Abstract
CD1d is a major histocompatibility complex class 1–like molecule that regulates the function and development of natural killer T (NKT) cells. Previously, we identified a critical role for the CD1d-NKT cell arm of innate immunity in promoting the development of UVB-induced p53 mutations, immune suppression, and skin tumors. Sunburn, an acute inflammatory response to UVB-induced cutaneous tissue injury, represents a clinical marker for non-melanoma skin cancer (NMSC) risk. However, the innate immune mechanisms controlling sunburn development are not considered relevant in NMSC etiology, and remain poorly investigated. Here we found that CD1d knockout (CD1d−/−) mice resist UVB-induced cutaneous tissue injury and inflammation compared with wild-type (WT) mice. This resistance was coupled with a faster epithelial tissue healing response. In contrast, the skins of UVB-irradiated invariant NKT cell-knockout (Jα18−/−) and NKT cell–deficient (TCRα−/−) mice, which express CD1d but are deficient in CD1d-dependent NKT cells, exhibited as much cutaneous tissue injury and inflammation as WT mice. In the absence of NKT cells, CD1d-deficient keratinocytes, dendritic cells, and macrophages exhibited diminished basal and stress-induced levels of pro-inflammatory mediators. Thus, our findings identify an essential role for CD1d in promoting UVB-induced cutaneous tissue injury and inflammation. They also suggest sunburn and NMSC etiologies are immunologically linked.
Introduction
CD1d is a non-classical major histocompatibility complex class I–like antigen-presenting molecule that presents lipids (Godfrey et al., 2004; Bendelac et al., 2007; Balato et al., 2009) and non-lipids (Van Rhijn et al., 2004) to natural killer T (NKT) cells. It is expressed by professional antigen-presenting cells (Porcelli, 1995; Brigl and Brenner, 2004) and epithelial cells of most tissues, including skin (Blumberg et al., 1991; Nickoloff et al., 1999; Bonish et al., 2000; Fishelevich et al., 2006). Functionally, NKT cells have the ability to suppress or activate innate and adaptive immune responses without the need for clonal expansion, by rapidly releasing significant quantities of cytokines and other mediators on primary CD1d recognition (Bendelac et al., 2007; Balato et al., 2009), thus closely resembling cells of the innate immune system. NKT cell function and development is dependent on CD1d expression because these cells fail to develop in transgenic mice with CD1d targeted gene disruption (Mendiratta et al., 1997; Godfrey et al., 2004). Invariant or type 1 NKT cells use a conserved, semi-invariant mouse Vα14-Jα18 or human Vα24-Jα18 TCR (Taniguchi et al., 2003; Godfrey and Kronenberg, 2004) whereas type 2 NKT cells have a more diverse TCR repertoire (Godfrey et al., 2004; Bendelac et al., 2007; Balato et al., 2009).
Wavelengths in the UVB radiation range (290–320 nm) of the solar spectrum are absorbed by the skin and responsible for causing physical cutaneous tissue injury and inflammation (Cavallo and DeLeo, 1986; Clydesdale et al., 2001), immune suppression (Fisher and Kripke, 1982; Ullrich, 2005), DNA mutations (Brash et al., 1996; Melnikova and Ananthaswamy, 2005), and eventually non-melanoma skin cancer (NMSC; Melnikova and Ananthaswamy, 2005). The principal causes of NMSC are considered to be the combined mutagenic and immune-suppressive effects of UVB exposure (Melnikova and Ananthaswamy, 2005; Ullrich, 2005). Previously, we showed that CD1d and NKT cells had a critical role in the development of UVB-induced skin tumors (Matsumura et al., 2004). We found that skin tumor incidence was significantly reduced in chronically UVB-irradiated CD1d knockout (CD1d−/−) mice compared with wild-type (WT) controls. Tumor resistance was associated with the skin of these mice being more sensitive to UVB-induced apoptosis and harboring significantly fewer p53 mutations compared with WT control skin, indicating an essential role for CD1d in controlling the tumor initiation phase of UV carcinogenesis. In adoptive transfer experiments, NKT cells isolated from the spleens of chronically UVB-irradiated donor mice were demonstrated to have a critical role in suppressing the anti-skin tumor immune response and permitting highly antigenic UVB-induced skin tumors to develop (Moodycliffe et al., 2000). Thus, these studies indicate an essential role for the CD1d-NKT cell arm of innate immunity in the etiology of UVB-induced skin tumors.
