Abstract
The rapid, reductive early divisions of many metazoan embryos are followed by the mid-blastula transition (MBT), during which the cell cycle elongates and zygotic transcription begins. It has been proposed that the increasing nuclear to cytoplasmic (N/C) ratio is critical for controlling the events of the mid-blastula transition (MBT). We show that four DNA replication factors, Cut5, RecQ4, Treslin, and Drf1, are limiting for replication initiation at increasing N/C ratios in vitro and in vivo in Xenopus laevis . The levels of these factors regulate multiple events of the MBT including the slowing of the cell cycle, the onset of zygotic transcription, and the developmental activation of the kinase Chk1. This work provides a mechanism for how the N/C ratio controls the MBT and shows that the regulation of replication initiation is fundamental for normal embryogenesis.
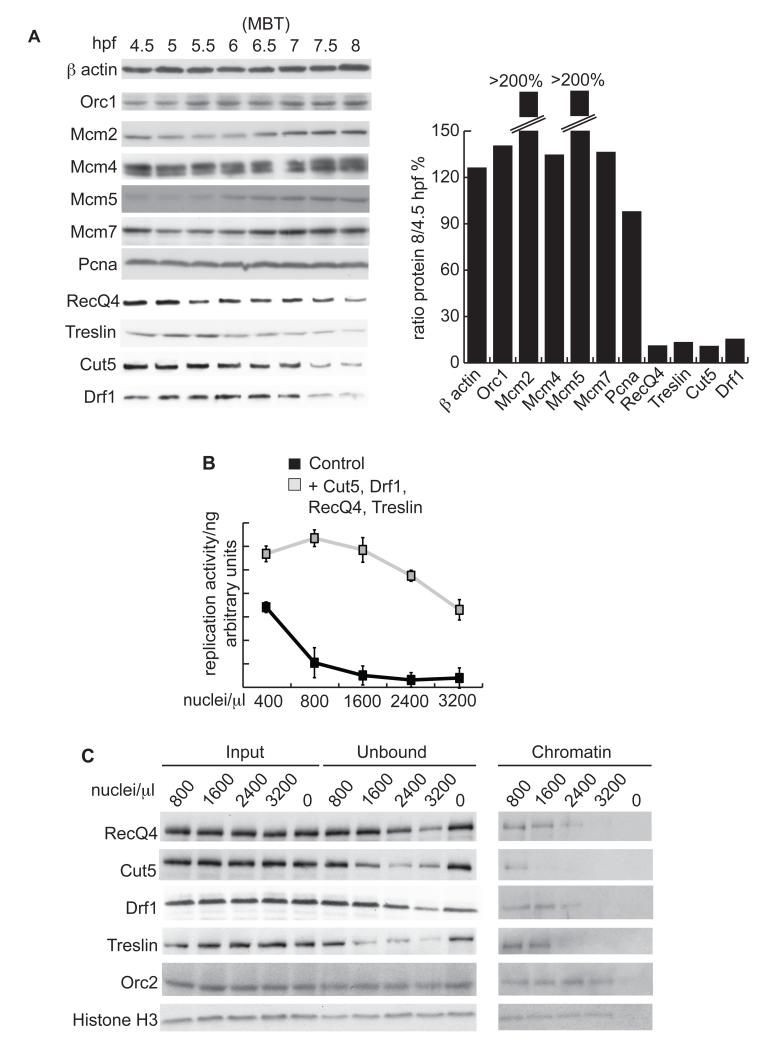
The early cell divisions in many metazoa are rapid and maintained by maternally supplied products. Bulk zygotic transcription begins at the mid-blastula transition (MBT), also referred to as the start of the maternal-zygotic transition (MZT) (1), and is accompanied by the onset of cell movement, the activation of cell cycle checkpoints and the elongation and asynchrony of cell divisions (2-6). This elongation of the cell cycle is coincident with a reduction in the density and synchrony of DNA replication initiation events (7-9), leading to the possibility that replication factors themselves might be important regulators of cell cycle duration during development. To test this hypothesis we measured the abundance of replication initiation factors in Xenopus laevis embryos. Components of the first step of replication initiation, a process called ‘licensing’ or pre-replicative complex (pre-RC) assembly, remained constant or increased in abundance during embryogenesis (Fig. 1A - Orc1 and Mcm2, 4, 5 and 7).
Figure 1. Initiation factors are limiting for replication in vitro in Xenopus laevis.
