Abstract
Background
Mutations in the PRRT2 gene have been identified as the major cause of benign familial infantile epilepsy (BFIE), paroxysmal kinesigenic dyskinesia (PKD) and infantile convulsions with paroxysmal choreoathetosis/dyskinesias (ICCA). Here, we analyzed the phenotypes and PRRT2 mutations in Chinese families with BFIE and ICCA.
Methods
Clinical data were collected from 22 families with BFIE and eight families with ICCA. PRRT2 mutations were screened using PCR and direct sequencing.
Results
Ninety-five family members were clinically affected in the 22 BFIE families. During follow-up, two probands had one seizure induced by diarrhea at the age of two years. Thirty-one family members were affected in the eight ICCA families, including 11 individuals with benign infantile epilepsy, nine with PKD, and 11 with benign infantile epilepsy followed by PKD. Two individuals in one ICCA family had PKD or ICCA co-existing with migraine. One affected member in another ICCA family had experienced a fever-induced seizure at 7 years old. PRRT2 mutations were detected in 13 of the 22 BFIE families. The mutation c.649_650insC (p.R217PfsX8) was found in nine families. The mutations c.649delC (p.R217EfsX12) and c.904_905insG (p.D302GfsX39) were identified in three families and one family, respectively. PRRT2 mutations were identified in all eight ICCA families, including c.649_650insC (p.R217PfsX8), c.649delC (p.R217EfsX12), c.514_517delTCTG (p.S172RfsX3) and c.1023A > T (X341C). c.1023A > T is a novel mutation predicted to elongate the C-terminus of the protein by 28 residues.
Conclusions
Our data demonstrated that PRRT2 is the major causative gene of BFIE and ICCA in Chinese families. Site c.649 is a mutation hotspot: c.649_650insC is the most common mutation, and c.649delC is the second most common mutation in Chinese families with BFIE and ICCA. As far as we know, c.1023A > T is the first reported mutation in exon 4 of PRRT2. c.649delC was previously reported in PKD, ICCA and hemiplegic migraine families, but we further detected it in BFIE-only families. c.904_905insG was reported in an ICCA family, but we identified it in a BFIE family. c.514_517delTCTG was previously reported in a PKD family, but we identified it in an ICCA family. Migraine and febrile seizures plus could co-exist in ICCA families.
Keywords: Benign familial infantile epilepsy, Infantile convulsions with paroxysmal choreoathetosis, Phenotype, PRRT2, Mutation
Background
Benign familial infantile epilepsy (BFIE), formerly called benign familial infantile seizures (OMIM 605751), is a benign familial focal epilepsy syndrome [1,2]. It is characterized by afebrile seizures with onset between 3 and 12 months of age. Seizures are partial, with or without secondary generalization, often occur in clusters and usually remit before 2 years of age. During childhood or adolescence, family members in BFIE may develop paroxysmal kinesigenic choreoathetosis or dyskinesias (PKC/D). The term “infantile convulsions with paroxysmal choreoathetosis” (ICCA, OMIM 602066) has been used to describe the phenotype of infantile convulsions and paroxysmal dyskinesias (choreoathetosis or dystonia) co-occurring in the same patient or family [3]. Paroxysmal kinesigenic dyskinesia (PKD, OMIM128200) was first described by Demirkiran, who suggested using the generic term “dyskinesia”, rather than dystonia, chorea, or choreoathetosis [4]. PRRT2 gene, encoding proline-rich transmembrane protein 2, was recently identified as a major causative gene for BFIE, PKD and ICCA [5-8]. The aim of this study was to analyze the clinical features and PRRT2 mutations in Chinese families with BFIE and ICCA.
Methods
Patients
This study was approved by the Ethics Committee of Peking University First Hospital. Written informed consent for publication of their clinical details was obtained from the patients or their parents in case of minors. We recruited 22 BFIE families and eight ICCA families with autosomal dominant inheritance at Peking University First Hospital from September 2006 to July 2013. Clinical information was collected from the probands and their family members.
The diagnostic criteria for BFIE were as follows [9,10]: (1) seizure onset between 3 and 12 months; (2) seizures in clusters; (3) focal seizures with or without secondary generalization, usually manifesting motor arrest, deviation of the head and eyes to one side, generalized hypertonia, cyanosis, and limb jerks; (4) the interictal electroencephalography (EEG) is normal while the ictal EEG shows abnormalities that may originate from various cerebral lobes; (5) normal brain imaging; (6) normal psychomotor development before, during and after the onset of seizures; (7) family history of seizures (similar age at onset); (8) good response to treatment and seizures remitted often before the age of 2 years. A diagnosis of PKD was determined according to the criteria proposed by Bruno as follows [11]: (1) age at onset between1 and 20 years; (2) identified kinesigenic trigger for the attacks; (3) short duration of attacks (<1 minute); (4) no loss of consciousness or pain during attacks; (5) control of attacks with phenytoin or carbamazepine; (6) exclusion of other organic diseases and normal neurologic examination. The diagnosis of ICCA was considered according to Szepetowski if the two clinical manifestations of BFIE and PKD were present in the same patient or different family members [3].
Genetic analysis
Blood samples were obtained from the probands and their family members where possible. Genomic DNA was extracted from peripheral blood by standard protocols. Mutation screening of PRRT2 was performed using PCR and direct sequencing. Primers for PRRT2 (NM_145239.2) were designed using Primer Premier 5.0 software. The three coding exons (containing the coding sequence of the three isoforms of the protein), 5′ untranslated region (containing exon 1), and their flanking introns of the PRRT2 gene were sequenced. Primer sequences and annealing temperatures for PCR are available upon request. Mutations found in a proband were examined for co-segregation in other family members. The PRRT2 mutations found in the patients were also screened in 100 Chinese unrelated healthy controls. Impact of an amino acid substitution of missense mutation was predicted by PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/). Patients were followed up at a pediatric neurology clinic at our hospital or by telephone.
