD40/CD80/86 KO and TCRα KO were stained with a combination of (left panel) anti-keratin 14 (medullary epithelium, red) and anti-keratin-8 (cortical and some medullary epithelium, green) or (right panel) with UEA1 (medullary epithelium, green) and anti-Ly51 (cortical epithelium, red). Bars, 2 mm. Thymic cross sections from 3 mice per strain were stained and representative images are shown. Mice were all 4 weeks of age.
Figure 2.
Number of thymic medullary epithelial cells is decreased in CD40/CD80/86 KO mice while number of CD4 SP thymocytes is increased. (A) Thymic stromal cells from BALB WT, CD80/86 KO, CD40 KO and CD40/CD80/86 KO mice were prepared as described in Materials and Methods and stained with anti-CD45, UEA-1, anti-Ly51 and anti-IA/IE. After first gating on CD45neg, IA/IE+ cells, mTECs were identified as UEA+ and cTEC as UEAnegLy51+. Flow cytometry dot plots are representative of > 9 mice per strain. (B) Thymic stromal cells were prepared as in (A). After gating on CD45neg, IA/IE+ cells, numbers of mTEC (UEA+) and cTEC (UEAneg, Ly51+) were calculated for each group. Data shown are mean ± SE for ≥ 9 mice per strain. All mice were between 3-5 weeks of age. **, p≤ 0.01; ***, p≤ 0.001. (C) Thymocytes from 4 week old WT, CD80/86 KO, CD40 KO and CD40/CD80/86 KO mice were stained with anti-CD4 and anti-CD8 and numbers of CD4 and CD8 SP were calculated after gating on the respective single positive populations. *, p≤ 0.05; **, p≤ 0.01; ***, p≤ 0.001 ****, p≤ 0.0001. (D) mTEC population in CD40/CD80/86 KO mice fails to expand after birth. Thymic stromal cells from BALB WT and CD40/80/86 KO mice were prepared from mice 1-32 days of age and stained as in B. After gating on CD45neg, IA/IE+ cells, numbers of mTEC (UEA-1+) were determined. Data shown are the mean ± SE for ≥ 3 mice per strain.
mTECs are a heterogeneous population consisting of MHCIIloCD80lo and MHCIIhi CD80hi cells. A subpopulation of the MHCIIhi CD80hi cells are AIRE+ and have a critical role in the induction of thymic tolerance (reviewed in (22)). Interestingly, while the total number of UEA+ mTECs was significantly decreased in CD40/CD80/86 KO mice, the frequencies of MHCIIhi and MHCIIlo mTEC, and the frequency of AIRE+ MHCIIhi cells within the mTEC population was similar to that of WT mice (Fig S2A, S2B). Overall, however, there was a dramatic reduction in the absolute number of AIRE+ mTEC in CD40/CD80/86 KO mice (Fig S2C).
To further characterize the decrease in mTECs observed in CD40/CD80/86 KO mice, we compared the number of mTECs present in WT and CD40/CD80/86 KO mice at various time points after birth. Numbers of mTECs were comparable in WT and CD40/CD80/86 KO mice shortly after birth (Fig. 2D). With increasing age, the number of mTEC increased progressively in WT mice, but not in KO mice. Thus the absence of CD40-CD40L and CD28-CD80/86 interactions does not affect the numbers of mTEC present until several days after birth, a time at which the number of SP thymocytes begins to dramatically increase.
Costimulation influences expression of genes critical for mTEC development and SP thymocyte recruitment to the medulla
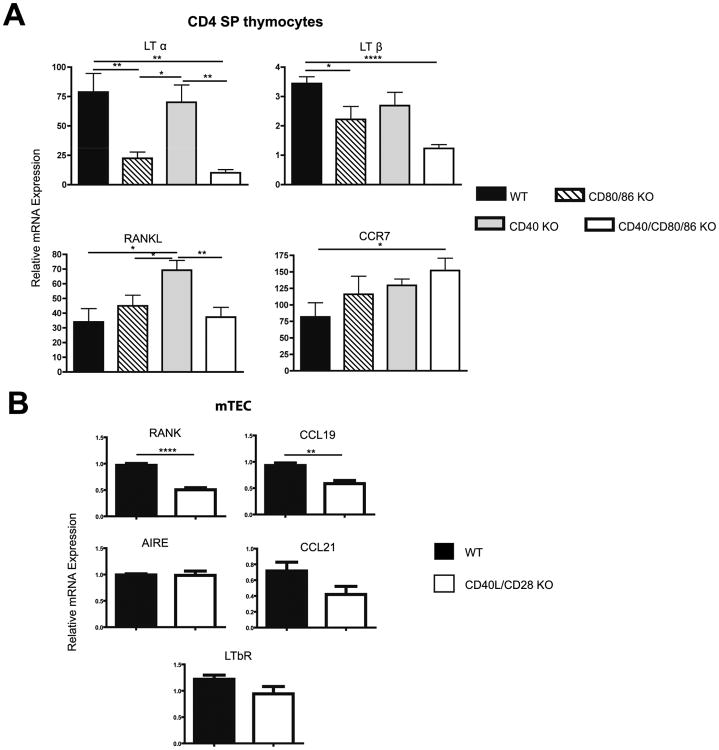
A number of the factors implicated in mTEC development are members of the TNF/TNFR family known to signal through the non-classical NF-κB pathway central to lympho-organogenesis and mTEC formation (reviewed in (23)). Therefore, to identify the mechanisms underlying collaborative effects of CD28 and CD40 pathways in mTEC development, we determined if disruption of CD28-CD80/86 and CD40-CD40L interactions affected expression of a panel of TNF/TNFR family members in both CD4 SP cells and mTEC. Relative to WT CD4 thymocytes, levels of LTα and LTβ mRNA were significantly decreased in the CD80/86 KO and CD40/CD80/86 KO but not in CD40 KO CD4 SPs (Fig. 3A). In addition, expression of the chemokine CCL19, known to be upregulated in mTEC in response to LTα/β (24), was decreased in CD40L/CD28 KO relative to WT mTEC. Expression of mTEC CCL21, was not significantly decreased (p=0.1). CD4 SP thymocyte expression of RANKL was not reduced in the absence of CD40-CD40L and/or CD28-CD80/86 interactions, while comparison of WT and CD40L/CD28 KO mTEC revealed that expression of RANK was significantly decreased in CD28/CD40L KOs (Fig. 3B). Expression of LTβR and AIRE in WT and CD28/CD40L KO mTECs was comparable. Taken together, the gene expression data from CD4 SP thymocytes and from mTEC indicate that mice lacking both CD28-CD80/86 and CD40-CD40L interactions have deficiencies in at least three TNFR family pathways which are known to promote mTEC development: CD40L-CD40, LT-LTβR and RANKL-RANK, as well as reduction in the CCL19 chemokine important for recruiting SP thymocytes to the medulla where they are subject to negative selection.
