Abstract
Aspergillus fumigatus is the major filamentous fungal pathogen in humans. Although A. fumigatus can be treated with many of the available antifungal drugs, including azole compounds, drug resistant isolates are being recovered at an increasing rate. In other fungal pathogens such as the Candida species, ATP-binding cassette (ABC) transporter proteins play important roles in development of clinically-significant azole resistance phenotypes. Central among these ABC transporter proteins are homologues of the Saccharomyces cerevisiae Pdr5 multidrug transporter. In this work, we test the two A. fumigatus genes encoding proteins sharing the highest degree of sequence similarity to S. cerevisiae Pdr5 for their ability to be function in a heterologous pdr5Δ strain of S. cerevisiae. Expression of full-length cDNAs for these two Afu proteins failed to suppress the drug sensitive phenotype of a pdr5Δ strain and no evidence could be obtained for their expression as green fluorescent protein (GFP) fusions. To improve the expression of one of these Afu ABC transporters (XP_755847), we changed the sequence of the cDNA to use codons corresponding to the major tRNA species in S. cerevisiae. This codon-optimized (CO Afu abcA) cDNA was efficiently expressed in pdr5Δ cells and able to be detected as a GFP fusion protein. The CO Afu abcA did not correct the drug sensitivity of the pdr5Δ strain and exhibited a high degree of perinuclear fluorescence suggesting that this fusion protein was localized to the S. cerevisiae ER. Interestingly, when these experiments were repeated at 37 °C, the CO Afu abcA was able to complement the drug sensitive phenotype of pdr5Δ cells and exhibited less intracellular fluorescence. Additionally, we found that the CO Afu abcA was able to reduce resistance to drugs like phytosphingosine that act via causing mislocalization of amino acid permeases in fungi. These data suggest that the Afu abcA protein can carry out two different functions of Pdr5: drug transport and regulation of protein internalization from the plasma membrane.
Keywords: Aspergillus fumigatus, ABC transporter, Heterologous expression, Saccharomyces cerevisiae, Functional complementation
1. Introduction
As the population of immunocompromised patients continues to increase, A. fumigatus-based fungal disease has increased in a similar fashion. Although existing antifungal drugs are efficacious in treating A. fumigatus infections, the inevitable rise in resistant isolates has begun (reviewed in Pfaller, 2012). The primary antifungal drug treatment involves azole compounds such as voriconazole. Most resistant A. fumigatus isolates contain changes in the target of these azole drugs, the lanosterol α14 demethylase encoded by the cyp51A gene. However, increasing numbers of azole resistant organisms are being recovered that possess no detectable change at their cyp51A locus, suggesting the possibility that alternative mechanisms of azole resistance are at work (reviewed in Bowyer et al., 2011; Escribano et al., 2012; Tashiro et al., 2012).
A likely contributor to drug resistance in A. fumigatus are membrane transporters of the ABC family (Dean et al., 2001). These proteins have been extensively studied in other fungal pathogens, especially in Candida albicans and Candida glabrata, where azole resistance occurs as a result of the overexpression of particular ABC transporter proteins (Morschhauser, 2010; Shahi and Moye-Rowley, 2009; Klein et al., 2011; Prasad and Goffeau, 2012). One of the most important of these Candida ABC transporters is the Cdr1 protein, first discovered on the basis of its ability to complement the drug sensitive phenotype of a pdr5Δ strain of S. cerevisiae (Prasad et al., 1995). Pdr5 is the founding member of these ABCG-type ABC transporters in fungi that act as strong drug resistance determinants (Balzi et al., 1994; Bissinger and Kuchler, 1994; Hirata et al., 1994). Pdr5 and its relatives are thought to act as broad specificity, ATP-dependent drug efflux pumps (recently reviewed in (Prasad and Goffeau, 2012). Overexpression of Pdr5 in S. cerevisiae results in a strain that exhibits resistance to a large number of different drugs.
The A. fumigatus genome encodes 50 different genes classified as ABC transporter proteins (Kovalchuk and Driessen, 2010). To facilitate the analysis of these A. fumigatus proteins, we employed the approach of heterologous expression in a pdr5Δ strain of S. cerevisiae. This strategy has been used extensively in the analysis of ABC transporter proteins from the Candida species (Moran et al., 1998; Nakamura et al., 2001; Sanglard et al., 1999; Wada et al., 2002) and allows a relatively simple evaluation of the function of foreign transporters in the well-characterized S. cerevisiae background.
Initial experiments aimed at producing the two ABC transporters from A. fumigatus that shared the highest degree of sequence similarity were unsuccessful likely owing to the presence of unfavorable codons in the A. fumigatus sequences. We selected the Afu ABC transporter sharing the highest degree of sequence similarity with Sc Pdr5 (XP_755847), which we designated abcA, and had this gene chemically synthesized in a form that was codon-optimized for strong expression in the S. cerevisiae background. When we combined this codon-optimized cDNA with a higher growth temperature than is typically employed in S. cerevisiae experiments, we found that the Afu abcA protein localized to the plasma membrane and complemented pdr5Δ drug sensitivity. From these experiments, we conclude that Afu abcA is capable of fulfilling at least two different functions of Pdr5 and that its efficient folding/trafficking requires expression at an elevated temperature. These data suggest that abcA and Pdr5 are likely to be carrying out similar roles in A. fumigatus and S. cerevisiae, respectively.