Results
Clinical findings
BFIE families
Among the 22 families with BFIE, 95 family members were affected. Families with at least two members affected by benign infantile epilepsy (BIE) were included and the largest one had 19 affected members over five generations (Family 1). Family pedigrees of BFIE families with a PRRT2 mutation are shown in Figure 1A and pedigrees of BFIE families without a PRRT2 mutation are shown in Figure 1B. Among the 22 probands, the age of seizure onset was 3–11 months (median: 4.5 months). Seizures remitted either spontaneously or after treatment with antiepileptic drugs. However, two probands of unrelated families had one afebrile seizure induced by diarrhea at the age of 2 years. The age at last follow-up of these two probands was 2 years and 9 months (Family 6: IV-3) and 3 years and 2 months (Family 11: III-1), respectively. In BFIE Family 6, the proband (IV-3) had seizure onset at 4.5 months of age and spontaneous remission at the age of 6 months without antiepileptic treatment, but she had one afebrile seizure at 31 months of age induced by diarrhea, manifesting as eye deviation to the left side without loss of consciousness, which lasted about one minute. In BFIE Family 11, the proband (III-1) had seizure onset at 3 months of age and remission after treatment with levetiracetam. He had one afebrile generalized tonic-clonic seizure at 25 months of age in conjunction with diarrhea. The interictal EEG of these two probands was normal.
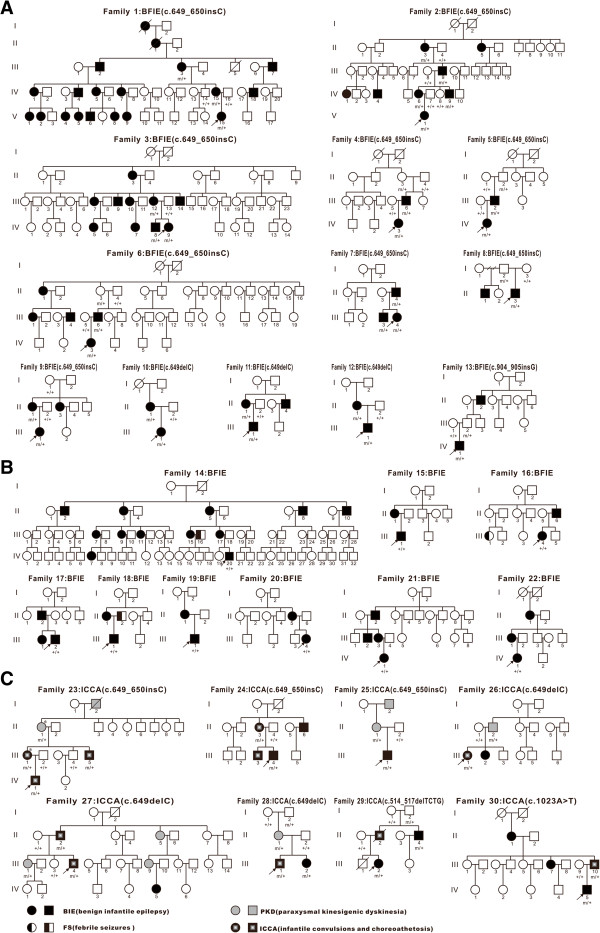
Figure 1.
Pedigrees of the 22 BFIE families and 8 ICCA families. The arrow indicates the proband. An individual with a heterozygous PRRT2 mutation is indicated by m/+, and an individual without a PRRT2 mutation is indicated by +/+. An individual with an asterisk is a patient with migraine. (A) Pedigrees of families 1-13: thirteen BFIE families with a PRRT2 mutation, (B) Pedigrees of families 14-22: nine BFIE families without a PRRT2 mutation, (C) Pedigrees of families 23-30: eight ICCA families with a PRRT2 mutation.
The clinical features of all the BFIE probands and the detected PRRT2 mutations are summarized in Table 1. The clinical data of other affected members in BFIE families were also collected but not included in the Table 1, because for some, a detailed clinical history of infantile seizures could not be obtained. The clinical history of the other 73 affected relatives were shown in Additional file 1: Table S1. Families 1–13 were PRRT2 mutation-positive and Families 14–22 were PRRT2 mutation-negative.
Table 1.
Clinical features and PRRT2 mutations in the probands of 22 BFIE families
| Family No. | Proband | Sex | Age of seizure onset | Age at last follow-up | Nucleotide change | Amino acid change |
|---|---|---|---|---|---|---|
| 1 |
V-15 |
F |
4.5 m |
4y5 m |
c.649_650insC |
p.R217PfsX8 |
| 2 |
V-1 |
F |
5 m |
10 m |
c.649_650insC |
p.R217PfsX8 |
| 3 |
IV-9 |
F |
4 m |
1y |
c.649_650insC |
p.R217PfsX8 |
| 4 |
IV-3 |
F |
4.5 m |
2y5m |
c.649_650insC |
p.R217PfsX8 |
| 5 |
IV-1 |
F |
7 m |
1y2m |
c.649_650insC |
p.R217PfsX8 |
| 6 |
IV-3 |
F |
4.5 m |
2y9m |
c.649_650insC |
p.R217PfsX8 |
| 7 |
III-4 |
F |
3.5 m |
4y |
c.649_650insC |
p.R217PfsX8 |
| 8 |
II-3 |
M |
3.5 m |
8 m |
c.649_650insC |
p.R217PfsX8 |
| 9 |
III-1 |
F |
5 m |
4y5m |
c.649_650insC |
p.R217PfsX8 |
| 10 |
III-1 |
F |
3.5 m |
5y8m |
c.649delC |
p.R217EfsX12 |
| 11 |
III-1 |
M |
3 m |
3y2m |
c.649delC |
p.R217EfsX12 |
| 12 |
III-1 |
M |
4 m |
8 m |
c.649delC |
p.R217EfsX12 |
| 13 |
IV-1 |
M |
6 m |
3y |
c.904_905insG |
p.D302GfsX39 |
| 14 |
IV-20 |
M |
3 m |
6y10m |
none |
none |
| 15 |
III-1 |
M |
3.5 m |
5y11m |
none |
none |
| 16 |
III-4 |
F |
6 m |
2y11m |
none |
none |
| 17 |
III-2 |
M |
4 m |
5y7m |
none |
none |
| 18 |
III-1 |
M |
4.5 m |
3y |
none |
none |
| 19 |
III-1 |
M |
3 m |
1y |
none |
none |
| 20 |
III-4 |
F |
10 m |
1y10m |
none |
none |
| 21 |
IV-1 |
F |
11 m |
2y1m |
none |
none |
| 22 | IV-1 | F | 8 m | 3y7m | none | none |
M: male, F: female, y: years, m: months.