Figure 3.
Quantitative RT-PCR analysis of sorted BALB WT, CD80/86 KO, CD40 KO, and CD40/CD80/86 KO CD4 SP thymocytes, and WT and CD28/CD40L mTECs. (A) mRNA expression of LTα, LTβ, RANKL, and CCR7 in CD4 SP thymocytes was normalized to GAPDH mRNA and values for the WT DP samples were arbitrarily set to 1. Data shown are mean ± SE for 5 independent measurements for each strain (*, p ≤0.05; **, p ≤0.01; ****, p ≤0.0001). All mice were 4 weeks of age. (B) mTEC were sorted as described in Material and Methods by gating on CD45neg, MHC II+, UEA+ cells. mRNA expression of RANK, CCL19, CCL21, AIRE, and LTβR was normalized to GAPDH. Data shown are mean ± SE for n=5 (WT) or n=4 (CD28/CD40L K0) independent measurements. For each independent measurement, RNA was prepared from mTECs sorted from a minimum of 4 pooled thymuses per strain. All mice were 2 weeks of age. (p=0.1 for CCL21; **, p<0.01; ****p<0.0001).
CD28 acts via regulation of LTα/β expression in cooperating with CD40 signals to promote mTEC development
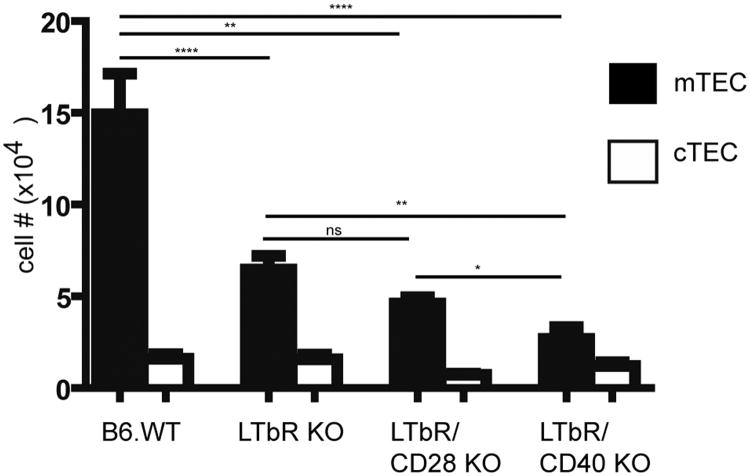
The observations that CD28-CD80/86 interactions contribute significantly to production of LTαβ in CD4 SP thymocytes and that CD40/CD80/86 KO mice have a profound reduction in mTEC prompted us to test the hypothesis that CD28 signals cooperate with CD40 signals via regulation of the LTβR pathway to promote mTEC development. To do this we examined the medullary phenotype of LTβR/CD40 KO mice. As previously described (25, 26), we observed a significant reduction in the number of UEA+ mTEC in LTβR KOs relative to WT mice (Fig. 4). Interestingly, mTEC were significantly further reduced when the LTβR KO is combined with CD40 KO in LTβR/CD40 KO mice but not when combined with CD28 KO in LTβR/CD28 KO mice (Fig. 4). The failure to observe a further deficit in mTEC numbers in LTβR/CD28 KOs relative to LTβR KO mice suggests that the major contribution of CD28-CD80/86 interactions to mTEC development is via increased SP thymocyte expression of LTαβ. Conversely, the finding that the LTβR/CD40 KO does have a more profound deficit in mTEC relative to the LTβR KO is consistent with CD28-CD80/86 cooperating with CD40-CD40L interactions to facilitate mTEC development by promoting SP thymocyte production of LTαβ.
Figure 4.
Number of thymic medullary epithelial cells in CD40/LTβR KO mice is reduced relative to LTβR KO mice. Thymic stromal cells from 3-5 week old mice were prepared as described in Materials and Methods and stained with anti-CD45, UEA-1, anti-Ly51 and anti-IA/IE. After gating on CD45neg, IA/IE+ cells, numbers of mTEC (UEA+), and cTEC (UEAneg, Ly51+) were calculated for each group. Data shown are mean ± SE for ≥ 3 mice per strain. (*, p<0.05; **, p<0.01, ****p<0.0001), ns – not significant.