Sunburn, clinically in its mildest form, consists of a transient reddening or erythema of the exposed skin (Johnson, 1978; Cavallo and DeLeo, 1986; Clydesdale et al., 2001). With increasing intensity of UVB exposure, sunburn is characterized by severe blistering, necrosis, redness, and pain. The inflammatory processes include the production and secretion of pro-inflammatory mediators, including cytokines, chemokines, and prostaglandins produced mostly by keratinocytes, and infiltration of neutrophils into the irradiated site (Kock et al., 1990; Luger and Schwarz, 1990; Barker et al., 1991). When erythema is minimal, epidermal tissue damage is restricted to isolated cells undergoing apoptosis, called sunburn cells (Ziegler et al., 1994; Brash et al., 1996). With more severe sunburn reactions, the number of damaged cells increases and basal layer cells may be involved. Complete epidermal necrosis is seen in blistering reactions and dermal tissue damage may be evident (Johnson, 1978; Cavallo and DeLeo, 1986; Clydesdale et al., 2001). Sunburn is recognized epidemiologically as an important clinical marker for skin cancer risk, since people with a history of repeated blistering sunburns are more likely to develop NMSC (Naylor, 1997; Armstrong and Kricker, 2001; Kennedy et al., 2003). However, the innate immune mechanisms that regulate sunburn development are not considered linked to UVB-induced skin tumor development, and remain poorly understood. Thus, the aim of our study here was to address if these two skin pathologies are immunologically linked, by investigating if the CD1d-NKT cell arm of innate immunity also regulates sunburn development.
Results
CD1d-knockout mice resist UVB-induced cutaneous tissue damage and inflammation
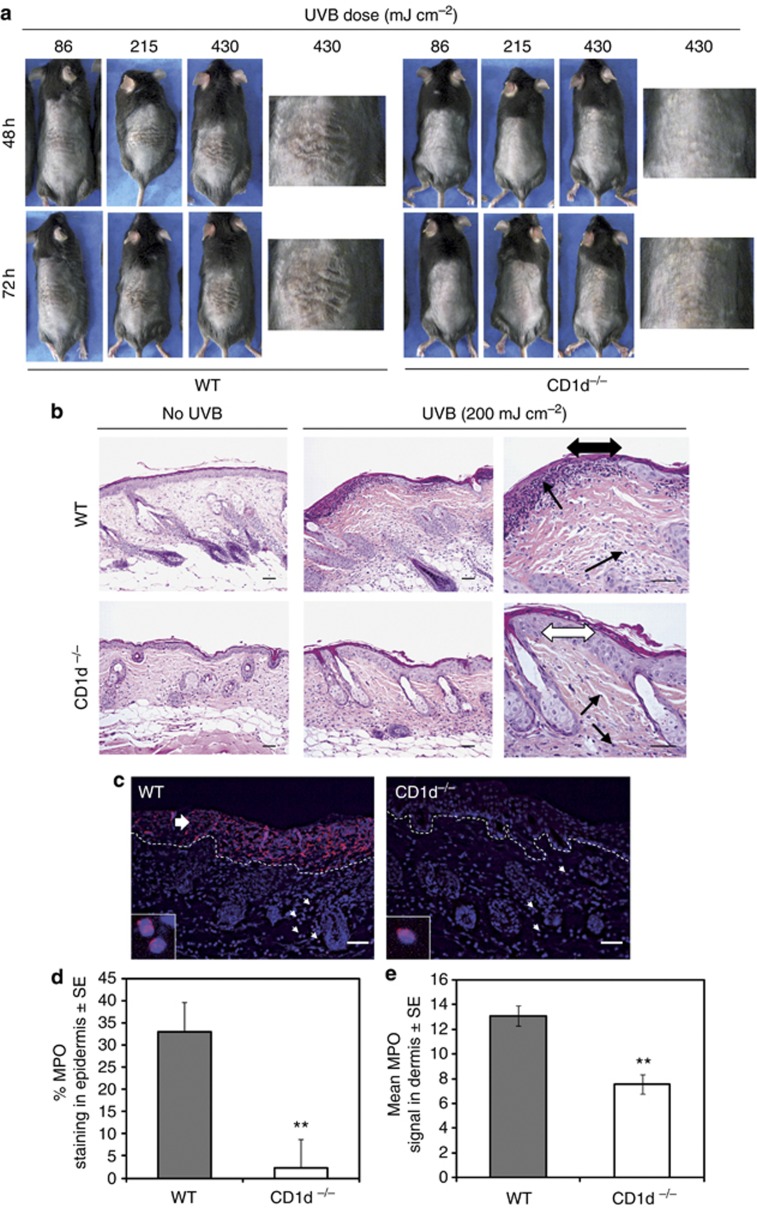
At the dermatoscopic level, signs of UVB-induced cutaneous tissue injury (referred to as necrotic crust formation) and inflammation (erythema/skin reddening) are very difficult to visualize in highly pigmented C57BL/6 murine skin. For this reason, we first studied susceptibility to UVB-induced skin injury and inflammation using WT and CD1d−/− mice maintained on a C57BL/6 × 129 mixed background, which have much less pigmented skin compared with C57BL/6 mice. At 48 and 72 hours after exposure to 86, 215, or 430 mJ cm–2 UVB, all C57BL/6 × 129 WT mice