ICCA families
The clinical features of the affected members in the ICCA families and the detected PRRT2 mutations are summarized in Table 2. In the eight ICCA families, 31 family members were affected, of which 11 individuals had BIE only, 9 individuals had PKD only, and 11 individuals had BIE followed by PKD. Pedigrees of ICCA families are shown in Figure 1C. In Family 23, the phenotypes of PKD or ICCA and migraine with aura co-existed (II-1 and III-1). In Family 27, the proband had a seizure during fever at 7 years old.
Table 2.
Clinical features and PRRT2 mutations in 31 affected members from 8 ICCA families
| |
|
|
|
Infantile convulsions |
Paroxysmal dyskinesia |
|
|
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family No | Individual | Phenotype | Sex | Age of seizure onset | Age of seizure remission | Age at onset | Trigger | Involuntary movements | Age at last follow-up | Nucleotide change | Amino acid change |
| 23 |
I-2 |
PKD |
M |
- |
- |
Adolescence |
SM |
D |
50y* |
na |
na |
| |
II-1 |
PKD |
F |
- |
- |
10y |
SM |
D |
65y |
c.649_650insC |
p.R217PfsX8 |
| |
III-1 |
ICCA |
F |
<12 m |
24 m |
9y |
SM |
D |
42y |
c.649_650insC |
p.R217PfsX8 |
| |
III-5 |
ICCA |
M |
<12 m |
24 m |
8y |
SM |
D |
38y |
c.649_650insC |
p.R217PfsX8 |
| |
IV-1 |
ICCA |
M |
7 m |
12 m |
10y |
SM |
D/C |
11y |
c.649_650insC |
p.R217PfsX8 |
| 24 |
II-3 |
ICCA |
F |
<12 m |
<24 m |
7y |
SM |
D |
34y |
c.649_650insC |
p.R217PfsX8 |
| |
II-6 |
BIE |
M |
<12 m |
<24 m |
- |
- |
- |
33y |
na |
na |
| |
III-3 |
ICCA |
M |
6 m |
8 m |
7y |
SM |
C |
9y |
na |
na |
| |
III-4 |
BIE |
M |
4.5 m |
5 m |
- |
- |
- |
6 m |
c.649_650insC |
p.R217PfsX8 |
| 25 |
I-2 |
PKD |
M |
- |
- |
14y |
SM |
C |
51y |
na |
na |
| |
II-1 |
PKD |
F |
- |
- |
12y |
SM |
C |
29y |
c.649_650insC |
p.R217PfsX8 |
| |
III-1 |
BIE |
M |
3.5 m |
5 m |
- |
- |
- |
5.5 m |
c.649_650insC |
p.R217PfsX8 |
| 26 |
II-2 |
PKD |
M |
- |
- |
11y |
SM |
D |
42y |
c.649delC |
p.R217EfsX12 |
| |
III-1 |
ICCA |
F |
8 m |
10 m |
8y |
SM/S |
D |
16y |
c.649delC |
p.R217EfsX12 |
| |
III-2 |
BIE |
F |
5 m |
9 m |
- |
- |
- |
14y |
na |
na |
| 27 |
II-2 |
ICCA |
M |
<12 m |
24 m |
15y |
SM/S |
D |
50y |
c.649delC |
p.R217EfsX12 |
| |
II-5 |
PKD |
F |
- |
- |
10y |
SM |
D |
42y |
na |
na |
| |
III-1 |
PKD |
F |
- |
- |
10y |
SM |
D/C |
26y |
c.649delC |
p.R217EfsX12 |
| |
III-4 |
ICCA |
M |
5.5 m |
12 m |
8y |
SM/S |
D/C |
13y |
c.649delC |
p.R217EfsX12 |
| |
III-9 |
PKD |
F |
- |
- |
10y |
SM |
D |
22y |
na |
na |
| |
IV-5 |
BIE |
F |
4.5 m |
24 m |
- |
- |
- |
3y |
na |
na |
| 28 |
II-1 |
PKD |
F |
- |
- |
5y |
SM |
C |
35y |
c.649delC |
p.R217EfsX12 |
| |
III-1 |
ICCA |
M |
4 m |
4.5 m |
5.5y |
SM, Ex |
C |
12y |
c.649delC |
p.R217EfsX12 |
| |
III-2 |
BIE |
F |
4 m |
5 m |
- |
- |
- |
5y |
c.649delC |
p.R217EfsX12 |
| 29 |
II-2 |
ICCA |
M |
4.5 m |
12 m |
16y |
SM |
D |
40y* |
na |
na |
| |
II-4 |
BIE |
M |
<12 m |
<24 m |
- |
- |
- |
36y |
c.514_517delTCTG |
p.S172RfsX3 |
| |
III-2 |
BIE |
F |
3 m |
5 m |
- |
- |
- |
5y |
c.514_517delTCTG |
p.S172RfsX3 |
| 30 |
II-1 |
BIE |
F |
<12 m |
<24 m |
- |
- |
- |
83y |
na |
na |
| |
III-7 |
BIE |
F |
3 m |
24 m |
- |
- |
- |
50y |
na |
na |
| |
III-10 |
ICCA |
M |
3 m |
9 m |
12y |
SM |
D |
41y |
c.1023A > T |
X341C |
| IV-5 | BIE | M | 4 m | 8 m | - | - | - | 3y9m | c.1023A > T | X341C | |
PKD: paroxysmal kinesigenic dyskinesias, BIE: benign infantile epilepsy, ICCA: infantile convulsions with paroxysmal choreoathetosis, M: male, F: female, y: years, m: months, -: negative, SM: sudden movement, S: startle, Ex: exercise, D: dystonia, C: choreoathetosis, na: not available, *deceased.