Expression of a TCR with high affinity for self does not overcome the requirement for costimulation to promote mTEC development
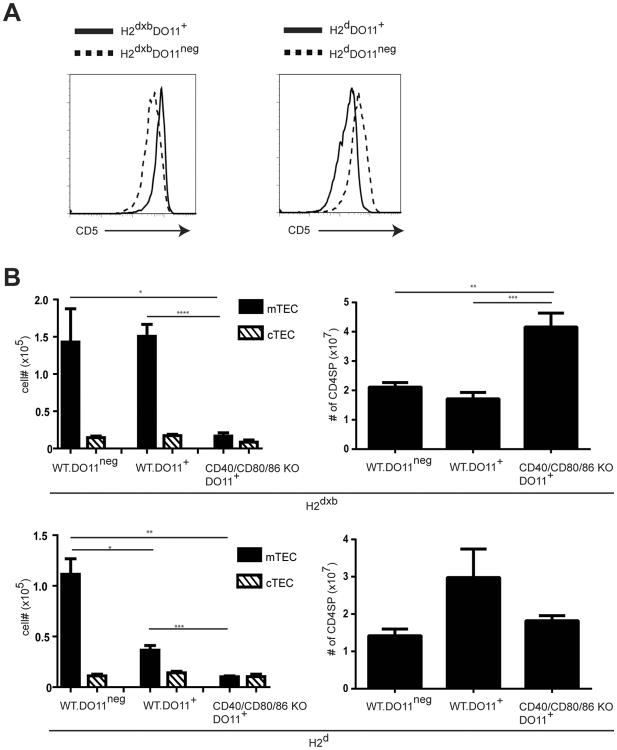
Requirements for costimulation in the activation of peripheral T cells are influenced by the strength of TCR-ligand interactions, such that strong TCR signaling can reduce or obviate the requirement for costimulation (27, 28). It has also been reported that high affinity TCR-ligand interactions are important in driving mTEC development (7). We therefore asked whether expression of a TCR with known high affinity for self antigen would overcome the requirement for CD40-CD40L and CD28-CD80/86 interactions in promoting mTEC development. To this end we generated DO11 TCRtg+ mice on either an H2d or H2b×d background and compared development of mTEC in the presence or absence of CD40-CD40L and CD28-CD80/86 interactions. The DO11 TCR transgene (tg) has been shown to have a high affinity for self I-Ab ligand, as evidenced by increased CD5 levels on DO11 tg+ in comparison with DO11tgneg CD4+ SP thymocytes in I-Ab-expressing H2b×d mice (29, 30), consistent with results shown in Fig.5A (left panel). The lower affinity of the DO11 tg for H-2d ligand is reflected in the reduced CD5 levels of DO11 tg+ T cells in H2d mice (Fig. 5A right panel). When we examined the thymic epithelial compartment of DO11tg+ H2b×d mice, we observed no significant difference in the numbers of mTECs recovered relative to those found in DO11tgneg H2b×d mice, whereas numbers of mTEC are profoundly reduced in DO11tg+ H2d mice compared to DO11tgneg H2d mice. These results are consistent with an enhanced ability of high affinity TCR to mediate the robust interactions required to support mTEC development (Fig 5B). Interestingly, we found that generation of the mTEC compartment even in the presence of high affinity TCR is strongly dependent on CD40-CD40L and CD28-CD80/86 costimulatory interactions, as mTEC numbers in DO11tg+ CD40/CD80/86 KO H2b×d mice are dramatically reduced relative to DO11tg+ WT H2b×d mice. Notably, the number of CD4 SP thymocytes in DO11tg+ CD40/CD80/86 KO H2b×d mice was not reduced, and in fact was significantly increased, in costimulation-deficient mice, indicating that defects in mTEC development were not the result of decreased SP thymocytes (Fig. 5B). Thus, the requirement for CD40-CD40L and CD28-CD80/86 costimulatory interactions is stringently maintained even in the presence of a high affinity TCR-ligand interaction.
Figure 5.
High affinity TCR-MHC interactions are not sufficient to overcome requirement for costimulation. (A) Thymocytes from CB6F1 DO11tgneg (H2d×b), CB6F1 DO11 TCRtg+(H2d×b), BALB DO11tgneg (H2d), and BALB DO11 TCR tg+(H2d) mice were stained with anti-CD4, anti-CD8, KJ126 (DO11 TCRtg+ samples only), and anti-CD5 and analyzed by flow cytometry. Histograms show CD5 expression on gated CD4 SP thymocytes from CB6F1 DO11tgneg (H2d×b) and BALB DO11tgneg (H2d) and KJ126+ CD4 SP thymocytes from and CB6F1 DO11 TCRtg+ (H2d×b), and BALB DO11 TCR tg+(H2d) mice. (B) Preparations of thymic stromal cells from costimulation intact as well as CD40/CD80/86 deficient CB6F1 DO11tgneg (H2d×b), CB6F1 DO11 TCRtg+ (H2d×b), BALB DO11 TCR tgneg(H2d), and BALB DO11 TCR tg+(H2d) mice were stained with anti-CD45, UEA-1, anti-Ly51 and anti-IA/IE to identify mTEC (CD45neg, IA/IE+, UEA+) and cTEC (CD45neg, IA/IE+,UEAneg, Ly51+). Thymocytes were stained with anti-CD4 and anti-CD8 to identify CD4 SP cells. Numbers of mTEC, cTEC, and CD4 SP thymocytes for each strain are shown. Bar graphs show the mean ± SE for ≥ 4 mice per strain (*, p < 0.05;**, p < 0.01; ***, p < 0.001).
