Abstract
Activation of the complement system has long been known to be regulatedby a series of steps involving fluid-phase convertases, in this issue of Immunity, Liszewski et al. (2013) report the discovery of a novel intracellular cathepsin-L dependent C3activation pathway.
The complement system can be activated by “hard-wired” pattern recognition receptors (PRRs) that have evolved to recognize pattern-associated molecular patterns (PAMPs). PRRs in the complement system recognize exogenous as well as endogenous “danger” motifs. Recognition receptors in the complement system (i.e. specific antibody, mannan-binding lectin (MBL), C1q, and natural IgM) activate three separate complement pathways referred to as the classical, lectin, and alternative. Although each of these pathways is activated by distinct PRRs, they all culminate in activation of the complement factor 3 (C3), the central step in complement activation. The C3 convertase (C3bBb) converts the inactive yet abundant C3 component into the biologically active effector fragments referred to as anaphylatoxins (C3a and C3b). C3a, in turn binds its G-protein coupled receptor, C3aR on the surface of cells, while C3b can either bind its receptor, CD46, or bind to more of the C3 convertase creating the C5 convertase [C3(H2O)BbP3b] which leads to the generation of C5a and C5b. C5b initiates the terminal enzymatic cascade of the lytic membrane attack complexwhich mediates lysis of pathogens and unprotected host cells.
Although liver-generated circulating anaphylatoxins undoubtedly play a role in pathogen control systemically, emerging evidence suggests that anaphylatoxinsare also produced by immune cells including Tcells. Once produced, they bind their receptors on the T cell surface and regulate adaptive T cell immunity (Heeger, 2012; Cardone, 2010). However, to date, the exact mechanism(s)governing anaphylatoxin production in T cellsare not well understood. In pursuit of the mechanism by which C3a is released from human T cells, Liszewski et al (2013)speculated that T cell intrinsic mechanisms might be regulating the rapid release of the C3-cleavage product C3a. Based on their observation that aseries of endosomal or lysosomal proteases were expressed in T cells, they first explored whetherthese cathepsins (B, G, L), may play a role in cleavage of C3 into C3a and C3b in human T cells. Theyfound that cathepsin L (CTSL), but no other cathepsin, cleaved C3 into its active fragments (C3a, C3b). Interestingly, the other cathepsins degradedC3 without generation of biologically active C3a and C3b-suggesting specificity of CTSL for this reaction. Similarly, CTSL-dependent cleavage appears to be specific to C3 and C4, as CTSL does not cleave C5 into its activation fragments. In support of an interaction between CTSL and C3 and C3a in intact cells, they demonstrated that CTSL and C3 co-localized to lysosomal compartments and that inhibition of CTSL activity with a cell-permeable CTSL inhibitor reduced intracellular levels of C3a. These results suggested that CTSL generates “tonic” C3a from intracellular pools of C3 in resting T cells. As neither the C3aR nor the C3b receptor, CD46 were expressed on resting T cells, the functional consequence of producing C3a intracellularly was unclear. However, the authors gained an important clue to the potential function when they noted that the C3aR was co-localized with C3 in lysosomes. Based on the proximity between the C3a and its receptor C3aR intracellularly, they proposeda scenario in which CTSL cleaves C3 into C3a and C3b and C3a in turn binds its receptor intracellularly to regulate basal T cell function (Figure 1). As C3aR engagement on CD4+ T cells activatesthe kinase mTOR, which is required for T cell survival in vivo (Strainic, 2008), they tested the hypothesis that intracellular C3a-C3aR engagement mediated mTOR and resting T cell survival. Indeed, CTSL inhibition and siRNA knockdown of C3aR in resting T cells resulted in reduced mTOR phosphorylation and reduced T cell viability. Although the exact logistics of C3a-C3aR engagement were not elucidated, these results suggest the intriguing possibility that C3a engages its G-protein coupled receptor, C3aR, on the surface of intracellular lysosomes, not on the plasma membrane. These fascinating findings suggesta new pathway of enzymatic cleavage of C3, challenging the traditionallyheld belief that complement activation only occurs through a series of serum convertases.