Genetic analysis
BFIE families
In our 22 BFIE families, three truncating mutations in PRRT2 were identified in 13 of 22 families (Figure 2), leading to a mutation rate of 59.1%. The mutation c.649_650insC (p.R217PfsX8) was found in nine BFIE families (Families 1–9), accounting for 69.2% (9/13) of BFIE families with a PRRT2 mutation. In four families, incomplete penetrance was observed (Family 4: II-3, Family 5: II-2, Family 6: II-3, and Family 8: I-2) (Figure 1A). The mutation c.649delC was identified in three BFIE families (Families 10–12). The mutation c.904_905insG (p.D302GfsX39) was detected in one BFIE family (Family 13). In this family, incomplete penetrance was also observed, the proband’s father (III-2) carried the mutation without seizures.
Figure 2.

Sequencing chromatograms showing the five PRRT2 mutations detected in BFIE or ICCA families, compared with wild-type traces. The arrow shows the position of the mutation.
ICCA families
In the eight ICCA families, four different PRRT2 mutations were identified (Figure 2). The mutation c.649_650insC (p.R217PfsX8) was identified in three families, one of whom had ICCA and co-existing migraine (Family 23). The mutation c.649delC (p.R217EfsX12) was found in three families (Families 26–28) and the mutation c.514_517delTCTG (p.S172RfsX3) was present in one family (Family 29). A novel mutation c.1023A > T (X341C) in exon 4 was found in one ICCA family (Family 30). This mutation would result in abrogation of the stop codon and elongation of the peptide by 28 amino acids at the C-terminal. This mutation was not found in public single nucleotide polymorphism databases.
Discussion
PRRT2 is an important gene recently identified in neurological paroxysmal disorders. PRRT2 is the major causative gene of PKD, BFIE, and ICCA [5-8,12-19], and is also responsible for several familial or sporadic cases with paroxysmal non-kinesigenic dyskinesia (PNKD), paroxysmal exercised-induced dyskinesia (PED), sporadic BIE, and hemiplegic migraine (HM) [20-24]. In this study, we observed that PRRT2 mutations are also common in Chinese families with BFIE and ICCA.
Including our mutations, 62 different PRRT2 mutations have been described in literature in B(F)IE (Additional file 2: Table S2). To date, 170 BFIE families and 32 sporadic BIE cases have been screened for PRRT2 mutations. PRRT2 mutations were identified in 74.1% (126/170) of BFIE families and 34.4% (11/32) of sporadic BIE cases (Additional file 3: Table S3). Fifteen different PRRT2 mutations were identified in BFIE or BIE (Additional file 2: Table S2), including two insertion mutations, five deletion mutations, four missense mutations, one nonsense mutation, and three splice site mutations. References cited in Additional file 2: Table S2 and Additional file 3: Table S3 were listed in the Additional file 4: Supplemental references. The mutation rates of PRRT2 in BFIE ranged from 54.5% to 85.7% in previous studies [6,12,17].
Our study provides the first report of the deletion mutation c.649delC and the insertion mutation c.904_905insG being associated with a phenotype of BFIE only. The mutation c.649delC has been previously reported in PKD, ICCA, and HM [13,22,25-27], but not in families with BFIE. This mutation was observed in our three BFIE families (Families 10–12). The mutation c.904_905insG was previously described in one ICCA family [21]. We detected this mutation in one BFIE family (Family 13). The most described mutation c.649_650insC is a hotspot for PRRT2 mutation. This was also present in our BFIE families, accounting for 69.2% (9/13) of PRRT2 mutation-positive families. In previous studies, the percentage of c.649_650insC in BFIE families with a PRRT2 mutation ranged from 85.7% to 92.9% [6,12,24]. This mutation was observed mainly with a BFIE, PKD and ICCA phenotype [6,8,12,13,28,29], and but also in a few familial or sporadic cases of PNKD, PED, HM and episodic ataxia [20-22]. This site appears to be particularly prone to mutation: it occurs in a tract of nine cytosines preceded by four guanines, facilitating the formation of a hairpin loop and slippage during DNA replication. Besides the deletion mutation c.649delC and the insertion mutation c.649_650insC, a nonsense mutation c.649C > T affecting the same nucleotide has been reported [13,15,25,30-33]. Incomplete penetrance was found in five of our BFIE families (Families 4, 5, 6, 8 and13). This phenomenon has also been described in other studies [6,12,28]. It was reported that a penetrance of PRRT2 mutations in BFIE was 82% [12].
In our study, two probands of BFIE families experienced a single diarrhea-induced seizure. We cannot differentiate whether the late relapse of seizures in these two patients is a manifestation of convulsions with mild gastroenteritis (CwG), or the presentation of BFIE itself. CwG is a well-recognized infant seizure disorder associated with mild diarrhea. It is characterized by [34]: (1) previously healthy infants and young children aged 6 months to 3 years having afebrile generalized convulsions associated with symptoms of gastroenteritis; (2) seizures occuring sometimes in cluster; (3) normal laboratory examination results including electrolytes, blood glucose and cerebrospinal fluid; (4) normal interictal electroencephalography; and (5) excellent seizure and developmental outcomes. The incidence of CwG is thought to be higher in Asian populations than in people from Western countries [35-38]. Okumura reported that approximately 10% of children with BIE experienced CwG [39]. To explore whether PRRT2 mutations are associated with CwG, several groups conducted PRRT2 mutation analysis in patients with CwG, but no PRRT2 mutation was found [18,24,40].
In our study, PRRT2 mutations were present in 59.1% of Chinese BFIE families, presumably relating to the ethnic background. For the absence of a mutation in PRRT2 in our remaining nine BFIE families, microdeletion of the PRRT2 gene or other BFIE-related genes (SCN2A, KCNQ2, and KCNQ3) should be to be screened [29]. Microdeletions of PRRT2 gene have been found in sporadic cases of PKD and ICCA [41], and also in one PKD family [33].
