Abstract
Mutants of Azotobacter vinelandii that fix N2 in the presence of excess NH4+ have been isolated. A mutant that was unable to synthesize component I and component II of nitrogenase was spontaneously reverted to the N2-fixing phenotype. Of 21 revertants picked, 7 revertants were not as sensitive as the wild type to repression. A derepressed mutant is as sensitive as the wild type to growth inhibition by 2-methylalanine in the presence of glucose.
Keywords: soil fertility, Azotobacter vinelandii, pleiotropic-negative, 2-methylalanine, derepressed
Full text
PDF
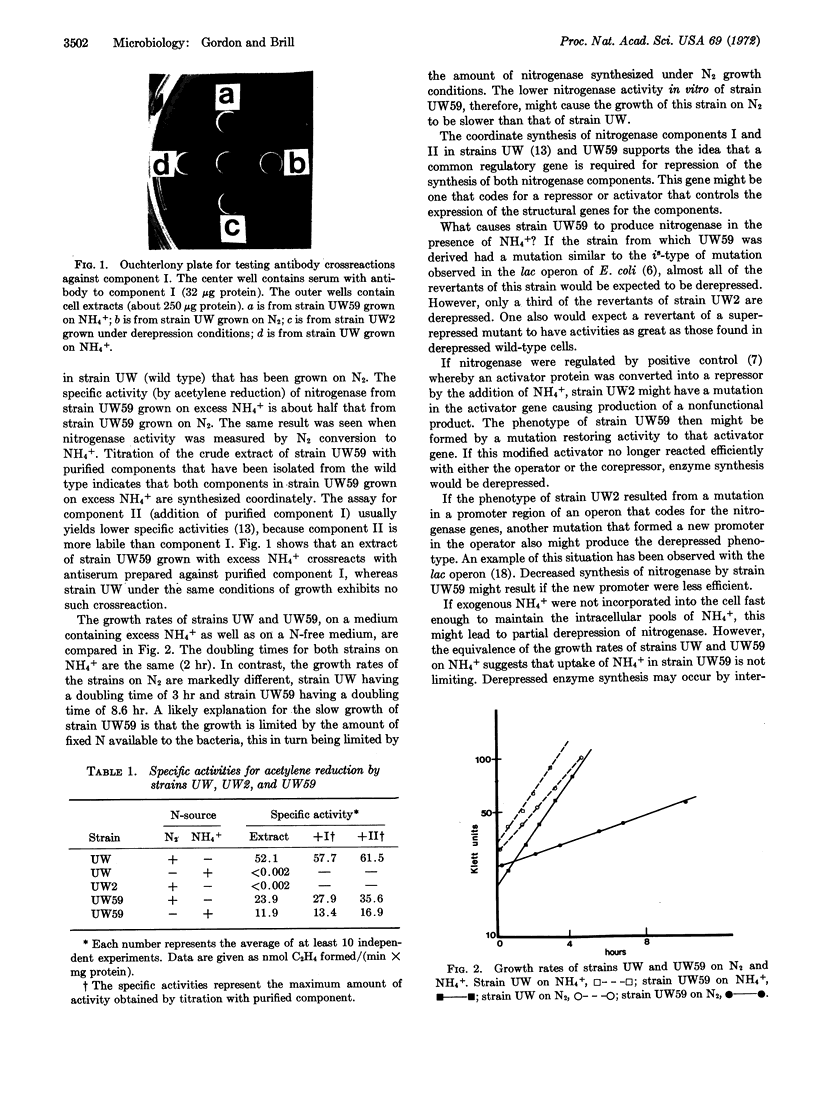

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditti R. R., Scaife J. G., Beckwith J. R. The nature of mutants in the lac promoter region. J Mol Biol. 1968 Dec;38(3):421–426. doi: 10.1016/0022-2836(68)90396-3. [DOI] [PubMed] [Google Scholar]
- DE WITT C. W., ROWE J. A. N,O-Diacetylneuraminic acid and N-acetylneuraminic acid in Escherichia coli. Nature. 1959 Aug 1;184(Suppl 6):381–382. doi: 10.1038/184381b0. [DOI] [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J., Orme-Johnson W. H. Nitrogenase. II. Changes in the EPR signal of component I (iron-molybdenum protein) of Azotobacter vinelandii nitrogenase during repression and derepression. Biochim Biophys Acta. 1972 Feb 28;256(2):512–523. doi: 10.1016/0005-2728(72)90079-5. [DOI] [PubMed] [Google Scholar]
- Englesberg E., Squires C., Meronk F., Jr The L-arabinose operon in Escherichia coli B-r: a genetic demonstration of two functional states of the product of a regulator gene. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1100–1107. doi: 10.1073/pnas.62.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. J., Brill W. J. Mutants of Azotobacter vinelandii unable to fix nitrogen. Biochim Biophys Acta. 1969 Jun 17;184(1):99–105. doi: 10.1016/0304-4165(69)90103-2. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Knight E., Jr ATP-dependent reduction of azide and HCN by N2-fixing enzymes of Azotobacter vinelandii and Clostridium pasteurianum. Biochim Biophys Acta. 1967 May 16;139(1):69–90. doi: 10.1016/0005-2744(67)90114-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munson T. O., Burris R. H. Nitrogen fixation by Rhodospirillum rubrum grown in nitrogen-limited continuous culture. J Bacteriol. 