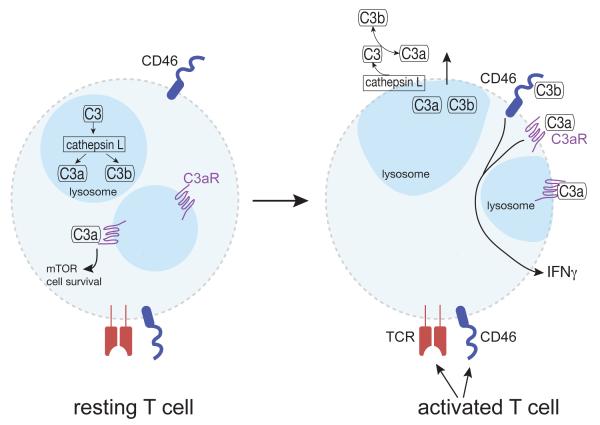
Figure 1. Cathepsin L-dependent intracellular and extracellular complement activation pathways.
In resting T cells, the complement factor C3 is contained intracellularly in performed stores and is proteolytically cleaved to C3a and C3b, by cathepsin L within lysosomes. Intracellularly generated C3a signals via engagement of intracellular C3aRs to promote cell survival via mTOR activation. Unstimulated T cells do not express surface C3, C3a, C3b, the C3aR or cathepsin L. However, upon engagement of the TCR alone or the TCR plus CD46, T cells release C3, C3a, C3b, as well as cathepsin L and express surface C3aR. On the cell surface, cathepsin L cleavesC3 to generate C3a and C3b. Subsequent binding of C3a to C3ar, and C3b to CD46 induces T cell secretion of cytokines such as IFN-γ and IL-17A.
To explore whether C3 mediates similar processes in activated T cells, the authors examined the levels and cellular localization of C3, C3a and C3aRupon TCRactivation. They show TCR activation induces shuttling of the intracellular stores of C3aR to thecell surface, and amplifies intracellular CTSL-mediated cleavage of C3 into C3a and C3b, and induces extracellular, cell surface CTSL-mediated C3 activation. Subsequent extracellular C3aR and CD46 engagement by C3a and C3b, respectively leads to induction of the Th1 cell cytokine, IFN-γ, and TNF-α (Figure 1). CTSL inhibition of TCR-activated T cells results in a reduction in the secretion of the Th1 cell cytokines, IFN-γ and TNF-α, and IL-17A, while it had no effect on Th2 cell cytokine production. This sequence of events is consistent with the lack of robust Th1 cell responses in CD46− and C3-deficient patients (Le Friec, 2012). Interestingly, this phenomenon was not observed in CTSL-deficient mice in which CTSL was maintained only in thymic epithelium, suggesting that mouse and human cells may differ in regards to the role of CTSL regulation of C3 cleavage. This species difference in CTSL regulation of C3a-C3aR may explain some of the conflicting reports of C3aR expression on mouse T cells. The differential expression of the C3aR between resting and activated T cells appears to represent a fail-safe mechanism designed to prevent unnecessary complement activation in the absence of pathogens, but at the same time allows maintenance of a supply of resting T cells which can be called into action if necessary. This elaborate rheostat mimics the protective mechanisms utilized to protect host cells against serum complement activation products.