2. Materials and methods
2.1. Strain and media
The two S. cerevisiae strains used in this study were pdr5Δ (Katzmann et al., 1994) and pdr5Δyor1 (Johnson et al., 2010) strains in the SEY6210 background. Untransformed strains were maintained on YPD (1% yeast extract, 2% peptone, 2% glucose) medium. Upon plasmid transformation by standard lithium acetate protocol (Ito et al., 1983), cells were maintained in selective SC-ura [6.7 g/L Difco yeast nitrogen base w/o amino acids, 2% glucose medium, 1.92 g/L Ura drop out mix (Sigma)], which was also used for spot testing of drugs on solid plates with 1.5% agar.
2.2. Plasmids
The list of oligonucleotides for PCR amplification and plasmids used in this study is given in Tables 1 and 2, respectively. The intronless abcA gene was PCR amplified from genomic DNA isolated from A. fumigatus Af293 strain as 5 exonic DNA fragments. Exons 1, 2, 3, 4 and 5 were PCR amplified using the following primer pairs: Exon 1 – CUP1pr-abcA-Ex1-F and abcA-Ex1-R; Exon 2 – abcA-Ex2-F and abcA-Ex2-R; Exon 3 – abcA-Ex3-F and abcA-Ex3-R; Exon 4 – abcA-Ex4-F and abcA-Ex4-R and Exon 5 – abcA-Ex5-F and abcA-Ex5-GFP-R. The primers were designed such that each exonic DNA fragment had a 50 bp overlap with the adjacent exon. 5′ termini of exon 1 DNA had a 50 bp overlap with the 3′ end of the S. cerevisiae CUP1 promoter (CUP1pr) DNA sequence while the 3′ termini of exon 5 DNA had a 50 bp overlap with the 5′ end of the GFP DNA sequence. In all cases, high fidelity Phusion (New England Biolabs) Pfu polymerase was used for PCR amplification. These PCR amplified exonic DNA fragments were co-transformed along with the pRS316-CUP1-FUR4-GFP gapped within FUR4 by HindIII/BamH1 digestion, into the SEY6210 pdr5Δ strain for the generation of pRS316-CUP1-Afu abcA-GFP by gap repair recombination in S. cerevisiae. Plasmids were isolated from S. cerevisiae, and recovered in E. coli DH10B cells by electroporation. Plasmids isolated from DH10B cells were used for restriction mapping, sequencing, and for further use.
Table 1.
Primers.
| Primer | Sequence |
|---|---|
| CUPpro-abcA-Ex1-F | ATATAGAAGTCATCGAAATAGATATTAAGAAAAACAAACTGTAACGtctagaatggcgatgcaaccatccta |
| abcA-Ex1-R | ACAAACCATGAGTTTCTCCCGCAATCGTCTTGAGGAAGGTTGAACATCCACTGCCTGGCCGGCCAAGTAC |
| abcA-Ex2-F | GGATTTGTCCGCAGCGGCGAAATGCTTGTTGTACTTGGCCGGCCAGGCAGTGGATGTTCAACCTTCCTCA |
| abcA-Ex2-R | TGATCATGAGAGACTCGAAAGCATAACCGATCGGATTCAAATAGTTCAGC |
| abcA-Ex3-F | GCTGAACTATTTGAATCCGATCGGTTATGCTTTCGAGTCTCTCATGATCA |
| abcA-Ex3-R | AAAATGTGAAGCCAATGAACAGAGGCGGAATGATACTCGTTGCAGCCTTT |
| abcA-Ex4-F | AAAGGCTGCAACGAGTATCATTCCGCCTCTGTTCATTGGCTTCACATTTT |
| abcA-Ex4-R | AAGAGCCGATGGACTTGCCAGCACACCATTGAAAATGAGACAAAGTGAGA |
| abcA-Ex5-F | TCTCACTTTGTCTCATTTTCAATGGTGTGCTGGCAAGTCCATCGGCTCTT |
| abcA-Ex5-GFP-R | AGACAGAAAATTTGTGACCATTAACATCACCATCTAATTCAACCAAggatccctcctccttgatcttgcgag |
| pRS316-Kpn1-p15A-F | CCATGCAAATGAGAAGATGCTTGTATATCGGAAAAATTTAGCGCTGGAttaataagatgatcttcttgag |
| pRS316-PmlI-Kan-R | TATTTTTAATTTATATATTTATATTAAAAAATTTAAATTATAATTATTTTTATAGccgtcccgtcaagtc |
Table 2.
Plamid.
| Plasmid | Description | Source |
|---|---|---|
| pRS316-CUP1 pro-FUR4-GFP | Robert Piper | |
| pSP1 | pRS316-Af abcA-GFP | This study |
| pSP4A | pJET2.1-Sc Af abcA N terminus | This study |
| pSP4B | pUC57-Sc Af abcA C terminus | This study |
| pSP13 | pRS316-p15A ori-kanR-CUP1 pro-FUR4-GFP | This study |
| pSP14 | pRS316-p15A ori-kanR-CUP1 pro-Sc Af abcA-GFP | This study |
The abcA gene adapted for optimal expression in S. cerevisiae was chemically synthesized (Genscript, Inc) as 2 DNA fragments that overlapped with each other by 200 bp. The 2.4 kb N-terminal abcA DNA fragment was cloned in the low copy pJET2.1/blunt vector in between the XhoI and XbaI sites within the polylinker. The 2.4 kb C-terminal abcA DNA fragment was cloned in the pUC57 vector in the EcoRV site. The N-terminal and C-terminal abcA DNA fragments were released from the respective vectors by XhoI/HindIII and KpnI/PstI digestions and were co-transformed along with the pRS316-CUP1-FUR4-GFP gapped within FUR4 by HindIII/BamH1 digestion, into the SEY6210 pdr5Δ strain for the generation of pRS316-CUP1-Sc Afu abcA-GFP by gap repair recombination in S. cerevisiae. Appropriate recombinants were identified on the basis of high level production of membrane-associated fluorescence and functional assays.