1969 Mar;97(3):1093–1098. doi: 10.1128/jb.97.3.1093-1098.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- Sen M., Sen S. P. Interspecific transformation in Azotobacter. J Gen Microbiol. 1965 Oct;41(1):1–6. doi: 10.1099/00221287-41-1-1. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Brill W. J. Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim Biophys Acta. 1972 Feb 28;256(2):498–511. doi: 10.1016/0005-2728(72)90078-3. [DOI] [PubMed] [Google Scholar]
- Sorger G. J. Regulation of nitrogen fixation in Azotobacter vinelandii OP and in an apparently partially constitutive mutant. J Bacteriol. 1968 May;95(5):1721–1726. doi: 10.1128/jb.95.5.1721-1726.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Brill W. J. Inhibitory effect of methylalanine on glucose-grown Azotobacter vinelandii. Biochim Biophys Acta. 1972 Jan 28;261(1):63–69. doi: 10.1016/0304-4165(72)90314-5. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Lex M. Nitrogenase activity in the blue-green alga Plectonema boryanum strain 594. Arch Mikrobiol. 1970;73(3):250–260. doi: 10.1007/BF00410626. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Molecular H2 and the PN2 function of azotobacter. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1404–1409. doi: 10.1073/pnas.58.4.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonomura B., Rabinowitz J. C. An investigation of the induction of beta-galactosidase in a broken spheroplast preparation of Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):177–202. doi: 10.1016/0022-2836(67)90325-7. [DOI] [PubMed] [Google Scholar]
- WILLSON C., PERRIN D., COHN M., JACOB F., MONOD J. NON-INDUCIBLE MUTANTS OF THE REGULATOR GENE IN THE "LACTOSE" SYSTEM OF ESCHERICHIA COLI. J Mol Biol. 1964 Apr;8:582–592. doi: 10.1016/s0022-2836(64)80013-9. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Hull J. F., Burris R. H. Competition between Free and Combined Nitrogen in Nutrition of Azotobacter. Proc Natl Acad Sci U S A. 1943 Sep;29(9):289–294. doi: 10.1073/pnas.29.9.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Simultaneous Use of Molecular Nitrogen and Ammonia by Clostridium Pasteurianum. Proc Natl Acad Sci U S A. 1951 Sep;37(9):559–565. doi: 10.1073/pnas.37.9.559. [DOI] [PMC free article] [PubMed] [Google Scholar]