exhibited signs of necrotic crust formation (skin damage) and erythema; the extent of sunburn increased with increasing UVB dose and time after exposure (Figure 1a). In contrast, C57BL/6 × 129 CD1d−/− mice were much less susceptible to the skin damaging and erythemic effects of UVB exposure, regardless of time or dose (Figure 1a). A histopathological and immunohistochemical analysis of the UVB (200 mJ cm–2)—irradiated skin of both strains confirmed these findings (Figure 1b and c). UVB-irradiated C57BL/6 WT skin sections displayed multi-focal sites of erosion (epidermal loss) and/or ulceration (epidermal and dermal loss), which was most evident 48 hours after UVB exposure. This tissue destruction was accompanied by extensive infiltration of inflammatory cells into the dermis and at sites of epidermal loss, indicating an inflammatory response. In contrast, C57BL/6 CD1d−/− mice were far more resistant to UVB-induced cutaneous tissue destruction and inflammation, since their epidermal layer remained intact and the relative amounts of dermal inflammatory cell infiltrates were low compared with UVB-irradiated WT control mice, at all time points analyzed (Figure 1b). Myloperoxidase staining of UVB-irradiated WT and CD1d−/− skin confirmed that the extent of neutrophil infiltration into the dermis and epidermis is severely diminished in CD1d−/− mice 48 hours after irradiation (Figure 1c–e).
Figure 1.
UVB-induced cutaneous tissue injury and inflammation are abolished in CD1d-knockout mice. (a) Photographs of single UVB-irradiated backs of CD1d knockout (CD1d−/−) and wild-type (WT) (C57BL/6 × 129) mice exposed to different UVB doses. (b) Hematoxylin and eosin staining of the backs of single UVB-irradiated C57BL/6 CD1d−/− and WT mice at 48 hours. Single arrowheads: inflammatory infiltrates; double arrowheads, black: epidermis erosion, white: intact epidermis. (c) Immunohistofluorescence of skin sections from (b) stained for myeloperoxidase (MPO) and 4,6-diamidino-2-phenylindole (DAPI). Dashed: epidermis–dermis junction. Small arrows: MPO-positive cells. Large arrow: epidermis ulceration. (d) Mean epidermal MPO staining (normalized to the total epidermal surface)±SE for WT (n=3) and CD1d−/− (n=4) skin sections; analysis of variance, **P<0.01. (e, d) Mean MPO-positive cells in the dermis (per 104 μm2)±SE. Scale bar=0.05 mm.