While the use of the codon-optimized Afu abcA provided strong expression in S. cerevisiae, we encountered serious problems when attempting to recover the correctly recombined fusion gene in E. coli. Bacterial transformants were found to contain only incorrect versions of the pRS316-CUP1-Sc Afu abcA-GFP fusion that appeared to represent deletion derivatives of this plasmid. We were concerned that the inability to retrieve this plasmid was due to inappropriate expression of the extremely hydrophobic abcA in bacteria as was previously described for the cystic fibrosis trans-membrane conductance regulator (Gregory et al., 1990). To reduce this expression, we replaced the high-copy-number bacterial replication origin of the pRS316 plasmid with a lower copy origin from the pACYC177 plasmid. The p15A origin of replication and KanR marker gene were PCR amplified using primers pRS316-Kpn1-p15A-F and pRS316-PmlI-KanR-R from pACYC177 DNA. The above primers were designed such that the 5′ terminus of the PCR product had a 48 bp overlap with pRS316 sequence upstream of KpnI site and the 3′ terminus had a 55 bp overlap with pRS316 sequence downstream of Pml1 site. This PCR product was co-transformed with pRS316-CUP1-FUR4-GFP digested with KpnI/PmlI (to remove the pBluescript origin and AmpR gene) into the S. cerevisiae SEY6210 strain to generate pRS316-p15A ori-kanR-CUP1-FUR4-GFP plasmid by gap repair recombination under uracil selection. The plasmid recovered from S. cerevisiae was then transformed into E. coli DH10B strain by electroporation and selected for kanamycin resistance. The structure of this plasmid was confirmed by restriction mapping. Using this reduced copy number plasmid, the gap repair recombination described above for the codon-optimized Afu abcA clone was repeated. The use of this lower copy number origin permitted the isolation of the desired recombinant.
2.3. Spot assays
Overnight cultures grown in SC-ura at 30 °C were re-inoculated in fresh media at an A600 of 0.05 and grown to mid-log phase cells (A600 of around 0.5) at either 30 °C or 37 °C, corresponding to the temperature at which the spotted drug plates were incubated. 500 cells were spotted onto drug plates at indicated drug concentrations and incubated at either 30 °C or 37 °C. When gradient drug plates were used for spot assays, the concentration of drug indicated correspond to maximum drug concentration on the plate.
2.4. Fluorescence microscopy
SEY6210 derivatives lacking either Pdr5 (pdr5Δ) or Pdr5 and Yor1 (pdr5Δ yor1) transformed with GFP-tagged FUR4 or abcA plasmids were grown overnight in SC-ura at 30 °C and subcultured from an A600 of 0.05–0.5 in SC-ura at either 30 °C or 37 °C. Cells were then washed and resuspended in sterile water and visualized for GFP fluorescence and Nomarski optics using an Olympus (Tokyo, Japan) BX-60 microscope with a 100× oil immersion objective. Images were captured using a Hamamatsu (Shizuoka, Japan) ORCA charge-coupled device camera.
2.5. Western blotting
For Western blot analysis of the GFP-tagged abcA or FUR4 transformants, cells were grown in SC-ura to an A600 of 0.8 in SC-ura, and whole cell extracts were prepared using the TWIRL buffer (8 M urea, 5% SDS, 10% glycerol, 50 mM Tris, pH 6.8, 5% β-mercaptoethanol) extraction method. Briefly, 2 A600 units of cells were resuspended in 100 μl of TWIRL buffer and vortexed for 3 min with glass beads at 4 °C and centrifuged for 5 min at 12,000 rpm in an Eppendorf microcentrifuge at 4 °C. 20 μl of supernatant were electrophoresed on 7.5% SDS–PAGE, transferred to a nitrocellulose membrane, blocked with 5% nonfat dry milk in phosphate-buffered saline, and then probed with polyclonal anti-GFP antiserum (1:200). Horseradish peroxidase conjugated secondary antibody and an ECL kit (Pierce) were used to visualize immunoreactive protein.
2.6. Rhodamine 6G assay
The rhodamine efflux study was essentially carried out as described by Kolaczkowski et al. (1996) with a few modifications. The strains were pregrown in SC-ura broth overnight at 30 °C and then reinoculated into fresh media to an initial A600 of 0.1. After incubation for 3 h at 37 °C, equal numbers of cells from the exponential-phase cultures were harvested, washed twice with water and resuspended in 0.5 ml HEPES buffer (50 mM HEPES-NaOH, pH 7-0). 2-Deoxy-β-glucose and rhodamine 6G were added to a final concentration of 5 mM and 5 μM, respectively, and incubated at 37 °C for 2 h. After incubation, rhodamine-6G-loaded cells were spun down, washed twice with HEPES buffer and finally resuspended in 4 ml HEPES buffer. 1 mM glucose was added to initiate the energy dependent efflux of rhodamine 6G. The cell suspension was incubated at 37 °C. At time zero, i.e. immediately after the addition of glucose, and after 15, 30 and 45 min of further incubation, 0.5 ml of sample was withdrawn, cells were removed by centrifugation and the fluorescence of rhodamine 6G extruded in the supernatant (100 μl) was measured at an excitation and emission wavelength of 529 nm and 553 nm, respectively, on a SpectraMAX GeminiXS microplate fluorimeter (Molecular Devices).