Altered thymocyte subpopulations in CD40/CD80/86 KO mice: increased CD4 SP cells and severe reductions of T regulatory and iNKT subpopulations
As shown above in Fig 2C, the proportion and number of CD4 SP thymocytes was increased in CD40 KO and CD80/86 KO mice, and was further increased in CD40/CD80/86 KO mice relative to WT, CD40 KO and CD80/86 KOs while numbers of CD8 SP thymocytes were not significantly different. The significant changes in the thymic medullary compartment of CD40/CD80/86 KO mice prompted us to further characterize the thymocyte subpopulations present in these animals.
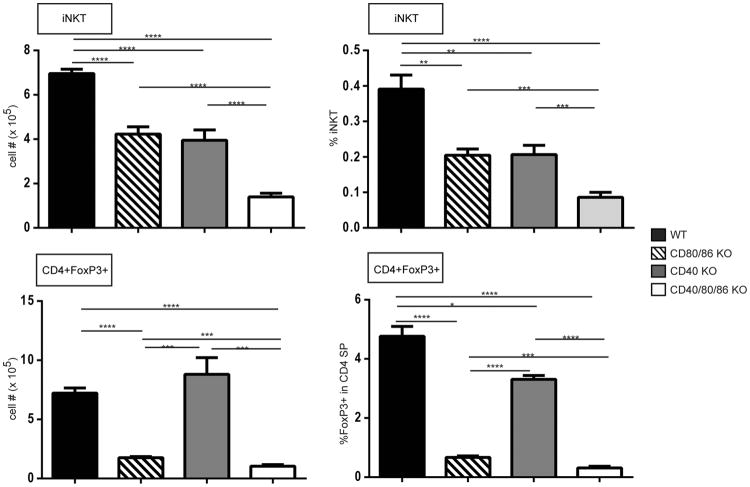
Using a CD1-tetramer to identify thymic iNKT cells, we observed a significant reduction in these cells in CD80/86 KO mice as previously reported (31, 32) and, interestingly, also found that the absence of CD40 was accompanied by a significant decrease in thymic iNKTs, a finding, to our knowledge, not previously reported (Fig. 6 ). A significantly more profound decrease of iNKT cells occurred in CD40/CD80/86 KO mice.
Figure 6.
Changes in number (left panels) and frequency (right panels) of thymocyte subpopulations in the absence of costimulatory interactions. Thymocytes from 4 week old BALB WT, CD80/86 KO, CD40 KO and CD40/CD80/86 KO mice were stained with anti-CD3 and CD1-PBS-57 tetramers to identify iNKT cells (top panels), or anti-CD4, anti-CD8 and anti-FoxP3 to identify Treg cells (bottom panels) and analyzed by flow cytometry. Bar graphs show the mean ± SE for 4-9 mice per strain. (*, p≤ 0.05; **, p≤ 0.01; ***, p≤ 0.001 ****, p≤ 0.0001).
CD4+FoxP3+ (Treg) thymocytes were clearly decreased in the absence of CD80/86, as previously reported (33, 34) (Fig. 6 ). Also consistent with previous reports, we found a decrease in the frequency of CD4+ Treg thymocytes in CD40 KO mice (35, 36), but we did not see a decrease in absolute Treg numbers. Notably, however, both Treg cell frequency and number were decreased in CD40/CD80/86 KO relative to wildtype, CD40 KO and even CD80/86 KOs (Fig. 6). Thus, iNKT and Treg thymic populations are dependent on both CD28-CD80/86 and CD40 signaling.
CD40/CD80/86 KO thymocytes are autoreactive
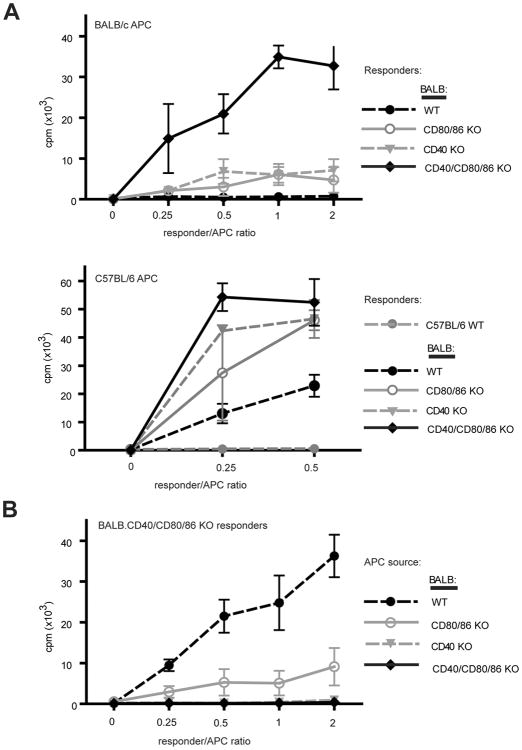
The increase in CD4 SP thymocytes observed in CD40/CD80/86 KO mice suggested that tolerance induction via clonal deletion may not be occurring normally in these mice. The deficiency in regulatory iNKT cells and Treg further suggested that defects in tolerance might occur in this setting. To determine whether or not these defects in CD40/CD80/86 KO mice result in the appearance of an autoreactive T cell repertoire, we first examined the TCR Vβ repertoire of the BALB CD40/CD80/86 KO to assess endogenous superantigen (SAg)-mediated negative selection. SAg-mediated deletion of CD4 SP cells expressing Vb5, Vb11 and Vb12 was severely compromised in BALB CD40 KO, consistent with previous reports (Fig. S3) (10, 11). BALB CD40/CD80/86 KO mice exhibited a similarly severe impairment. We next assessed self-reactivity in mixed lymphocyte cultures with responder CD4-enriched thymocytes derived from BALB WT, CD40 KO, CD80/86 KO or CD40/CD80/86 KO animals and syngeneic BALB WT APCs or allogeneic B6 WT APCs. CD4-enriched thymocytes from BALB WT mice did not proliferate in response to BALB APC (Fig. 7A). Strikingly, in contrast to the limited magnitude of syngeneic responses of CD40KO or CD80/86KO thymocytes, CD40/CD80/86 KO thymocytes generated a much stronger syngeneic response. All four BALB background strains responded vigorously to allogeneic B6 APCs. CD40/CD80/86 KO CD4 thymocytes failed to generate a proliferative response to BALB CD40 KO or CD40/CD80/86 KO splenic APCs and mounted a much reduced response to CD80/86 KO APCs, indicating that both CD40-CD40L and CD80/86-CD28 interactions are required for this syngeneic response (Fig. 7B).