Including our ICCA families, 95 families with ICCA has been reported (Additional file 3: Table S3). 23 different PRRT2 mutations have been reported in the ICCA phenotype (Additional file 2: Table S2): seven insertion mutations, four deletion mutations, four missense mutations, six nonsense mutations, one splice site mutation, and one microdeletion. Overall, PRRT2 is reported to be mutated in 83.3%-100% of ICCA families [6,8,18,31], although one study reported that the much lower rate of 37.5% [17].We reviewed the PRRT2 mutation rate in BFIE and ICCA families described in literature and our study (Additional file 3: Table S3), and found that it was higher (91.6%, 87/95) in ICCA families than in BFIE families (74.1%, 126/170).
The mutation c.514-517delTCTG has been previously observed in one family with PKD [5], and we found this mutation in an ICCA family. The mutation c.649delC has been reported in one sporadic ICCA case. In our study, we found three BFIE (Families 10–12) and three ICCA families (Families 26–28) with this mutation. This suggests that this mutation is the second most common mutation in Chinese families with BFIE and ICCA. We identified a novel stop codon mutation c.1023A > T (X341C) in one ICCA family. As far as we know, this mutation is the first reported mutation of PRRT2 exon 4. It is predicted to change the stop codon into a cysteine, and would introduce a new stop codon after a 28-amino acids elongation of the C-terminal tail, which forms the extracellular domain of the PRRT2 protein. Functional studies of this mutation should be performed in the future.
We observed some uncommon phenotypes in our ICCA families. Family 23 is an ICCA family with c.649_650insC mutation. In this family, migraine co-existed with ICCA in one individual and with PKD in another. Both relatives had the 649_650inC mutation. The phenotype of migraine (with or without aura) has been reported in BFIE, PKD or ICCA families with a PRRT2 mutation [17,19,20,22,33,42,43]. PRRT2 mutation was identified in one large family with isolated HM [20]. The reported frequency of migraine among PRRT2 mutation carriers is significantly higher (27.1%) than in the overall population with epilepsy (8%-15%) [19,44]. Therefore, the association of migraine with PRRT2 mutation may not be coincidental. In Family 27, the proband (III-4) with ICCA also had an episode of febrile seizure (FS) at 7 years old, conforming to the diagnosis of febrile seizures plus (FS+). Patients with FS or FS + have been reported in families with BFIE [12,23,29,40,45], PKD [25,30,46] or ICCA [15,17,18]. Some carried PRRT2 gene mutations. However, FS may not be associated with PRRT2 mutations in ICCA families. For example, PRRT2 mutation did not co-segregate with FS in a large ICCA family [15]. In a previously described ICCA family, only two of the four FS patients had a PRRT2 mutation [18]. FS are more common in patients with epilepsy than in the general population [47]. It is therefore plausible that the occurrence of febrile seizures in patients with a PRRT2 gene mutation is not solely caused by this mutation, but by the occurrence of epilepsy itself. It could also be caused by a mutation in another gene. The proband in Family 28 had PED. In ICCA families, paroxysmal dyskinesias are mostly of the kinesigenic type, but families with PED have also been reported [48]. A phenotype of PED with PRRT2 mutation has been reported in two Chinese ICCA families [21].
Conclusions
In summary, we confirm that PRRT2 is the major causative gene for BFIE and ICCA in Chinese families. Site c.649 is a mutation hotspot: c.649_650insC is the most common mutation, and c.649delC is the second most common mutation in Chinese families with BFIE and ICCA. We also report a novel mutation, c.1023A > T (X341C), in one ICCA family. Migraine with aura and febrile seizures plus could co-exist in ICCA families.
Abbreviations
BFIE: Benign familial infantile epilepsy; ICCA: Infantile convulsions with paroxysmal choreoathetosis; PKD: Paroxysmal kinesigenic dyskinesia; PKC: Paroxysmal kinesigenic choreoathetosis; EEG: Electroencephalogram; CwG: Benign convulsions with mild gastroenteritis; PNKD: Paroxysmal non-kinesigenic dyskinesia; PED: Paroxysmal exertion-induced dyskinesia; HM: Hemiplegic migraine; BIE: Benign infantile epilepsy; EA: Episodic ataxia; FS+: Febrile seizures plus; FS: Febrile seizures.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YZ designed the study and contributed to the initial draft of the manuscript. YZ, XX, SW, ZY, YW, XL and XW assessed the patients clinically, performed the phenotyping, and collected the DNA samples. XY extracted DNA from peripheral blood, analyzed the clinical and genetic data, and drafted the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Clinical features and PRRT2 mutations in the 73 affected relatives from 22 BFIE families.
Reported PRRT2 mutations allocated to the different phenotypes of BFIE, PKD, ICCA and others.
Reported familial or sporadic cases screened PRRT2 gene in different phenotypes.
Contributor Information
Xiaoling Yang, Email: yxl18b@gmail.com.
Yuehua Zhang, Email: zhangyhd@hotmail.com.
Xiaojing Xu, Email: clairexxj@gmail.com.
Shuang Wang, Email: drwangshuang@sina.com.
Zhixian Yang, Email: zhixian.yang@163.com.
Ye Wu, Email: dryewu@263.net.
Xiaoyan Liu, Email: dr_lxy@sina.com.
Xiru Wu, Email: wxrwwwn@public.bta.net.cn.
Acknowledgements
We thank all the patients and their family members who participated in this study.