3. Results
3.1. Generation of S. cerevisiae adapted A. fumigatus abcA expressing plasmid in S. cerevisiae
Since A. fumigatus contains 15 different ABCG class ABC transporter-encoding genes (Kovalchuk and Driessen, 2010), we first aligned these with Sc Pdr5 in order to select Afu proteins with the highest sequence similarity. The homologues closest to Sc Pdr5 are shown in Fig. 1A. If the stringency of the alignment is further increased, only two Afu proteins share adequate sequence similarity to satisfy this criterion: XP_755847 and XP_752803. We designated these two ABC transporters as abcA and abcB.
Fig. 1.
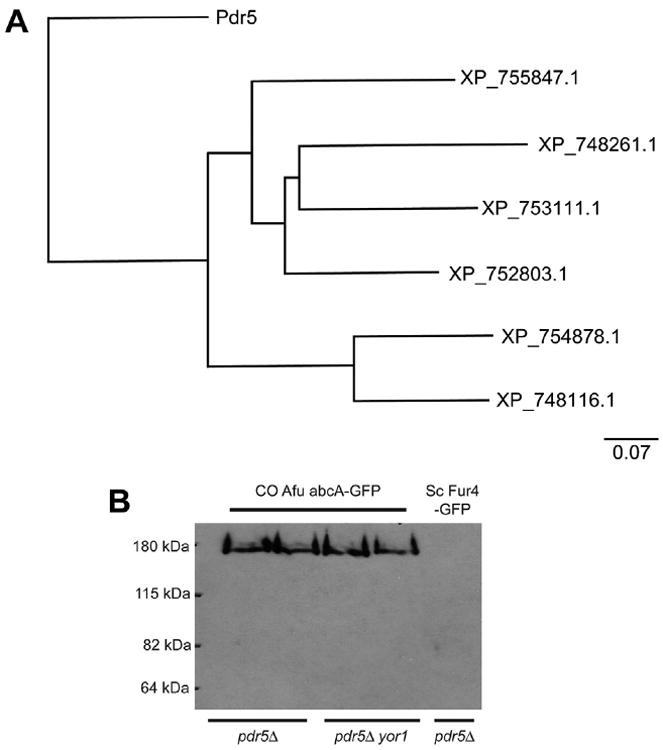
Relationship of A. fumigatus ABCG homologues to S. cerevisiae Pdr5. (A) The relatedness of the 6 ABCG homologues from A. fumigatus sharing the most sequence similarity to S. cerevisiae Pdr5 is shown. This alignment was generated using Blast Tree View at the NCBI Database (Maximum Sequence Difference = 0.6). The two ABCG proteins with the highest degree of sequence similarity are XP_755847.1 (abcA) and XP_752803.1 (abcB). (B) Western blot analysis of codon-optimized (CO) cDNA encoding A. fumigatus abcA expressed in S. cerevisiae. Strains with the indicated relevant genotypes listed at the bottom were transformed with low-copy-number vectors expressing either CO abcA or Sc Fur4 from the CUP1 promoter as GFP fusion proteins. Transformants were grown to mid-log phase, protein extracts prepared and subjected to western blotting using anti-GFP antibodies. At this level of exposure, Fur4-GFP is difficult to visualize.
To express these Afu genes in Sc, we first constructed full-length cDNAs based on the predicted structures of each gene. These were prepared by PCR amplification across all predicted intron-exon boundaries directly from genomic DNA. Efforts to construct full-length cDNAs from the respective mRNAs were complicated by the large size of these transcripts (data not shown). The different amplification products were linked together by homologous recombination in S. cerevisiae cells (see Supplementary Fig. 1 for a description of this strategy for abcA). Both abcA and abcB were inserted into a pRS316 plasmid backbone. This insertion placed both cDNAs under control of the Sc CUP1 promoter and fused GFP to their C-terminal amino acid. These expression clones were introduced into pdr5Δ cells. Transformants tested for their ability to correct the drug hypersensitive phenotype caused by loss of Pdr5 as well as for the expected fluorescence signal from the GFP fusion proteins. Neither cDNA produced any detectable change in these two parameters (data not shown).
The failure of these cDNAs to function in the S. cerevisiae strain prompted us to more closely examine the sequences of the two A. fumigatus clones. In each case, a large number (19 in the case of abcA) of inefficiently translated codons were present in the native A. fumigatus coding sequence that could contribute to undetectable production of the polypeptide in the heterologous S. cerevisiae background (data not shown). In order to address this possibility, we purchased a chemically synthesized cDNA that was codon-optimized (CO) in order to use the most abundant S. cerevisiae tRNAs. This CO Afu abcA cDNA clone was constructed by recombination in S. cerevisiae (strategy shown in Supplementary Fig. 1). This cDNA was again placed under control of the Sc CUP1 promoter and expressed as a GFP fusion protein. The proper construction of this clone was verified by DNA sequencing and then transformed into pdr5Δ cells to assess its function.
3.2. Confirmation of expression of heterologous abcA in S. cerevisiae
We transformed low-copy-number plasmids expressing either Fur4-GFP or the CO Afu abcA-GFP from the Sc CUP1 promoter into strains lacking the PDR5 gene. FUR4 encodes the Sc uracil permease and has no known influence on drug resistance. Transformants were grown to mid-log phase at 30 °C, protein extracts prepared and then subjected to western blotting analysis using anti-GFP antiserum (Fig. 1B).