As T cell hyperactivation and aberrant complement activation are prominent features of several autoimmune disorders, the authors sought to determine whether modulation of cathepsin L pathways normalized T cell cytokine production in T cells from patients with autoimmune arthritis.Strikingly, they found that intracellular C3a levels; mTOR activity and IFN-γ levels were higher in blood T cells from patients with autoimmune arthritis as compared to those obtained from healthy individuals. Importantly, pharmacological targeting of CTSL reversed the heightened IFN-γ production observed in the patient’s T cells. The normalization of their IFN-γ productive capacity was accompanied with a reduction in intracellular C3a levels and mTOR activity.While these findings will need to be confirmed in a larger study, the insights afforded by this study raise the possibility that aberrant regulation of the steps involved in intracellular C3 activation may underlie susceptibility to autoimmune arthritis. More broadly, these findings have implications for a wide spectrum of human disorders associated with complement dysregulation including: other autoimmune diseases, sepsis, age-related macular degeneration, graft rejection, and asthma to name a few.
The current study highlights a role for CTSL-dependent C3a-driven production of the Th1 cell cytokines. Although these studies suggest that CTSL-mediated C3a generation is specifically associated with enhanced Th1 cells and IFN-γ production, other studies have shown that C3a regulates the production of the signature cytokines of other CD4+ T cell subsets such as Th17 (Lajoie, 2010), and Th2 cells (Zhang, 2010). Studies have also shown that Treg cells express C3aR and C5aR and that signaling through these receptors inhibits Foxp3+ expression and Tregcell function. Moreover, blockade of these complement pathways in both mouse and human CD4+ T cells favored their transformation to Foxp3+Tregcells and as a consequence limits the clinical expression of graft-versus-host disease(Van der Touw, 2013). The known ability of Treg cells to suppress the expansion and cytokine production of other CD4+ T cell subsets, suggests that the effect of C3a blockade on IFN-γ levels in autoimmune arthritis patients may be secondary to C3aR-mediated suppression of Treg cell cytokine production and function.
Evidence supporting the conceptual model that intracellular T cell production of C3 and C3a occurs independently of that generated in the liver, was provided by the observation that T cells derived from C3-deficient patients, which do not have measurable serum C3 or C3a levels, contain both C3 mRNA and C3a protein. Although the T cells from all the patients examined contained C3a proteins, the levels were variable between patients and appeared to be dependent upon specific genetic polymorphisms in the respective C3 genes. Theseresults highlight the possibility that previous assumptions made about the role of C3 in immunoregulation, which were based on serum complement component deficiencies in humans, may need to be re-evaluated.
Although pursuit of CTSL and C3 pathways as therapeutic targets for the treatment of T cell-mediated disorders is a tempting option, much remains to be learned about the role of intracellular activation of C3 in T cell-mediated disorders. For example, it remains to be shown whether enhanced T cell survival and cytokine production of T cells from autoimmune patients and/or other complement-associated diseases is due to enhanced basal T cell expression of C3 or to enhanced CTSL cleavage of C3 into C3a. It also remains to be determined whether specific SNPs in C3 preferentially render it susceptible or resistant to CTSL-mediated cleavage. These results will undoubtedly fuel further investigation into the role of dysregulated CTSL-mediated intracellular C3 activation in health and disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–224. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Friec G, Sheppard D, Whiteman P, Karsten CM, Shamoun SA-T, Laing A, Bugeon L, Dallman MJ, Melchionna T, Chillakuri C, et al. The CD46-Jagged interaction is critical for human TH1 immunity. Nat Immunol. 2012;13:1213–1221. doi: 10.1038/ni.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liszewski, et al. 2013. In this issue.
- 6.Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song W-C. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strainic M, Liu J, Huang d., An F, Lalli P, Muqim N, Shapiro V, Dubyak G, Heeger P, Medof M. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naïve CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-B1 signaling and induction of Foxp3+ regulatory T cells. Nat Immunol. 2012;14:162–171. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Kohl J. A complex role for complement in allergic asthma. Expert Rev ClinImmunol. 2010;6:269–277. doi: 10.1586/eci.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS. Cutting edge: Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory cells. J Immunol. 2013;190:5921–5925. doi: 10.4049/jimmunol.1300847. [DOI] [PMC free article] [PubMed] [Google Scholar]