UVB-induced pro-inflammatory gene expression is severely diminished in CD1d-knockout mouse skin
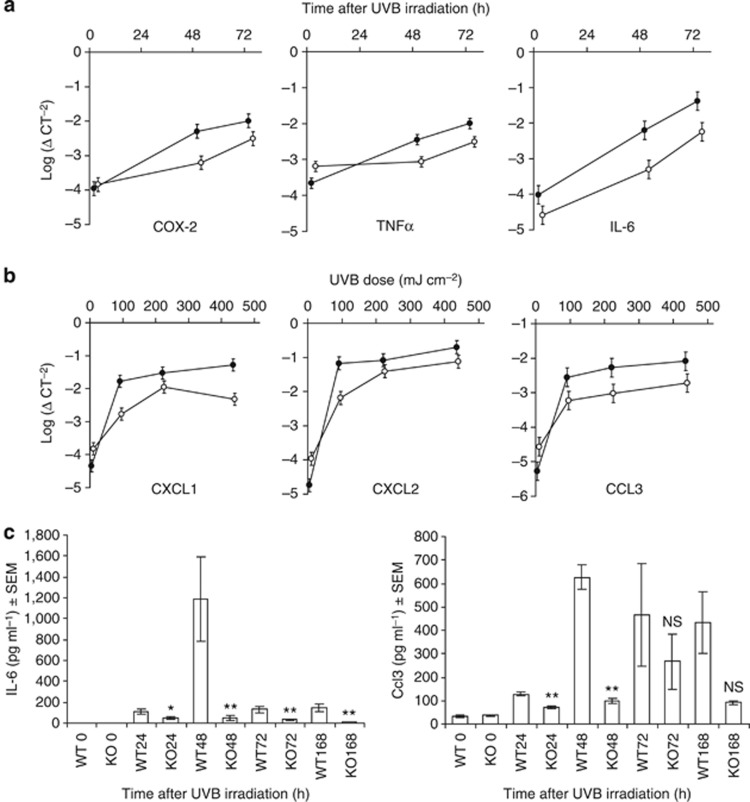
To quantify the severity of the inflammation, we measured and compared the mRNA expression level of key pro-inflammatory genes in UVB-exposed C57BL/6 × 129 WT and CD1d−/− mouse skin. COX-2, tumor necrosis factor α (TNFα) and IL-6 mRNA levels were measured in skin exposed to high-dose UVB whereas CXCL1, CXCL2, and CCL3 mRNA levels were measured in skin exposed to low-, medium-, and high-dose UVB. In response to high-dose (430 mJ cm–2) UVB exposure, COX-2, TNFα, and IL-6 mRNA levels increased in the skin of WT and CD1d−/− mice in a time-dependent manner, compared with non-irradiated controls (Figure 2a). However, these levels were significantly less in the skin of UVB-irradiated CD1d−/− mice compared with WT mice 48 and 72 hours after UVB irradiation (Figure 2a). Seventy-two hours after UVB exposure, there was a UVB dose-dependent increase in the levels of CXCL1, CXCL2, and CCL3 mRNA in the skin of WT and CD1d−/− mice, compared with non-UVB-exposed controls, but again the mRNA levels of each and every one of these chemokines was considerably significantly lower in CD1d−/− skin compared with WT skin, regardless of the dose (Figure 2b).
Figure 2.
UVB-induced pro-inflammatory gene expression is severely diminished in CD1d-knockout skin. (a, b) COX-2, tumor necrosis factor α (TNFα), IL-6, CXCL1, CXCL2, and CCL3 mRNA expression in the skin of UVB-irradiated C57BL/6 × 129 CD1d−/− (○) and wild-type (WT; •) mice. (a) mRNA levels measured at different time points after high-dose UVB (430 mJ cm–2) exposure. Non-irradiated (0 hours). (b) mRNA levels 72 hours after exposure to 86, 215, or 430 mJ cm–2 UVB. (c) Skin IL-6 and Ccl3 protein levels of C57BL/6 CD1d−/− (KO) versus WT mice at different times after exposure to 200 mJ cm–2 UVB. Non-irradiated (0 hours). *P<0.05 and **P⩽0.02. NS, no significance. Data are the means±SEM of triplicate wells; three independent experiments with n=5 per group.
To further quantify the severity of the inflammatory response, we measured the level of IL-6 and Ccl3 protein in 200 mJ cm–2 UVB-exposed skin of C57BL/6 CD1d−/− and WT mice at different time points after irradiation. IL-6 protein levels remained significantly lower in CD1d−/− compared with WT skin, 24 (P<0.05), 48 (P<0.02), 72 (P<0.02), and 168 hours (P<0.01) after UVB exposure (Figure 2c). Similarly, the protein level of Ccl3 also remained significantly lower in CD1d−/− skin compared with WT skin, 24 (P<0.005) and 48 hours (P<0.005) following UVB exposure (Figure 2c).