References
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;13:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Engel J Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;13:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Szepetowski P, Rochette J, Berquin P, Piussan C, Lathrop GM, Monaco AP. Familial infantile convulsions and paroxysmal choreoathetosis: a new neurological syndrome linked to the pericentromeric region of human chromosome 16. Am J Hum Genet. 1997;13:889–898. doi: 10.1086/514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirkiran M, Jankovic J. Paroxysmal dyskinesias: clinical features and classification. Ann Neurol. 1995;13:571–579. doi: 10.1002/ana.410380405. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Lin Y, Xiong ZQ, Wei W, Ni W, Tan GH, Guo SL, He J, Chen YF, Zhang QJ, Li HF, Lin Y, Murong SX, Xu J, Wang N, Wu ZY. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;13:1252–1255. doi: 10.1038/ng.1008. [DOI] [PubMed] [Google Scholar]
- Heron SE, Grinton BE, Kivity S, Afawi Z, Zuberi SM, Hughes JN, Pridmore C, Hodgson BL, Iona X, Sadleir LG, Pelekanos J, Herlenius E, Goldberg-Stern H, Bassan H, Haan E, Korczyn AD, Gardner AE, Corbett MA, Gecz J, Thomas PQ, Mulley JC, Berkovic SF, Scheffer IE, Dibbens LM. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet. 2012;13:152–160. doi: 10.1016/j.ajhg.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Cao L, Li XH, Hu ZM, Li JD, Zhang JG, Liang Y, San A, Li N, Chen SQ, Guo JF, Jiang H, Shen L, Zheng L, Mao X, Yan WQ, Zhou Y, Shi YT, Ai SX, Dai MZ, Zhang P, Xia K, Chen SD, Tang BS. Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesias. Brain. 2011;13:3493–3501. doi: 10.1093/brain/awr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Huang Y, Bruneau N, Roll P, Roberson ED, Hermann M, Quinn E, Maas J, Edwards R, Ashizawa T, Baykan B, Bhatia K, Bressman S, Bruno MK, Brunt ER, Caraballo R, Echenne B, Fejerman N, Frucht S, Gurnett CA, Hirsch E, Houlden H, Jankovic J, Lee WL, Lynch DR, Mohammed S, Muller U, Nespeca MP, Renner D, Rochette J. et al. Mutations in the gene PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions. Cell Rep. 2012;13:2–12. doi: 10.1016/j.celrep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigevano F, Fusco L, Di Capua M, Ricci S, Sebastianelli R, Lucchini P. Benign infantile familial convulsions. Eur J Pediatr. 1992;13:608–612. doi: 10.1007/BF01957732. [DOI] [PubMed] [Google Scholar]
- Okumura A, Hayakawa F, Kato T, Kuno K, Negoro T, Watanabe K. Early recognition of benign partial epilepsy in infancy. Epilepsia. 2000;13:714–717. doi: 10.1111/j.1528-1157.2000.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Bruno MK, Hallett M, Gwinn-Hardy K, Sorensen B, Considine E, Tucker S, Lynch DR, Mathews KD, Swoboda KJ, Harris J, Soong BW, Ashizawa T, Jankovic J, Renner D, Fu YH, Ptacek LJ. Clinical evaluation of idiopathic paroxysmal kinesigenic dyskinesia: new diagnostic criteria. Neurology. 2004;13:2280–2287. doi: 10.1212/01.WNL.0000147298.05983.50. [DOI] [PubMed] [Google Scholar]
- Schubert J, Paravidino R, Becker F, Berger A, Bebek N, Bianchi A, Brockmann K, Capovilla G, Dalla Bernardina B, Fukuyama Y, Hoffmann GF, Jurkat-Rott K, Anttonen AK, Kurlemann G, Lehesjoki AE, Lehmann-Horn F, Mastrangelo M, Mause U, Muller S, Neubauer B, Pust B, Rating D, Robbiano A, Ruf S, Schroeder C, Seidel A, Specchio N, Stephani U, Striano P, Teichler J. et al. PRRT2 mutations are the major cause of benign familial infantile seizures. Hum Mutat. 2012;13:1439–1443. doi: 10.1002/humu.22126. [DOI] [PubMed] [Google Scholar]
- Meneret A, Grabli D, Depienne C, Gaudebout C, Picard F, Durr A, Lagroua I, Bouteiller D, Mignot C, Doummar D, Anheim M, Tranchant C, Burbaud P, Jedynak CP, Gras D, Steschenko D, Devos D, Billette De Villemeur T, Vidailhet M, Brice A, Roze E. PRRT2 mutations: a major cause of paroxysmal kinesigenic dyskinesia in the European population. Neurology. 2012;13:170–174. doi: 10.1212/WNL.0b013e31825f06c3. [DOI] [PubMed] [Google Scholar]
- Ono S, Yoshiura K, Kinoshita A, Kikuchi T, Nakane Y, Kato N, Sadamatsu M, Konishi T, Nagamitsu S, Matsuura M, Yasuda A, Komine M, Kanai K, Inoue T, Osamura T, Saito K, Hirose S, Koide H, Tomita H, Ozawa H, Niikawa N, Kurotaki N. Mutations in PRRT2 responsible for paroxysmal kinesigenic dyskinesias also cause benign familial infantile convulsions. J Hum Genet. 2012;13:338–341. doi: 10.1038/jhg.2012.23. [DOI] [PubMed] [Google Scholar]
- van Vliet R, Breedveld G, de Rijk-van Andel J, Brilstra E, Verbeek N, Verschuuren-Bemelmans C, Boon M, Samijn J, Diderich K, van de Laar I, Oostra B, Bonifati V, Maat-Kievit A. PRRT2 phenotypes and penetrance of paroxysmal kinesigenic dyskinesia and infantile convulsions. Neurology. 2012;13:777–784. doi: 10.1212/WNL.0b013e3182661fe3. [DOI] [PubMed] [Google Scholar]
- de Vries B, Callenbach PM, Kamphorst JT, Weller CM, Koelewijn SC, ten Houten R, de Coo IF, Brouwer OF, van den Maagdenberg AM. PRRT2 mutation causes benign familial infantile convulsions. Neurology. 2012;13:2154–2155. doi: 10.1212/WNL.0b013e3182752c30. [DOI] [PubMed] [Google Scholar]