Fig 7.
CD40/CD80/86 KO thymocytes proliferate in syngeneic MLRs in a costimulation-dependent manner. (A) CD8-depleted thymocytes from BALB WT, CD80/86 KO, CD40 KO and CD40/CD80/86 KO mice were cultured with T cell-depleted BALB or B6 WT splenocytes for 72 hr prior to addition of 3H-thymidine for 16 hr. Results shown are means of triplicate wells ± SE and are representative of 8 independent experiments. (B) CD8-depleted BALB CD40/CD80/86 KO thymocytes were cultured with T-depleted BALB WT, CD80/86 KO, CD40 KO and CD40/CD80/86 KO splenocytes for 72 hr prior to addition of 3H-thymidine for 16 hr. Results shown represent means of triplicate wells ± SE and are representative of 2 independent experiments.
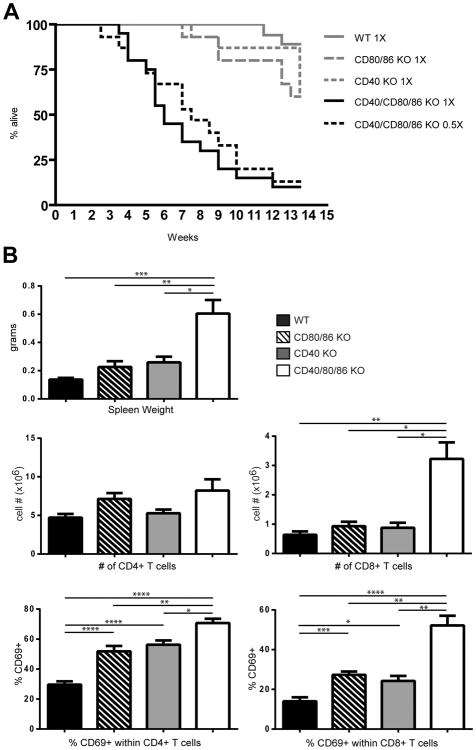
CD40/CD80/86 KO mice appeared healthy, with no apparent signs of overt autoimmunity, despite the in vitro autoreactivity of these cells. However, since in vitro autoreactivity was dependent upon expression of CD40 and CD80/86 costimulatory ligands on APC, it was possible that the absence of in vivo manifestations of autoreactivity in CD40/CD80/86 KO mice was due to the absence of CD40-CD40L and CD28-CD80/86 costimulatory interactions required to drive an autoimmune response. To overcome the absence of costimulatory interactions and to test whether the autoreactive response displayed by CD40/CD80/86 KO thymocytes in vitro would be manifest in vivo, thymocytes from BALB WT, CD40 KO, CD80/86 KO and CD40/CD80/86 KO mice were adoptively transferred to congenic athymic BALB nude CD40+/CD80/86+ recipients. Transfer of CD40/CD80/86 KO thymocytes resulted in death of the majority of recipient mice by 2 months post-transfer, a timepoint at which no deaths had occurred in recipients of BALB WT, CD40 KO or CD80/86 KO thymocytes (Fig 8A). Detailed histological examination revealed no consistent cause of death, but striking lymphadenopathy and splenomegaly were significantly more pronounced in recipients of CD40/CD80/86 KO thymocytes, and splenic CD8 T cells from these mice were significantly increased in numbers relative to BALB nudes receiving WT, CD80/86 KO or CD40 KO thymocytes (Fig 8B). In addition, the proportion of CD69+ CD4 and CD8 T cells was significantly higher in spleens of recipients of CD40/CD80/86 KO thymocytes relative to recipients of individual KO or WT thymocytes. Taken together, the autoproliferative phenotype observed in the BALB CD40/CD80/86 KO thymocytes in vitro and the high mortality rates accompanied by significant T cell activation observed in in vivo transfer indicate that there has been a breakdown of functional self-tolerance in the CD40/CD80/86 KO thymus.
Figure 8.