A. (Left) Western blot of DNA replication factors from Xenopus embryos at the indicated hours post fertilization (hpf) (. The MBT occurs at 6 hours 30 mins (6.5). (Right) Quantification of western blots using Image J. Protein levels are represented as the ratio between 8/4.5 hpf. Pcna is required for DNA replication elongation and did not change in abundance during early embryogenesis.
B. Replication of Xenopus sperm chromatin nuclei in IVT diluted egg extract. Sperm chromatin was added at the indicated concentrations either supplemented with rabbit reticulocyte lysate + empty vector (control) or + vectors encoding cut5, drf1, recq4 and treslin (grey). The reactions were quantified relative to input DNA to represent replication activity per ng of sperm DNA. n=3, error bars show standard deviation.
C. Western blot of chromatin binding of replication factors at increasing sperm nuclei to extract ratios. Xenopus sperm nuclei of the indicated ratios were replicated for 15 mins in undiluted egg extract. Input and chromatin unbound fractions were equally loaded by volume, while the chromatin fractions were normalized to the same DNA concentration.
After pre-RC formation, two kinases are required to assemble active replisomes, Cyclin-Dependent Kinase (CDK) and Dbf4-Dependent kinase (DDK). The abundance of the non-catalytic subunit of DDK, which in Xenopus embryos is Drf1 (Dbf4-related factor 1), becomes greatly reduced by the time of gastrulation (10, 11). Both the mRNA and protein levels of three other replication factors, cut5, treslin and recq4 also decreased during embryogenesis (Fig. 1A and Fig. S1). Treslin and RecQ4 are orthologues of Sld3 and Sld2, the Cyclin-Dependent Kinase (CDK) targets required for replication initiation in yeast, whereas Cut5 is the orthologue of their interaction partner Dpb11 (12-14). Reduction in the levels of these replication factors during early cell divisions suggested that their abundance might be a determinant of embryonic replication initiation rates. To test this, we added in vitro translated (IVT) RecQ4, Treslin, Cut5, and Drf1 to Xenopus egg extracts and analyzed the replication of sperm nuclei in vitro. Increased expression of these proteins in extracts resulted in a corresponding increase in DNA synthesis when these factors were overexpressed in combination (Fig. S2A-E). Furthermore, increasing the nuclei:extract ratio, to mimic the increasing N/C ratio of the early embryo, caused an exponential decline in DNA synthesis, which was suppressed by overexpression of RecQ4, Treslin, Cut5, and Drf1 (Fig. 1B and Fig. S2C). In addition, whereas neither the pre-RC component Orc2 nor histone H3 were titrated out from extracts or on chromatin at increasing nuclei:extract ratios (Fig. 1C) (15), RecQ4, Cut5, Drf1 and Treslin were significantly depleted from extracts (unbound) and less abundant on chromatin at nuclei:extract ratios that resulted in reduced replication activity (Fig. 1C and Fig. S2F). We conclude that these four factors are limiting for DNA synthesis rates in Xenopus egg extracts in a N/C ratio-dependent manner.
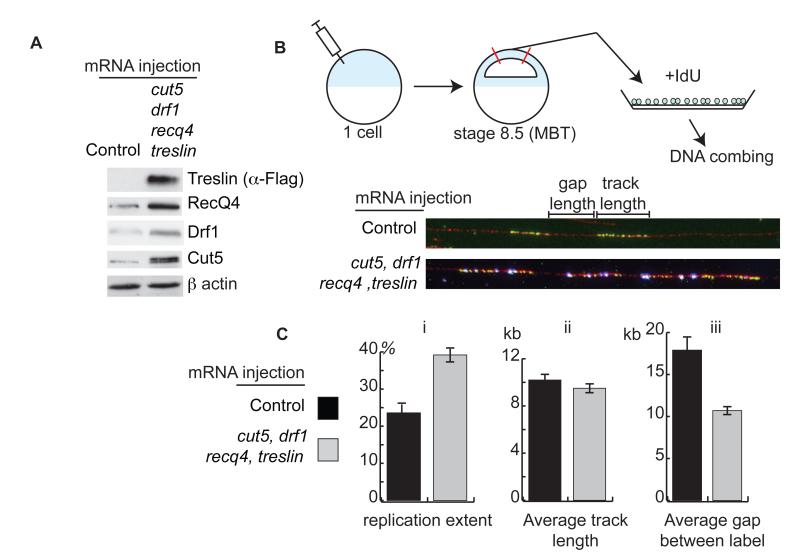
To test whether these factors are limiting for replication initiation in vivo, we increased the levels of all four factors in embryos by injection of mRNA into the fertilized egg (Fig. 2A). To analyze replication rates directly we dissected the animal caps of embryos at stage 8.5 (MBT), dissociated the cells, and pulse-labelled replication forks with IdU (Fig. 2B top). Anti-IdU immuno-fluorescence of labeled DNA stretched onto slides (Fig. 2B bottom) revealed that overexpression of RecQ4, Treslin, Drf1, and Cut5 resulted in an almost twofold increase in total replication (Fig. 2C i). The length of the IdU incorporation tracks remained constant after overexpression of the 4 factors (Fig. 2C ii), suggesting that replication elongation was not affected. By contrast, the gap between labels was reduced nearly twofold by overexpression of these factors (Fig. 2C iii), which is indicative of an increase in origin firing. From this analysis we conclude that RecQ4, Treslin, Drf1, and Cut5 are limiting for the frequency of initiation at the MBT in Xenopus embryos.