Thus, collectively these data indicate that sunburn etiology is largely dependent on the CD1d-NKT cell arm of innate immunity.
CD1d-knockout mice exhibit a faster epithelial tissue healing response following UVB-induced injury
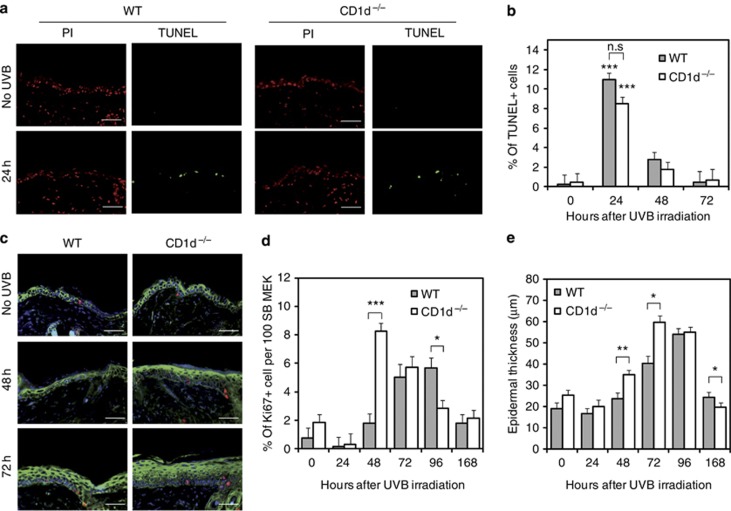
In addition to causing cutaneous tissue injury and inflammation, acute UVB overexposure also induces apoptotic cell death and epidermal regeneration (Johnson, 1978). We therefore next addressed if these processes differed between C57BL/6 CD1d−/− and WT mice exposed to a single UVB dose of 200 mJ cm–2. The number of TUNEL-positive cells peaked at 24 hours after UVB irradiation mainly in the epidermis of WT and CD1d−/− mice and declined thereafter (Figure 3a and b). However, no significant difference in the number of TUNEL-positive cells was observed between WT and CD1d−/− mice at 24, 48, and 72 hours after UVB exposure. Very few apoptotic cells were detected in the dermis of WT and CD1d−/− mice (Figure 3a). Significant differences were, however, observed in the levels of UVB-induced epidermal proliferation between WT and CD1d−/− mice. Specifically, there was a dramatic rapid increase in the level of basal epidermal proliferation 48 hours after UVB irradiation in CD1d−/− mice compared with WT mice (Figure 3c and d). Although WT mice also exhibited an increased proliferative response to UVB injury, this arose later at 72 hours and was of a lower magnitude. These differences were mirrored in the levels of epidermal thickness, which were significantly higher at 48 and 72 hours in CD1d −/− mice compared with WT mice (Figure 3e).
Figure 3.
UVB-induced epidermal proliferation but not apoptosis is enhanced in CD1d-knockout skin. (a–d) TUNEL and immunofluorescence of UVB-irradiated skin sections from C57BL/6 wild-type (WT; n=3) and CD1d knockout (CD1d−/−) (n=4) mice after exposure to a single UVB dose (200 mJ cm–2). (a) Apoptotic cells (green), total cells (red). (b) Quantification of (a) mean % of apoptotic cells 24–72 hours after UVR, non-irradiated (0 hours); analysis of variance, ***P<0.001 compared with non-UV conditions; nonsignificant (NS) compared with the cell type. (c) Skin sections stained for Ki67 (red), epidermal cytokeratin (green), and 4,6-diamidino-2-phenylindole (DAPI). (d) Quantification of (c) mean % of Ki67+ keratinocytes in the stratum basal 24–168 hours after UVR, non-irradiated (0 hours). (e) Epidermal thickness (μm) of (c) 24–168 hours after UVR. Analysis of variance, ***P<0.001; **P<0.01; *P<0.05. Scale bar=0.05 mm. PI, propidium iodide.