Transfer of CD40/80/86 KO thymocytes into syngeneic BALB nu/nu mice results in rapid death. Total thymocytes were purified from BALB WT, CD80/86 KO, CD40 KO and CD40/80/86 KO mice and 1 × 107 (1×) or 0.5 × 107 (0.5×) cells were transferred i.v. to BALB nu/nu recipient mice. (A) Mice were monitored daily for 13. 5 weeks and the proportion of surviving mice are shown plotted against days post transfer. 15-20 mice were included in each group. (B) Splenomegaly and increased numbers of activated CD8 T cells in BALB nu/nu mice receiving CD40/CD80/86 KO thymocytes. Total thymocytes were purified from BALB WT, CD80/86 KO, CD40 KO and CD40/80/86 KO mice and transferred i.v. to BALB nu/nu recipient mice as in Fig. 8A. Single cell suspensions were prepared from spleens harvested from BALB nu/nu recipient mice 4-6.5 weeks after receiving thymocytes and stained with anti-CD4, anti-CD8 and anti-CD69. Data shown are means ± SE for WT (n=9), CD80/86 KO (n=6), CD40 KO (n=5) and CD40/CD80/86 KO (n=17). 1 × 107 thymocytes were transferred for WT, CD80/86 KO and CD40 KO, while the data for the CD40/CD80/86 KO transfers represent both 1× (1 × 107) and 0.5× (0.5 × 107 ) cells transferred (*, p≤ 0.05; **, p≤ 0.01; ***, p≤ 0.001 ****, p≤ 0.0001).
Discussion
In the studies reported here, we found that simultaneous disruption of CD28-CD80/86 and CD40-CD40L costimulatory pathways results in a profound defect in mTEC development that is as severe as that which results from absence of all SP thymocytes. Moreover, CD4 SP thymocytes that matured in the altered thymic epithelial environment of CD40/CD80/86 KO mice were highly autoreactive in in vitro proliferative responses and were lethal when adoptively transferred to congenic athymic recipients in vivo. Collectively, these findings demonstrate a critical and cooperative role for these costimulatory pathways in the bidirectional interactions between medullary epithelium and developing thymocytes that are critical for self-tolerance as well as thymic epithelial development.
Consistent with previous reports, we observed that disruption of the CD40-CD40L pathway alone resulted in a significant defect in mTEC development, but one that was far less severe than that in mice deficient in both CD40-CD40L and CD28-CD80/86. We found no effect of disruption of CD28-CD80/86, although a recent report employing an elegant technique of 3-D reconstruction did identify an effect of CD80/86 deletion on mTEC(37).
Interestingly, the deficit which we observed in CD40/CD80/86 KO mTEC cell numbers was not evident in newborn mice and did not become apparent until approximately day 6 after birth, following the appearance of SP thymocytes. In the embryonic thymus, mTEC are critically dependent on RANKL-expressing LTi cells (38) and Vγ5 γδ T cells (39). Our data suggest that this early LTi-dependent mTEC development is occurring normally in the CD40/CD80/86 KO mice, with defects in the mTEC compartment only being manifest in 5-6 day old thymus. Expression of CD40L in the thymus is not detectable until the appearance of CD4 SP thymocytes (40) and is not detectable on LTi cells (41). Although CD28 is expressed on late DN and DP thymocytes, it is unlikely that these immature thymocytes would have access to or interact with CD80/86-expressing mTEC or thymic dendritic cells which are primarily localized to the medulla (42). The migration of developing thymocytes to the medulla occurs only once developing DP thymocytes receive a positive selection signal and the chemokine receptor, CCR7, is upregulated, allowing positively selected thymocytes to migrate towards CCL19/21-producing mTECs (43). Thus CD80/86-CD28 and CD40-CD40L interactions are expected to become critical components of the circuitry that promotes mTEC development only after CD4 SP thymocytes begin to appear, precisely as we observed when tracking mTEC numbers at birth and shortly thereafter.
mTECs express a number of TNFR family members including CD40, LTβR, and RANK (6). A feature shared by the TNFR family members expressed on mTECs is the capacity to activate RelB (44-46), a critical component of the non-classical NF-κB pathway. The importance of this pathway in mediating development of a normal mTEC compartment is evidenced by the absence of UEA+ medullary epithelial cells in mice deficient for crucial components involved in the alternative NF-κB pathway including RelB, IKKα, and NIK (47-51). The mechanism(s) by which SP thymocytes promote mTEC development have been shown to include important roles for members of the TNFR family ligands. In particular, CD40L, RANKL and LTαβ have all been shown to be upregulated on SP thymocytes relative to DN and DP thymocytes (6); and mice lacking RANK-RANKL, LTαβ-LTβR or CD40-CD40L interactions have mTEC compartment defects of varying severities (6, 25, 38, 40, 41). Consistent with recent reports (37, 52), we found that expression of LTαβ on CD4 SP thymocytes is decreased in the absence of CD28-CD80/86 interactions. Our finding that LTβR/CD40 combined KO mice have a more profound mTEC defect than either the LTβR or CD40 single KOs suggests that CD28-CD80/86 cooperates with CD40-CD40L interactions to promote mTEC development, in part via increased LTαβ production by CD4 SP thymocytes. These findings are consistent with the recent report that combined stimulation of 2-dGUO-treated thymic lobes with agonist anti- LTβR antibody and CD40L synergized in the in vitro induction of mTEC (52), importantly extending these findings by demonstrating that LTβR and CD40L pathways in fact interact cooperatively during in vivo thymic development. Furthermore, we found that RANK expression is decreased on mTEC in mice deficient for both CD40-CD40L and CD28-CD80/86 interactions, possibly a reflection of the decrease in LTαβ observed in the absence of CD28 signals (37, 52). Thus, in the CD40/CD80/86 KO thymus, RANK-RANKL, LTαβ-LTβR and CD40-CD40L interactions, all shown to cooperate in promoting development of mTEC, are either significantly reduced (RANK-RANKL and LTαβ-LTβR) or eliminated (CD40-CD40L), providing a basis for explaining the dramatic reduction in mTEC numbers we observe in the these mice.