Figure 2. Cut5, Drf1, RecQ4 and Treslin are limiting for replication in vivo in Xenopus laevis.
A. Western blot from stage 9 (post MBT) embryos either injected at the 1 cell stage with water (control) or with 300 pg mRNA each of treslin, cut5, recq4, and drf1.
B. (Top) sSchematic representation of experimental design. Xenopus embryos were injected at the one cell stage as in A in the animal pole. At 6.5 hpf (stage 8.5) animal caps were cut and their cells dissociated. The cells were given a pulse with IdU and incorporation was visualized by anti IdU immunofluorescence after stretching DNA onto slides (bottom, DNA-red, IdU-green).
C. Analysis from B of (i) replication extent (%), (ii) average IdU track length (kb) and (iii) average gap between label (kb). n = 96 and 93 fibers for control and overexpressing embryos, respectively.
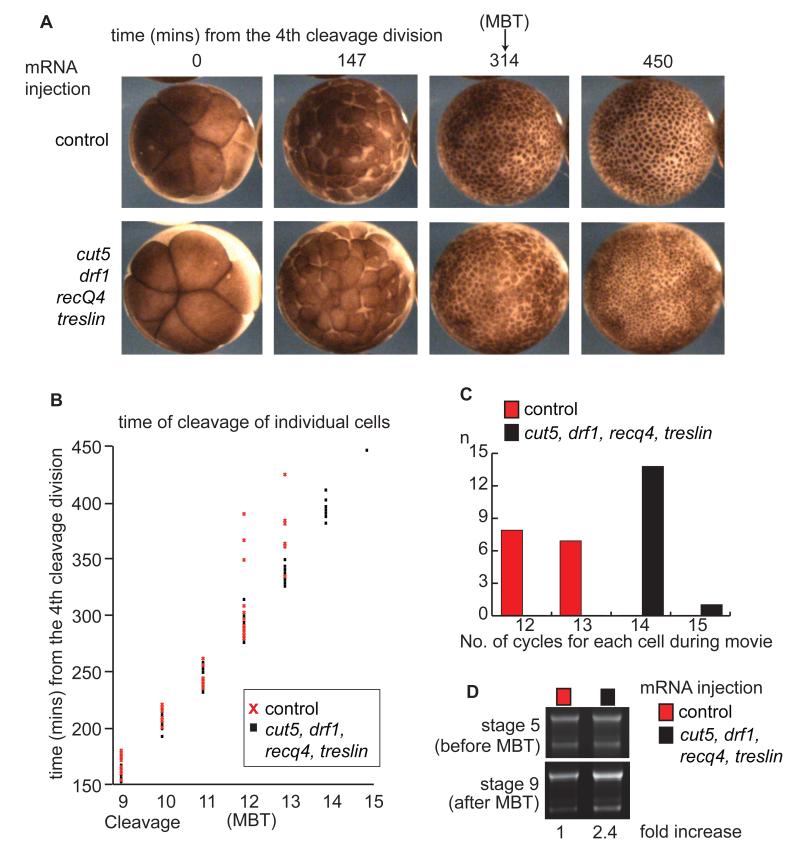
A major transition at the MBT is the lengthening of the cell cycle (4, 16). To test the importance of these limiting replication factors for cell cycle control at the MBT, we analyzed time-lapse movies of injected Xenopus laevis embryos (Movie S1). In control embryos, rapid synchronous divisions occurred until the MBT at cycle 12, after which cell divisions slowed and became asynchronous (Fig. 3A-C and Fig. S3), as previously described (17). In embryos that overexpress RecQ4, Treslin, Drf1, and Cut5, the rapid, synchronous cell divisions continued beyond cycle 12 (Fig. 3A-C and Fig. S3) and as a result these embryos had a considerably greater number of cells (Fig. 3A) and a higher DNA content after the MBT (Fig. 3D).