Invariant NKT cell knockout and NKT cell-deficient mice are not resistant to UVB-induced cutaneous tissue damage and inflammation
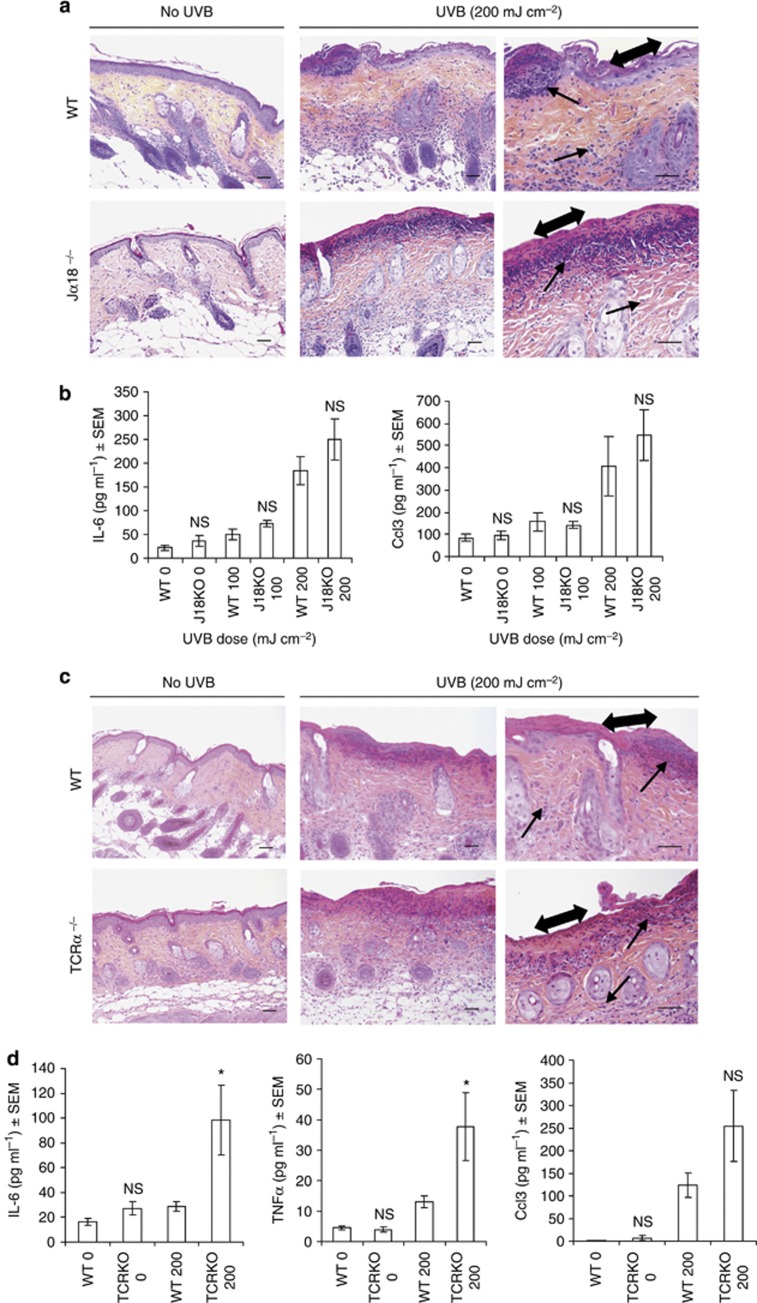
Considering CD1d's primary recognized role is the presentation of lipids to NKT cells (type 1 and 2) (Godfrey et al., 2004; Balato et al., 2009), the most likely candidate responsible for sunburn development are CD1d-activated NKT cells. We therefore next addressed the causal role of these cells in the induction of skin inflammation (Figure 4a and b). Unexpectedly, we found that Jα18−/− mice, which express CD1d but are deficient only in type 1 NKT cells, exhibited as much cutaneous erosion and ulceration (tissue necrosis) and dermal infiltration of mixed inflammatory cells as UVB-irradiated WT mouse skin (Figure 4a). A comparison of the protein levels of CCL3 and IL-6 between UVB-irradiated WT and Jα18−/− skin confirmed that type 1 NKT cell-deficient skin is not diminished in its capacity to initiate a skin inflammatory response (Figure 4b). Unlike type 1 NKT cells, little is known about type 2 NKT cells as we lack specific reagents to directly identify them (Terabe and Berzofsky, 2007), therefore making a direct assessment of their in vivo role in UVB-induced skin inflammation very difficult. In an attempt to address this question, we compared the histology and pro-inflammatory gene expression levels of UVB-irradiated WT skin with the skin of TCRα−/− mice, which express CD1d but are deficient in both types of NKT cells (Figure 4c and d). As with Jα18−/− mice we observed that the skins of UVB-irradiated TCRα−/− mice were as susceptible to UVB-induced cutaneous tissue injury and inflammation as WT mice (Figure 4c). Furthermore, regardless of UVB dose or time after exposure, the protein levels of IL-6, CCL3, and TNFα in the skin of UVB-irradiated TCRα−/− mice were not significantly different compared with WT controls and, in some cases, were actually higher (Figure 4d). Thus, contrary to our expectations these findings suggest that CD1d-dependent NKT cells are not important in promoting sunburn development.