Strong in vitro and lethal in vivo autoreactivity was observed in CD4 SP thymocytes that develop in CD40/CD80/86 KO thymus. A number of factors may contribute to the observed failure to induce self-tolerance in the absence of these costimulatory pathways. Autoreactivity may result, at least in part, from the defective thymic medullary epithelial compartment found in these costimulation-deficient animals, as defective tolerance induction has been shown to accompany disruption of the mTEC compartment. RelB-, Traf6-, Bcl3/NFκB2-, and CD40/RANK-deficient mice all provide examples of this; in each of these cases, severe defects in development of the mTEC compartment are coupled with an autoreactive T cell repertoire (40, 47, 48, 53, 54). In the RelB-, Traf6- and Bcl3/NF-κB2-deficient animals, clear signs of autoreactivity were present in the knockouts themselves whereas in the CD40/RANK-deficient mice, similar to our CD40/CD80/86 KOs, autoreactivity was revealed only when the T cells were placed in an environment where the ligands for T cell activation (i.e. CD40L and CD80/86) were supplied (40). However, while the severe disruption of the thymic medullary compartment may have a role in allowing self-reactive thymocytes to develop in the CD40/CD80/86 KOs, interactions between the costimulatory receptor-ligand pairs CD28-CD80/CD86 and CD40-CD40L have additional roles in shaping the repertoire of developing thymocytes that could, in their absence, lead to the emergence of autoreactive thymocytes. CD40-CD40L interactions play an essential role in negative selection of autoreactive thymocytes (10, 11), while CD28-CD80/CD86 interactions are required for thymic development of FoxP3+ Treg cells (33, 34) as well as a reported role in negative selection (8, 19). It is therefore possible that the failure to appropriately tolerize developing thymocytes in CD40/CD80/86 KO mice is multifaceted and reflects a failure of negative selection compounded by a deficit in mTECs and the absence of a T regulatory cell population.
The results reported here demonstrate that the effects of CD40-CD40L and CD28-CD80/86 interactions in the thymus are bidirectional and influence both CD28- and CD40L-expressing T cells and the CD80/86- and CD40-expressing cells with which these T cells interact; in the absence of these costimulatory pathways, a normal mTEC compartment fails to be formed and autoreactive thymocytes are allowed to persist. Thus, these costimulatory molecules that are vital components in the dialogue that occurs between T cells and the antigen presenting cells with which they interact in the periphery are also critical elements of the crosstalk that shapes the development of thymocytes and the epithelial cells of the thymus.
Supplementary Material
Acknowledgments
The authors wish to dedicate this paper to the memory of Dr. David Klug. David's intense intellectual curiosity, keen insight and infectious enthusiasm were an inspiration to all his colleagues. We thank Dr. Alfred Singer and Karen Hathcock for critical reading of the manuscript.
References
- 1.Heino M, Peterson P, Kudoh J, Nagamine K, Lagerstedt A, Ovod V, Ranki A, Rantala I, Nieminen M, Tuukkanen J, Scott HS, Antonarakis SE, Shimizu N, Krohn K. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257:821–825. doi: 10.1006/bbrc.1999.0308. [DOI] [PubMed] [Google Scholar]
- 2.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 4.Shores EW, Van Ewijk W, Singer A. Disorganization and restoration of thymic medullary epithelial cells in T cell receptor-negative scid mice: evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. Eur J Immunol. 1991;21:1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 5.Surh CD, Ernst B, Sprent J. Growth of epithelial cells in the thymic medulla is under the control of mature T cells. J Exp Med. 1992;176:611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foy TM, Page DM, Waldschmidt TJ, Schoneveld A, Laman JD, Masters SR, Tygrett L, Ledbetter JA, Aruffo A, Claassen E, Xu JC, Flavell RA, Oehen S, Hedrick SM, Noelle RJ. An essential role for gp39, the ligand for CD40, in thymic selection. J Exp Med. 1995;182:1377–1388. doi: 10.1084/jem.182.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JA, Sharrow SO, Adams AJ, Hodes RJ. CD40 ligand functions non-cell autonomously to promote deletion of self-reactive thymocytes. J Immunol. 2002;168:2759–2765. doi: 10.4049/jimmunol.168.6.2759. [DOI] [PubMed] [Google Scholar]
- 12.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 14.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 15.Gray DH, Fletcher AL, Hammett M, Seach N, Ueno T, Young LF, Barbuto J, Boyd RL, Chidgey AP. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods. 2008;329:56–66. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Hubert FX, Kinkel SA, Webster KE, Cannon P, Crewther PE, Proeitto AI, Wu L, Heath WR, Scott HS. A specific anti-Aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180:3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
- 17.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 18.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, Malefyt Rde W, Nitta T, Takahama Y. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noel PJ, Alegre ML, Reiner SL, Thompson CB. Impaired negative selection in CD28-deficient mice. Cell Immunol. 1998;187:131–138. doi: 10.1006/cimm.1998.1332. [DOI] [PubMed] [Google Scholar]