Figure 3. Overexpression of Cut5, Drf1, RecQ4 and Treslin causes extra rapid cell divisions after the MBT.
A. Images taken from a time-lapse movie (Movie S1) of embryos injected at the 1 cell stage as in Fig.2A. Cleavage 4 at the 16-cell stage was set to time zero.
B. 15 individual cells from the embryos in Movie S1 were followed through early divisions until 450 minutes after cleavage 4. Each time point corresponds to the cleavage of an individual cell. Cleavages 1-8 are excluded for simplicity.
C. Representation of the number of cell cycles each cell in B undergoes until 450 minutes after cleavage 4.
D. Embryos were injected as in A and half the DNA content of a single embryo at stages 5 and 9 was loaded onto an agarose gel and stained with ethidium bromide. This gel is representative of triplicate repeats. Below - DNA amount at stage 9 was quantified using ImageJ and fold increase relative to control is represented.
In accordance with a direct role for the N/C ratio in the titration of these factors in embryos, we observed a dose-dependency between the levels of overexpression of these factors and the number of rapid cell cycles that were induced after the MBT (Fig. S4). Furthermore, partial depletion of RecQ4, Treslin, Drf1, and Cut5 had the opposite effect to the overexpression and resulted in longer cell cycles at the MBT (Fig. S5C). We conclude that the titration of at least 4 replication factors, RecQ4, Treslin, Drf1, and Cut5 controls cell cycle length at the MBT in Xenopus laevis.
Shortening of the cell cycle at the MBT by overexpression of these limiting factors is unlikely due to indirect effects on the cell-autonomous developmental clock (18, 19) because the timing of Cyclin E degradation was unaffected in injected embryos (Fig. S6A). We also tested whether the limiting factors affected the checkpoint kinase Chk1, which becomes transiently activated at the time of the MBT (2, 3, 6). The combined overexpression of RecQ4, Treslin, Drf1 and Cut5 together caused much earlier activation of Chk1 in Xenopus embryos (Fig. S6B,E).
We have previously shown that increasing the rates of replication initiation in budding yeast leads to checkpoint activation by the transient depletion of nucleotides (20). Since it has been shown that deoxyribonucleotide levels are reduced at the onset of the MBT in Xenopus laevis (21), we wondered whether the early activation of Chk1 upon overexpression of RecQ4, Treslin, Drf1, and Cut5 is due to the premature depletion of nucleotides. To test this, we co-injected a fourfold excess (200pmols) of dNTPs into the fertilized egg (22). Whereas injection of dNTPs alone had no effect on the developmental activation of Chk1 (Fig. S6C), co-injection of dNTPs together with overexpression of the 4 factors greatly reduced the activation of Chk1, particularly at the time of the MBT (Fig. S6D). This suggests that the developmental activation of Chk1 is, at least in part, caused both by the depletion of initiation factors and by limiting dNTP levels at the MBT. Co-injection of dNTPs together with the 4 factors, while partially suppressing the developmental Chk1 activation, did not affect the continuation of rapid cell divisions in these embryos (Fig. S7). This indicates that overexpression of RecQ4, Treslin, Drf1 and Cut5 sustains rapid cell divisions at the MBT independently of Chk1 activation levels.
Previous studies have suggested that elongation of the cell cycle is a requirement for the onset of zygotic transcription (23). To address directly the importance of coordinating replication with the onset of transcription, we analyzed the Xenopus laevis transcriptome by high-throughput sequencing of staged embryos. Overexpression of limiting replication factors delayed the expression of a large number of genes at the MBT (Fig. S8). Therefore the regulation of replication initiation densities and cell cycle length at the MBT is a primary event that controls the timely transcription of a subset of genes in Xenopus embryos. As is the case in flies (24), the dynamics of expression of several genes remained unaffected by the extra rapid cell cycles (Fig. S8A), underlining that several mechanisms regulate mRNA abundance at the MBT (1).