- Marini C, Conti V, Mei D, Battaglia D, Lettori D, Losito E, Bruccini G, Tortorella G, Guerrini R. PRRT2 mutations in familial infantile seizures, paroxysmal dyskinesia, and hemiplegic migraine. Neurology. 2012;13:2109–2114. doi: 10.1212/WNL.0b013e3182752ca2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Grinton BE, Heron SE, Kivity S, Afawi Z, Iona X, Goldberg-Stern H, Kinali M, Andrews I, Guerrini R, Marini C, Sadleir LG, Berkovic SF, Dibbens LM. PRRT2 phenotypic spectrum includes sporadic and fever-related infantile seizures. Neurology. 2012;13:2104–2108. doi: 10.1212/WNL.0b013e3182752c6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloarec R, Bruneau N, Rudolf G, Massacrier A, Salmi M, Bataillard M, Boulay C, Caraballo R, Fejerman N, Genton P, Hirsch E, Hunter A, Lesca G, Motte J, Roubertie A, Sanlaville D, Wong SW, Fu YH, Rochette J, Ptacek LJ, Szepetowski P. PRRT2 links infantile convulsions and paroxysmal dyskinesia with migraine. Neurology. 2012;13:2097–2103. doi: 10.1212/WNL.0b013e3182752c46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner AR, Bhatia KP, Stamelou M, Dale RC, Kurian MA, Schneider SA, Wali GM, Counihan T, Schapira AH, Spacey SD, Valente EM, Silveira-Moriyama L, Teive HA, Raskin S, Sander JW, Lees A, Warner T, Kullmann DM, Wood NW, Hanna M, Houlden H. PRRT2 gene mutations: from paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology. 2012;13:2115–2121. doi: 10.1212/WNL.0b013e3182752c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Qi Z, Wan XH, Li JY, Shi L, Lu Q, Zhou XQ, Qiao L, Wu LW, Liu XQ, Yang W, Liu Y, Cui LY, Zhang X. Mutations in PRRT2 result in paroxysmal dyskinesias with marked variability in clinical expression. J Med Genet. 2012;13:79–82. doi: 10.1136/jmedgenet-2011-100653. [DOI] [PubMed] [Google Scholar]
- Riant F, Roze E, Barbance C, Meneret A, Guyant-Marechal L, Lucas C, Sabouraud P, Trebuchon A, Depienne C, Tournier-Lasserve E. PRRT2 mutations cause hemiplegic migraine. Neurology. 2012;13:2122–2124. doi: 10.1212/WNL.0b013e3182752cb8. [DOI] [PubMed] [Google Scholar]
- Specchio N, Terracciano A, Trivisano M, Cappelletti S, Claps D, Travaglini L, Cusmai R, Marras CE, Zara F, Fusco L, Bertini E, Vigevano F. PRRT2 is mutated in familial and non-familial benign infantile seizures. Eur J Paediatr Neurol. 2013;13:77–81. doi: 10.1016/j.ejpn.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Ishii A, Yasumoto S, Ihara Y, Inoue T, Fujita T, Nakamura N, Ohfu M, Yamashita Y, Takatsuka H, Taga T, Miyata R, Ito M, Tsuchiya H, Matsuoka T, Kitao T, Murakami K, Lee WT, Kaneko S, Hirose S. Genetic analysis of PRRT2 for benign infantile epilepsy, infantile convulsions with choreoathetosis syndrome, and benign convulsions with mild gastroenteritis. Brain Dev. 2013;13:524–530. doi: 10.1016/j.braindev.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Groffen AJ, Klapwijk T, van Rootselaar AF, Groen JL, Tijssen MA. Genetic and phenotypic heterogeneity in sporadic and familial forms of paroxysmal dyskinesia. J Neurol. 2013;13:93–99. doi: 10.1007/s00415-012-6592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LC, Methawasin K, Teng EW, Ng AR, Seah SH, Au WL, Liu JJ, Foo JN, Zhao Y, Tan EK. Clinico-genetic comparisons of paroxysmal kinesigenic dyskinesia patients with and without PRRT2 mutations. Eur J Neurol. 2013. in press. [DOI] [PubMed]
- Lee YC, Lee MJ, Yu HY, Chen C, Hsu CH, Lin KP, Liao KK, Chang MH, Liao YC, Soong BW. PRRT2 mutations in paroxysmal kinesigenic dyskinesia with infantile convulsions in a Taiwanese cohort. PLoS One. 2012;13:e38543. doi: 10.1371/journal.pone.0038543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlein OK, Villain M, Korenke C. The PRRT2 mutation c.649dupC is the so far most frequent cause of benign familial infantile convulsions. Seizure. 2012;13:740–742. doi: 10.1016/j.seizure.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Zara F, Specchio N, Striano P, Robbiano A, Gennaro E, Paravidino R, Vanni N, Beccaria F, Capovilla G, Bianchi A, Caffi L, Cardilli V, Darra F, Bernardina BD, Fusco L, Gaggero R, Giordano L, Guerrini R, Incorpora G, Mastrangelo M, Spaccini L, Laverda AM, Vecchi M, Vanadia F, Veggiotti P, Viri M, Occhi G, Budetta M, Taglialatela M, Coviello DA. et al. Genetic testing in benign familial epilepsies of the first year of life: clinical and diagnostic significance. Epilepsia. 2013;13:425–436. doi: 10.1111/epi.12089. [DOI] [PubMed] [Google Scholar]
- Liu XR, Wu M, He N, Meng H, Wen L, Wang JL, Zhang MP, Li WB, Mao X, Qin JM, Li BM, Tang B, Deng YH, Shi YW, Su T, Yi YH, Tang BS, Liao WP. Novel PRRT2 mutations in paroxysmal dyskinesia patients with variant inheritance and phenotypes. Genes Brain Behav. 2013;13:234–240. doi: 10.1111/gbb.12008. [DOI] [PubMed] [Google Scholar]
- Becker F, Schubert J, Striano P, Anttonen AK, Liukkonen E, Gaily E, Gerloff C, Muller S, Heussinger N, Kellinghaus C, Robbiano A, Polvi A, Zittel S, von Oertzen TJ, Rostasy K, Schols L, Warner T, Munchau A, Lehesjoki AE, Zara F, Lerche H, Weber YG. PRRT2-related disorders: further PKD and ICCA cases and review of the literature. J Neurol. 2013;13:1234–1244. doi: 10.1007/s00415-012-6777-y. [DOI] [PubMed] [Google Scholar]