- 20.Vacchio MS, Williams JA, Hodes RJ. A novel role for CD28 in thymic selection: elimination of CD28/B7 interactions increases positive selection. Eur J Immunol. 2005;35:418–427. doi: 10.1002/eji.200424918. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 22.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 23.Nitta T, Ohigashi I, Nakagawa Y, Takahama Y. Cytokine crosstalk for thymic medulla formation. Curr Opin Immunol. 2011;23:190–197. doi: 10.1016/j.coi.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Seach N, Ueno T, Fletcher AL, Lowen T, Mattesich M, Engwerda CR, Scott HS, Ware CF, Chidgey AP, Gray DH, Boyd RL. The lymphotoxin pathway regulates Aire-independent expression of ectopic genes and chemokines in thymic stromal cells. J Immunol. 2008;180:5384–5392. doi: 10.4049/jimmunol.180.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouri Y, Yano M, Shinzawa M, Shimo Y, Hirota F, Nishikawa Y, Nii T, Kiyonari H, Abe T, Uehara H, Izumi K, Tamada K, Chen L, Penninger JM, Inoue J, Akiyama T, Matsumoto M. Lymphotoxin signal promotes thymic organogenesis by eliciting RANK expression in the embryonic thymic stroma. J Immunol. 2011;186:5047–5057. doi: 10.4049/jimmunol.1003533. [DOI] [PubMed] [Google Scholar]
- 27.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 28.Lumsden JM, Prasad SJ, Peach RJ, Ronchese F. The effects of B7-dependent costimulation on T cell division and survival in vivo and in vitro are dependent on antigen concentration. Eur J Immunol. 2003;33:2074–2082. doi: 10.1002/eji.200323929. [DOI] [PubMed] [Google Scholar]
- 29.Love PE, Lee J, Shores EW. Critical relationship between TCR signaling potential and TCR affinity during thymocyte selection. J Immunol. 2000;165:3080–3087. doi: 10.4049/jimmunol.165.6.3080. [DOI] [PubMed] [Google Scholar]
- 30.Liu CP, Kappler JW, Marrack P. Thymocytes can become mature T cells without passing through the CD4+ CD8+, double-positive stage. J Exp Med. 1996;184:1619–1630. doi: 10.1084/jem.184.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng X, Zhang H, Yin L, Wang CR, Liu Y, Zheng P. Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation. PLoS One. 2008;3:e2703. doi: 10.1371/journal.pone.0002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JA, Lumsden JM, Yu X, Feigenbaum L, Zhang J, Steinberg SM, Hodes RJ. Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway. J Immunol. 2008;181:907–917. doi: 10.4049/jimmunol.181.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 34.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 35.Guiducci C, Valzasina B, Dislich H, Colombo MP. CD40/CD40L interaction regulates CD4+CD25+ T reg homeostasis through dendritic cell-produced IL-2. Eur J Immunol. 2005;35:557–567. doi: 10.1002/eji.200425810. [DOI] [PubMed] [Google Scholar]
- 36.Spence PJ, Green EA. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A. 2008;105:973–978. doi: 10.1073/pnas.0709071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irla M, Guenot J, Sealy G, Reith W, Imhof BA, Serge A. Three-dimensional visualization of the mouse thymus organization in health and immunodeficiency. J Immunol. 2013;190:586–596. doi: 10.4049/jimmunol.1200119. [DOI] [PubMed] [Google Scholar]
- 38.Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, Lane PJ, Anderson G. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, Cunningham AF, Paolino M, Penninger JM, Simon AK, Nitta T, Ohigashi I, Takahama Y, Caamano JH, Hayday AC, Lane PJ, Jenkinson EJ, Anderson G. Rank signaling links the development of invariant gammadelta T cell progenitors and Aire(+) medullary epithelium. Immunity. 2012;36:427–437. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 41.White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJ, Jenkinson EJ, Anderson G. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol. 2008;38:942–947. doi: 10.1002/eji.200738052. [DOI] [PubMed] [Google Scholar]
- 42.Williams JA, Hathcock KS, Klug D, Harada Y, Choudhury B, Allison JP, Abe R, Hodes RJ. Regulated costimulation in the thymus is critical for T cell development: dysregulated CD28 costimulation can bypass the pre-TCR checkpoint. J Immunol. 2005;175:4199–4207. doi: 10.4049/jimmunol.175.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coope HJ, Atkinson PG, Huhse B, Belich M, Janzen J, Holman MJ, Klaus GG, Johnston LH, Ley SC. CD40 regulates the processing of NF-kappaB2 p100 to p52. Embo J. 2002;21:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 47.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 48.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 49.Lomada D, Liu B, Coghlan L, Hu Y, Richie ER. Thymus medulla formation and central tolerance are restored in IKKalpha-/- mice that express an IKKalpha transgene in keratin 5+ thymic epithelial cells. J Immunol. 2007;178:829–837. doi: 10.4049/jimmunol.178.2.829. [DOI] [PubMed] [Google Scholar]
- 50.Kinoshita D, Hirota F, Kaisho T, Kasai M, Izumi K, Bando Y, Mouri Y, Matsushima A, Niki S, Han H, Oshikawa K, Kuroda N, Maegawa M, Irahara M, Takeda K, Akira S, Matsumoto M. Essential role of IkappaB kinase alpha in thymic organogenesis required for the establishment of self-tolerance. J Immunol. 2006;176:3995–4002. doi: 10.4049/jimmunol.176.7.3995. [DOI] [PubMed] [Google Scholar]
- 51.Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, Takahama Y, Sakaguchi S, Mitani T, Matsumoto M. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 52.Irla M, Guerri L, Guenot J, Serge A, Lantz O, Liston A, Imhof BA, Palmer E, Reith W. Antigen recognition by autoreactive CD4(+) thymocytes drives homeostasis of the thymic medulla. PLoS One. 2012;7:e52591. doi: 10.1371/journal.pone.0052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308:248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Wang H, Claudio E, Brown K, Siebenlist U. A role for the IkappaB family member Bcl-3 in the control of central immunologic tolerance. Immunity. 2007;27:438–452. doi: 10.1016/j.immuni.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.