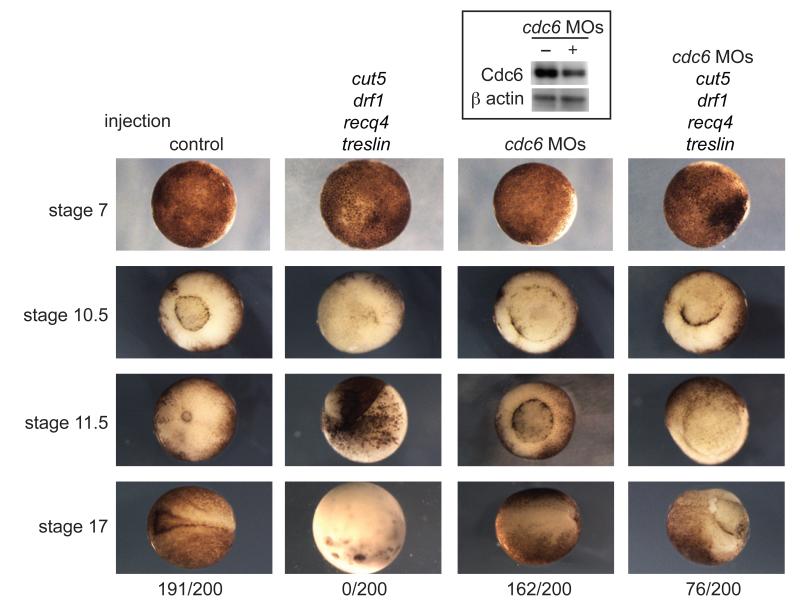
To understand the physiological importance of the regulation of DNA replication during development we analyzed the phenotype of embryos overexpressing RecQ4, Treslin, Drf1, and Cut5. Embryos that overexpress these 4 factors failed to complete gastrulation and blastopore closure at stage 10.5 (Fig. 4) and underwent cell death by stage 17 (neurula), showing that titration of these factors is critical for embryogenesis.
Figure 4. Increasing inter-origin distances at the MBT is critical for gastrulation and neurulation.
Images of embryos injected with water (control - first column), treslin, cut5, recq4, and drf1 mRNA (second column), cdc6 morpholinos (MOs – third column) and embryos injected with a mixture of the cdc6 morpholinos and mRNA for treslin, cut5, recq4, and drf1 (fourth column). Stage 7 = pre-MBT, 10.5 = gastrulation, 11.5 = mid-gastrulation, 17 = neurula. The number of embryos out of 200 surviving at stage 17 is indicated at the bottom. (Top) Western blot of knockdown of Cdc6 after morpholino injection.
If this developmental defect upon overexpression of RecQ4, Treslin, Drf1 and Cut5 is indeed due to increased rates of initiation, we hypothesized that a partial reduction in origin licensing might suppress this phenotype. Injection of embryos with morpholinos against the pre-RC component cdc6 resulted in partial depletion of the Cdc6 protein (Fig. 4, top) and although this caused a slight delay in blastopore closure, 81% of these morpholino-injected embryos reached the neurula stage (Fig. 4). However, co-injection of these cdc6 morpholinos together with overexpression of RecQ4, Treslin, Drf1 and Cut5 partially rescued the ability of these embryos to undergo gastrulation, and 38% of embryos survived until stage 17. This indicates that the developmental deficiency caused by overexpression of RecQ4, Treslin, Drf1 and Cut5 is at least in part due to the resulting increase in replication initiation. The cdc6 morpholinos in combination with the overexpression of limiting initiation factors not only partially rescued the embryonic defect, but also restored normal cell cycle elongation and Chk1 activation at the MBT (Fig. S9). Together these experiments demonstrate that the regulation of replication initiation rates is necessary for several of the critical events of the MBT and for normal development in Xenopus laevis.
This study provides a mechanistic basis for the hypothesis put forward thirty years ago that passive depletion of limiting factors by the N/C ratio is the primary mechanism controlling events at the MBT (4). We speculate that the interplay between the limiting replication factors RecQ4, Treslin, Drf1, and Cut5, together with Chk1 activation and CDK inactivation forms a feedback loop (Fig. S10), which in accordance with work in flies (3, 6, 25, 26), underlies how S-phase length is regulated during development across eukaryotes.