- van Strien TW, van Rootselaar AF, Hilgevoord AA, Linssen WH, Groffen AJ, Tijssen MA. Paroxysmal kinesigenic dyskinesia: cortical or non-cortical origin. Parkinsonism Relat Disord. 2012;13:645–648. doi: 10.1016/j.parkreldis.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Gardiner AR, Meyer E, King MD, Smith M, Rakshi K, Parker A, Mallick AA, Brown R, Vassallo G, Jardine PE, Guerreiro MM, Lees AJ, Houlden H, Kurian MA. Clinical features of childhood-onset paroxysmal kinesigenic dyskinesia with PRRT2 gene mutations. Dev Med Child Neurol. 2013;13:327–334. doi: 10.1111/dmcn.12056. [DOI] [PubMed] [Google Scholar]
- Uemura N, Okumura A, Negoro T, Watanabe K. Clinical features of benign convulsions with mild gastroenteritis. Brain Dev. 2002;13:745–749. doi: 10.1016/S0387-7604(02)00097-9. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Nanni G, Agostinelli S, Parisi P, Capovilla G, Beccaria F, Iannetti P, Spalice A, Coppola G, Franzoni E, Gentile V, Casellato S, Veggiotti P, Malgesini S, Crichiutti G, Balestri P, Grosso S, Zamponi N, Incorpora G, Savasta S, Costa P, Pruna D, Chiarelli F. Benign convulsions associated with mild gastroenteritis: a multicenter clinical study. Epilepsy Res. 2011;13:107–114. doi: 10.1016/j.eplepsyres.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Hung JJ, Wen HY, Yen MH, Chen HW, Yan DC, Lin KL, Lin SJ, Lin TY, Hsu CY. Rotavirus gastroenteritis associated with afebrile convulsion in children: clinical analysis of 40 cases. Chang Gung Med J. 2003;13:654–659. [PubMed] [Google Scholar]
- Chen SY, Tsai CN, Lai MW, Chen CY, Lin KL, Lin TY, Chiu CH. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin Infect Dis. 2009;13:849–855. doi: 10.1086/597256. [DOI] [PubMed] [Google Scholar]
- Li T, Hong S, Peng X, Cheng M, Jiang L. Benign infantile convulsions associated with mild gastroenteritis: An electroclinical study of 34 patients. Seizure. 2013. in press. [DOI] [PubMed]
- Okumura A, Watanabe K, Negoro T, Hayakawa F, Kato T, Maruyama K, Kubota T, Suzuki M, Kurahashi H, Azuma Y. Long-term follow-up of patients with benign partial epilepsy in infancy. Epilepsia. 2006;13:181–185. doi: 10.1111/j.1528-1167.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Okumura A, Shimojima K, Kubota T, Abe S, Yamashita S, Imai K, Okanishi T, Enoki H, Fukasawa T, Tanabe T, Dibbens LM, Shimizu T, Yamamoto T. PRRT2 mutation in Japanese children with benign infantile epilepsy. Brain Dev. 2013;13:641. doi: 10.1016/j.braindev.2012.09.015. 646. [DOI] [PubMed] [Google Scholar]
- Dale RC, Grattan-Smith P, Nicholson M, Peters GB. Microdeletions detected using chromosome microarray in children with suspected genetic movement disorders: a single-centre study. Dev Med Child Neurol. 2012;13:618–623. doi: 10.1111/j.1469-8749.2012.04287.x. [DOI] [PubMed] [Google Scholar]
- Dale RC, Gardiner A, Antony J, Houlden H. Familial PRRT2 mutation with heterogeneous paroxysmal disorders including paroxysmal torticollis and hemiplegic migraine. Dev Med Child Neurol. 2012;13:958–960. doi: 10.1111/j.1469-8749.2012.04394.x. [DOI] [PubMed] [Google Scholar]
- Sheerin UM, Stamelou M, Charlesworth G, Shiner T, Spacey S, Valente EM, Wood NW, Bhatia KP. Migraine with aura as the predominant phenotype in a family with a PRRT2 mutation. J Neurol. 2013;13:656–660. doi: 10.1007/s00415-012-6747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrotti A, Coppola G, Spalice A, Di Fonzo A, Bruschi R, Tozzi E, Iannetti P, Villa MP, Parisi P. Peri-ictal and inter-ictal headache in children and adolescents with idiopathic epilepsy: a multicenter cross-sectional study. Childs Nerv Syst. 2011;13:1419–1423. doi: 10.1007/s00381-011-1428-7. [DOI] [PubMed] [Google Scholar]
- Labate A, Tarantino P, Palamara G, Gagliardi M, Cavalcanti F, Ferlazzo E, Sturniolo M, Incorpora G, Annesi G, Aguglia U, Gambardella A. Mutations in PRRT2 result in familial infantile seizures with heterogeneous phenotypes including febrile convulsions and probable SUDEP. Epilepsy Res. 2013;13:280–284. doi: 10.1016/j.eplepsyres.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Castiglioni C, Lopez I, Riant F, Bertini E, Terracciano A. PRRT2 mutation causes paroxysmal kinesigenic dyskinesia and hemiplegic migraine in monozygotic twins. Eur J Paediatr Neurol. 2013;13:254–258. doi: 10.1016/j.ejpn.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. 2004;13:751–756. doi: 10.1136/adc.2003.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette J, Roll P, Fu YH, Lemoing AG, Royer B, Roubertie A, Berquin P, Motte J, Wong SW, Hunter A, Robaglia-Schlupp A, Ptacek LJ, Szepetowski P. Novel familial cases of ICCA (infantile convulsions with paroxysmal choreoathetosis) syndrome. Epileptic Disord. 2010;13:199–204. doi: 10.1684/epd.2010.0328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical features and PRRT2 mutations in the 73 affected relatives from 22 BFIE families.
Reported PRRT2 mutations allocated to the different phenotypes of BFIE, PKD, ICCA and others.
Reported familial or sporadic cases screened PRRT2 gene in different phenotypes.