Detection of the CO Afu abcA-GFP protein was easily accomplished under these conditions. This fusion protein accumulates at the appropriate molecular weight. Expression of the CO Afu abcA-GFP is much stronger than Fur4-GFP under these experimental conditions.
3.3. Functional status of heterologous abcA in S. cerevisiae
Having confirmed that our CO Afu abcA-eGFP construct is expressed in S. cerevisiae, we wanted to examine the functional status of this protein. The transformants described above were grown to mid-log phase and then placed as spots of 500 cells on rich medium containing fluconazole. These plates were then incubated at 30 °C for 48 h and then photographed (Fig. 2A).
Fig. 2.
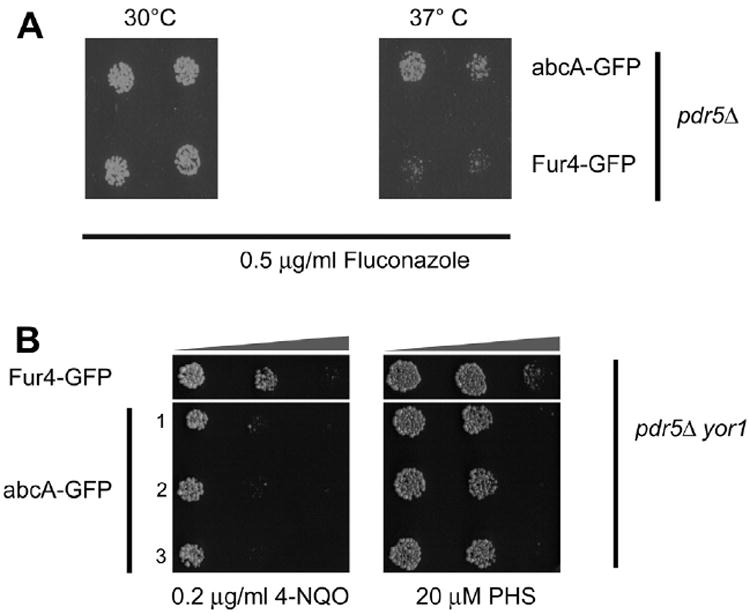
Function of abcA expressed in S. cerevisiae. Plasmids directing the expression of either abcA-GFP or Fur4-GFP from the CUP1 promoter were introduced into the indicated S. cerevisiae strains. (A) Transformants were grown to mid-log phase and then placed in duplicate on YPD medium containing 0.5 μg/ml fluconazole. Two identical plates were prepared and then placed at either 30 °C or 37 °C as noted. These plates were incubated for 48 h and then photographed. (B) Reversal of resistance phenotypes caused by loss of Pdr5. A pdr5Δ yor1 strain was used to enhance the relevant phenotypes as described (Kolaczkowska et al., 2008; Johnson et al., 2010). Transformants from above were placed on YPD medium containing the indicated concentrations of 4-nitroquinoline-N-oxide (4-NQO) or phytosphingosine (PHS). Resistance to these compounds is elevated in the absence of Pdr5 (Kolaczkowska et al., 2008). Transformants were incubated at 37 °C and the plates photographed.
We were surprised to see that there was no significant difference in fluconazole resistance between pdr5Δ cells expressing either abcA-GFP or Fur4-GFP. We wondered if the very different standard growth conditions of A. fumigatus (37 °C) and S. cerevisiae (30 °C) might have an impact on the relative lack of function of the abcA-GFP protein expressed in this heterologous environment. We repeated this fluconazole resistance assay but allowed the plates to develop at 37 °C.
As can be seen (Fig. 2A), increasing the incubation temperature led to a readily discernable increase in fluconazole resistance supported by abcA-GFP expressed in S. cerevisiae. It is important to note that the level of fluconazole resistance provided by abcA-GFP is still quite low compared to native Pdr5. Wild-type cells are capable of growth in the presence of 20 μg/ml fluconazole while these transformed cells grow very poorly at concentrations as low as 1 μg/ml (data not shown). However, these findings support the view that abcA is capable of complementing the fluconazole sensitivity caused by loss of Pdr5.
While the drug efflux activity of Pdr5 is well-described, more recent findings have implicated this transporter in other event at the membrane. The presence of Pdr5 appears to down-regulate other drug transporters (Kolaczkowska et al., 2008) and amino acid permeases (Johnson et al., 2010). Resistance to the DNA damaging agent 4-nitroquinoline-N-oxide (4-NQO) is mediated in large part by the ABCG homologue Snq2 (Decottignies et al., 1995) and enhanced upon loss of Pdr5 (Kolaczkowska et al., 2008). To determine if abcA was capable of correcting this other Pdr5-dependent phenotypes, the transformants described above were placed on media containing either 4-NQO or PHS (Fig. 2B). Expression of abcA was able to lower the enhanced 4-NQO resistance while expression of Fur4 was not. Similarly, loss of Pdr5 has been shown to increase resistance to the sphingolipid intermediate phytosphingosine (PHS) (Kihara and Igarashi, 2004). PHS causes mistargeting of amino acid permeases, including the tryptophan-specific permease Tat2, to the vacuole where these proteins are degraded (Chung et al., 2001; Skrzypek et al., 1998). We found that loss of Pdr5 also inhibits the vacuolar turnover of Tat2, likely due to a lowered rate of endocytosis (Johnson et al., 2010). As seen for 4-NQO, expression of abcA was able to reduce PHS tolerance while the presence of the CUP1-driven FUR4 gene had no effect. These data indicate that abcA can carry out functions of Pdr5 related to drug transport and also control of permease function.