Supplementary Material
Acknowledgments
We are grateful to T. Takahashi, J. Walter, V. Costanzo, J. Diffley, H. Takisawa, and R. Laskey for reagents. DNA combing analysis was performed with assistance from V. Gaggioli. We are grateful to J. Pines, R. Laskey and members of the Zegerman group for critical reading of this manuscript and S. Di Talia for discussions. PZ is funded by AICR 10-0908. JCS and CC were supported by the Wellcome Trust and by the UK Medical Research Council (Programme number U117597140). All authors acknowledge the core support provided by CRUK C6946/A14492 and the Wellcome Trust 092096.
Footnotes
The supporting online material consists of 10 figures, 1 movie and methods.
References
- 1.Tadros W, Lipshitz HD. Development. 2009 Sep;136:3033. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- 2.Shimuta K, et al. EMBO J. 2002 Jul 15;21:3694. doi: 10.1093/emboj/cdf357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sibon OC, Stevenson VA, Theurkauf WE. Nature. 1997 Jul 3;388:93. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- 4.Newport J, Kirschner M. Cell. 1982 Oct;30:675. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- 5.Newport J, Kirschner M. Cell. 1982 Oct;30:687. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- 6.Fogarty P, et al. Curr Biol. 1997 Jun 1;7:418. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- 7.Hyrien O, Maric C, Mechali M. Science (New York, N.Y) 1995 Nov 10;270:994. doi: 10.1126/science.270.5238.994. [DOI] [PubMed] [Google Scholar]
- 8.McKnight SL, Miller OL., Jr. Cell. 1977 Nov;12:795. doi: 10.1016/0092-8674(77)90278-1. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal AB, Kriegstein HJ, Hogness DS. Cold Spring Harb Symp Quant Biol. 1974;38:205. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi TS, Walter JC. Genes Dev. 2005 Oct 1;19:2295. doi: 10.1101/gad.1339805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva T, Bradley RH, Gao Y, Coue M. J Biol Chem. 2006 Apr 28;281:11569. doi: 10.1074/jbc.M510278200. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai A, Shevchenko A, Dunphy WG. J Cell Biol. 2011 Jun 13;193:995. doi: 10.1083/jcb.201102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuno K, Kumano M, Kubota Y, Hashimoto Y, Takisawa H. Mol Cell Biol. 2006 Jul;26:4843. doi: 10.1128/MCB.02267-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sangrithi MN, et al. Cell. 2005 Jun 17;121:887. doi: 10.1016/j.cell.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Walter J, Newport JW. Science (New York, N.Y) 1997 Feb 14;275:993. doi: 10.1126/science.275.5302.993. [DOI] [PubMed] [Google Scholar]
- 16.Edgar BA, Kiehle CP, Schubiger G. Cell. 1986 Jan 31;44:365. doi: 10.1016/0092-8674(86)90771-3. [DOI] [PubMed] [Google Scholar]
- 17.Newport J, Dasso M. J Cell Sci Suppl. 1989;12:149. doi: 10.1242/jcs.1989.supplement_12.13. [DOI] [PubMed] [Google Scholar]
- 18.Howe JA, Newport JW. Proc Natl Acad Sci U S A. 1996 Mar 5;93:2060. doi: 10.1073/pnas.93.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley RS, Sible JC, Lewellyn AL, Maller JL. Dev Biol. 1997 Aug 15;188:312. doi: 10.1006/dbio.1997.8647. [DOI] [PubMed] [Google Scholar]
- 20.Mantiero D, Mackenzie A, Donaldson A, Zegerman P. EMBO J. 2011 Nov 30;30:4805. doi: 10.1038/emboj.2011.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vastag L, et al. PLoS One. 2011;6:e16881. doi: 10.1371/journal.pone.0016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodland HR, Pestell RQ. Biochem J. 1972 Apr;127:597. doi: 10.1042/bj1270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimelman D, Kirschner M, Scherson T. Cell. 1987 Feb 13;48:399. doi: 10.1016/0092-8674(87)90191-7. [DOI] [PubMed] [Google Scholar]
- 24.Lu X, Li JM, Elemento O, Tavazoie S, Wieschaus EF. Development. 2009 Jun;136:2101. doi: 10.1242/dev.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shermoen AW, McCleland ML, O’Farrell PH. Curr Biol. 2010 Dec 7;20:2067. doi: 10.1016/j.cub.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCleland ML, Shermoen AW, O’Farrell PH. J Cell Biol. 2009 Oct 5;187:7. doi: 10.1083/jcb.200906191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boos D, et al. Curr Biol. 2011 Jul 12;21:1152. doi: 10.1016/j.cub.2011.05.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.