3.4. Efflux activity supported by heterologous abcA
To examine the quantitative function of Afu abcA expressed in S. cerevisiae, we compared the ability of this A. fumigatus transporter to efflux the fluorescent dye rhodamine 6G. Transport of this dye is carried out by Pdr5 and provides a convenient assay for function of this transporter (Kolaczkowski et al., 1996).
Wild-type cells were used as a control for normal levels of Pdr5 function. The expression plasmids producing either Fur4- or abcA-GFP were introduced into a pdr5Δ strain as above. These three different strains were grown to mid-log phase at 37 °C and assayed for their relative ability to extrude rhodamine 6G into the culture supernatant as described (Kolaczkowski et al., 1996). Wild-type cells rapidly exported rhodamine 6G while pdr5Δ transformants expressing abcA-GFP produced a lower but easily detectable rate of rhodamine 6G transport (Fig. 3). The Fur4-GFP transformants exhibited a rate of transport that was barely measurable over the entire time course of the experiment. As the plate assays indicated, these biochemical measurements confirm that abcA-GFP is capable of restoring Pdr5-like function to cells lacking their normal copy of PDR5.
Fig. 3.
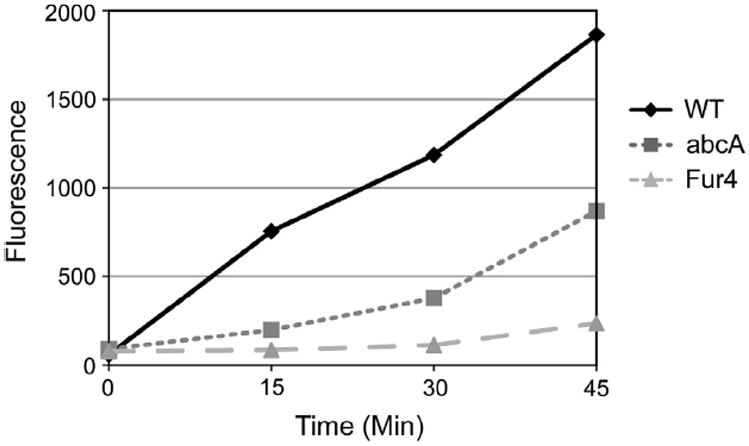
Rhodamine 6G transport by abcA. Either wild-type or pdr5Δ cells carrying the indicated expression plasmids were grown to mid-log phase. Cells were deenergized, loaded with rhodamine 6G and then washed. Cells were then resupplied with glucose and aliquots removed at various times. Samples were centrifuged and the level of rhodamine 6G in the supernatant determined using a fluorimeter.
3.5. Differential steady-state localization of heterologous abcA in response to growth temperature
Since our functional assays described above indicate the importance of the growth temperature in the function of the abcA, we wanted to determine if the A. fumigatus protein was localized similarly at 30 °C and 37 °C. We visualized the location of both the abcA- and Fur4-GFP fusion proteins using their fused GFP moieties. Transformants expressing one of these fusion proteins were grown to mid-log phase at either 30 °C or 37 °C and then photographed under visible or fluorescent light (Fig. 4).
Fig. 4.
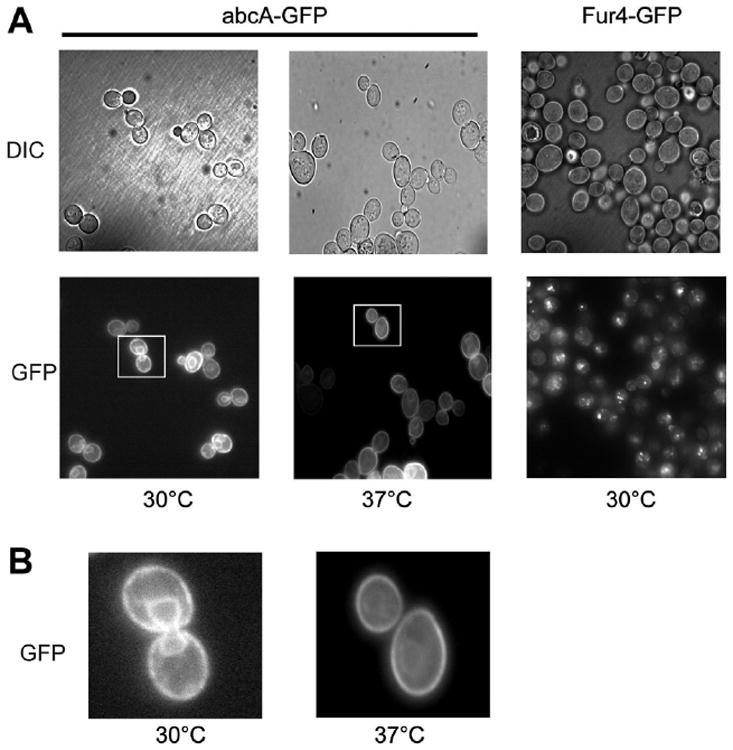
Temperature-regulated trafficking of abcA in S. cerevisiae. A. Transformants expressing either abcA- or Fur4-GFP were grown in selective medium at the indicated temperatures to mid-log phase. Cells were then visualized using Nomarski optics (DIC) or under fluorescent light (GFP). B. The indicated fields in the abcA-GFP panels above (Left = 30°, Right = 37°) were magnified 4× in order to see the fluorescence signal more clearly.
Growth at 37 °C led to a clear reduction in the level of intracellular fluorescence seen in abcA-GFP-expressing cells. The strong perinuclear fluorescence pattern produced by abcA-GFP at 30 °C is likely caused by much of this protein accumulating at the endoplasmic reticulum while this fluorescence signature was reduced when cells were grown at 37 °C. Pdr5 functions at the plasma membrane and the increased function of abcA-GFP seen at 37 °C correlates with this observed reduction in intracellular localization of the transporter. Together, these data suggest that trafficking of abcA-GFP to the plasma membrane is enhanced at the higher temperature. Fur4-GFP accumulated in intracellular compartments; a behavior that did not change in response to temperature (data not shown).
4. Discussion
The role of ABC transporters in drug resistance in fungal pathogens is best established in the Candida species (reviewed in Cannon et al., 2009; Morschhauser, 2010). Azole-resistant patient isolates of Candida albicans and Candida glabrata are commonly found that overproduce their respective Pdr5 homologue called Cdr1 (recently reviewed in Prasad and Goffeau, 2012). To date, the role of ABC transporters in azole tolerance in A. fumigatus has not assumed the importance seen in Candida. The vast majority of azole tolerant A. fumigatus isolates contain mutations in the promoter and/or coding sequence of the cyp51A gene encoding the target of this drug, lanosterol α14 demethylase (Denning et al., 1997; Diaz-Guerra et al., 2003). However, other data suggest that mechanisms with no involvement of cyp51A are at work in the population of azole resistance A. fumigatus (Nascimento et al., 2003). Based on the presence of Cdr1 homologues with high sequence similarity in A. fumigatus, we believe it is only a matter of time before azole resistance mechanisms involving these transporters are discovered. Very recently, patient isolates with enhanced azole resistance dependent on overproduction of a Cdr1 homologue have been identified (Fraczek et al., 2013) strongly supporting this prediction. This places a premium on understanding the shared and unique features of the A. fumigatus transporter proteins.
To begin the systematic analysis of the Afu Pdr5/Cdr1 homologues, we wanted to develop a facile system in which to analyze the function of these transporters. The pdr5Δ background in S. cerevisiae has been used to analyze a number of different transporters, ranging from Candida to human (Kiser et al., 2001; Nakamura et al., 2001). We were surprised that our initial attempts to produce the Afu transporter sharing the highest degree of sequence similarity with Pdr5, abcA, were unsuccessful. We resolved the initial expression problems by using a chemically-synthesized form of abcA cDNA that employed codons corresponding to the major S. cerevisiae tRNAs. Even these measures were not sufficient to produce functional abcA in S. cerevisiae until we grew the cultures at higher temperatures. Indeed, A. fumigatus thrives at elevated temperatures, suggesting that proper folding and localization may require higher temperatures for certain proteins derived from this organism. These experiences are useful guides for future experiments involving production of A. fumigatus proteins in the foreign environment of S. cerevisiae.
These experiments confirm that abcA is capable of correcting several defects caused by loss of Pdr5 in S. cerevisiae. Extensive literature links Pdr5 in particular and ABC transporters in general to drug resistance through their action as efflux pumps (reviewed in Prasad and Goffeau, 2012). Based on the high degree of sequence similarity between abcA and Pdr5, we anticipated that the A. fumigatus transporter would correct the azole sensitivity of the pdr5Δ strain. The putative function of Pdr5 as a regulator of the activity of other plasma membrane proteins is a more recently discovered function and much less well documented. PHS resistance of strains lacking pdr5Δ correlates with defects in the removal of the high-affinity tryptophan permease Tat2 from the plasma membrane (Johnson et al., 2010). Additionally, expression of the SNQ2 gene encoding an ABC transporter that confers 4-NQO resistance is elevated in pdr5Δ cells (Kolaczkowska et al., 2008). Although the understanding of the molecular mechanisms underlying these Pdr5-dependent functions is incomplete, abcA expressed heterologously can reverse both of these different phenotypes. We speculate that the regulatory function of Pdr5 is due to the action of this protein as a regulator of phospholipid asymmetry that in turn influences homeostasis of membrane proteins embedded in this bilayer (Kean et al., 1997; Pomorski et al., 2003). This suggests that in its native A. fumigatus environment, abcA can also provide similar functions. We are currently analyzing the behavior of A. fumigatus strains both lacking and overproducing abcA.
Supplementary Material
Acknowledgments
This work was supported in part by NIH GM49825 and AI92331. We thank Dr. Stacey Klutts for important discussions and a critical reading of this manuscript.
Abbreviations
- ABC
ATP-binding cassette
- ABCG
class G ABC transporter
- Afu
Aspergillus fumigatus
- CO
codon-optimized
- Sc
Saccharomyces cerevisiae
- SC-ura
synthetic complete medium lacking uracil
- YPD
yeast-peptone-dextrose growth medium
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.fgb.2013.06.004.
References
- Balzi E, et al. PDR5: a novel yeast multidrug resistance transporter controlled by the transcription regulator PDR1. J Biol Chem. 1994;269:2206–2214. [PubMed] [Google Scholar]
- Bissinger PH, Kuchler K. Molecular cloning and expression of the S. cerevisiae STS1 gene product. J Biol Chem. 1994;269:4180–4186. [PubMed] [Google Scholar]
- Bowyer P, et al. Azole antifungal resistance today: focus on Aspergillus. Curr Infect Dis Rep. 2011;13:485–491. doi: 10.1007/s11908-011-0218-4. [DOI] [PubMed] [Google Scholar]
- Cannon RD, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22:291–321. doi: 10.1128/CMR.00051-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung N, et al. Phytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae. J Biol Chem. 2001;276:35614–35621. doi: 10.1074/jbc.M105653200. [DOI] [PubMed] [Google Scholar]
- Dean M, et al. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- Decottignies A, et al. Identification and characterization of SNQ2, a new multidrug ABC transporter of the yeast plasma membrane. J Biol Chem. 1995;270:18150–18157. doi: 10.1074/jbc.270.30.18150. [DOI] [PubMed] [Google Scholar]
- Denning DW, et al. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Guerra TM, et al. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 2003;47:1120–1124. doi: 10.1128/AAC.47.3.1120-1124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano P, et al. In vitro acquisition of secondary azole resistance in Aspergillus fumigatus isolates after prolonged exposure to itraconazole: presence of heteroresistant populations. Antimicrob Agents Chemother. 2012;56:174–178. doi: 10.1128/AAC.00301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraczek MG, et al. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother. 2013 doi: 10.1093/jac/dkt075. [DOI] [PubMed] [Google Scholar]
- Gregory RJ, et al. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990;347:382–386. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Hirata D, et al. Saccharomyces cerevisiae YDR1, which encodes a member of the ATP-binding cassette (ABC) superfamily, is required for multidrug resistance. Curr Genet. 1994;26:285–294. doi: 10.1007/BF00310491. [DOI] [PubMed] [Google Scholar]
- Ito H, et al. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SS, et al. Regulation of yeast nutrient permease endocytosis by ATP-binding cassette transporters and a seven-transmembrane protein, RSB1. J Biol Chem. 2010;285:35792–35802. doi: 10.1074/jbc.M110.162883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, et al. Transcriptional control of the yeast PDR5 gene by the PDR3 gene product. Mol Cell Biol. 1994;14:4653–4661. doi: 10.1128/mcb.14.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean LS, et al. Plasma membrane translocation of fluorescent-labeled phosphatidylethanolamine is controlled by transcription regulators, PDR1 and PDR3. J Cell Biol. 1997;138:255–270. doi: 10.1083/jcb.138.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Igarashi Y. Cross talk between sphingolipids and glycerophospholipids in the establishment of plasma membrane asymmetry. Mol Biol Cell. 2004;15:4949–4959. doi: 10.1091/mbc.E04-06-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser GL, et al. Expression and degradation of the cystic fibrosis transmembrane conductance regulator in Saccharomyces cerevisiae. Arch Biochem Biophys. 2001;390:195–205. doi: 10.1006/abbi.2001.2385. [DOI] [PubMed] [Google Scholar]
- Klein C, et al. ABC proteins in yeast and fungal pathogens. Essays Biochem. 2011;50:101–119. doi: 10.1042/bse0500101. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska A, et al. Compensatory activation of the mutidrug transporters Pdr5p, Snq2p and Yor1p by Pdr1p in Saccharomyces cerevisiae. FEBS Lett. 2008;582:977–983. doi: 10.1016/j.febslet.2008.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowski M, et al. Anticancer drugs, ionophoric peptides and steroids as substrates of the yeast multidrug transporter Pdr5p. J Biol Chem. 1996;271:31543–31548. doi: 10.1074/jbc.271.49.31543. [DOI] [PubMed] [Google Scholar]
- Kovalchuk A, Driessen AJ. Phylogenetic analysis of fungal ABC transporters. BMC Genomics. 2010;11:177. doi: 10.1186/1471-2164-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran GP, et al. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob Agents Chemother. 1998;42:1819–1830. doi: 10.1128/aac.42.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhauser J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol. 2010;47:94–106. doi: 10.1016/j.fgb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Nakamura K, et al. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob Agents Chemother. 2001;45:3366–3374. doi: 10.1128/AAC.45.12.3366-3374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento AM, et al. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob Agents Chemother. 2003;47:1719–1726. doi: 10.1128/AAC.47.5.1719-1726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Pomorski T, et al. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, et al. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- Prasad R, Goffeau A. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu Rev Microbiol. 2012:39–63. doi: 10.1146/annurev-micro-092611-150111. [DOI] [PubMed] [Google Scholar]
- Sanglard D, et al. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob Agents Chemother. 1999;43:2753–2765. doi: 10.1128/aac.43.11.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi P, Moye-Rowley WS. Coordinate control of lipid composition and drug transport activities is required for normal multidrug resistance in fungi. Biochim Biophys Acta. 2009;1794:852–859. doi: 10.1016/j.bbapap.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek MS, et al. Inhibition of amino acid transport by sphingoid long chain bases in Saccharomyces cerevisiae. J Biol Chem. 1998;273:2824–2829. doi: 10.1074/jbc.273.5.2829. [DOI] [PubMed] [Google Scholar]
- Tashiro M, et al. Correlation between triazole treatment history and susceptibility in clinically isolated Aspergillus fumigatus. Antimicrob Agents Chemother. 2012;56:4870–4875. doi: 10.1128/AAC.00514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S, et al. Candida glabrata ATP-binding cassette transporters Cdr1p and Pdh1p expressed in a Saccharomyces cerevisiae strain deficient in membrane transporters show phosphorylation-dependent pumping properties. J Biol Chem. 2002;277:46809–46821. doi: 10.1074/jbc.M207817200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