Figure 4.
NKT cell-deficient mice are not resistant to UVB-induced cutaneous tissue injury and inflammation. (a) Hematoxylin and eosin (H&E) staining of single UVB-irradiated type I NKT cell-knockout mice (Jα18−/−) and wild-type (WT) mice backs 48 hours after exposure to 200 mJ cm–2 UVB. (b) Skin IL-6 and Ccl3 protein levels of (a) after exposure to 100 or 200 mJ cm–2 UVB. (c) H&E staining of UVB-irradiated type I and II NKT cell-deficient mice (TCRα−/−) and WT mice backs 48 hours after exposure to 200 mJ cm–2 UVB. (d) Skin IL-6, CCL3, and tumor necrosis factor α (TNFα) protein levels of (c). *P<0.05; NS, no significance. Data are the means±SEM of triplicate wells, two independent experiments with n=7–10 per group. Single arrowhead: inflammatory infiltrates. Double arrowhead: epidermis erosion. Non-irradiated (0 hours). Scale bar=0.05 mm.
Independently of NKT cells, CD1d controls the innate immune response of epithelial and differentiated myeloid cells by enhancing the basal expression of CXCL1
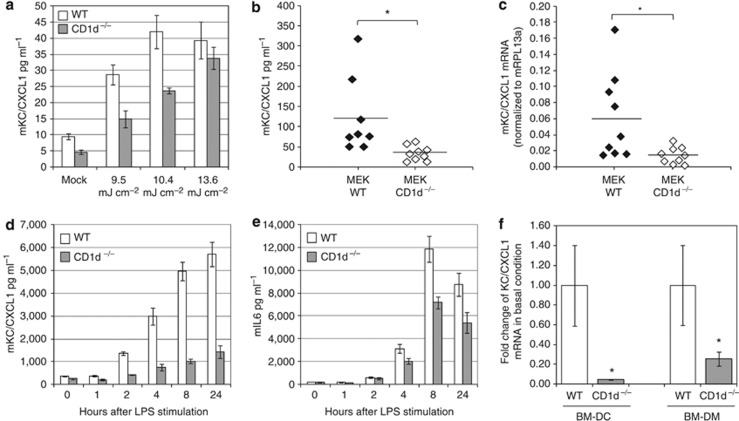
Keratinocytes have an essential role in the initiation of the innate immune response, in response to external stressors like UVB (Kock et al., 1990; Luger and Schwarz, 1990; Barker et al., 1991). Thus, to address if CD1d regulates the innate immune function of these cells independently of NKT cells, we next compared the innate immune response of WT versus CD1d−/− cultured primary neonatal keratinocytes. Although WT neonatal keratinocytes secreted significant levels of CXCL1 24 hours after UVB irradiation, the absolute level of CXCL1 secreted by CD1d−/− keratinocytes was significantly lower, regardless of the UVB dose (Figure 5a). This was not the result of increased UVB-induced cell death because no significant differences in cell viability were observed between WT and CD1d−/− keratinocytes, irrespective of UVB dose (Supplementary Figure S1 online). An assessment of the relative UVB induction of CXCL1 protein by WT and CD1d−/− keratinocytes compared with their respective basal levels revealed no significant differences, suggesting that the control of CXCL1 gene expression by CD1d in mouse keratinocyte is mainly occurring under resting conditions, irrespective of UVB exposure (data not shown). Indeed, a comparison of the basal protein expression levels of CXCL1 in nine independent WT and CD1d−/− keratinocyte cultures revealed that CXCL1 protein (Figure 5b) and mRNA (Figure 5c) levels are significantly reduced in CD1d-deficient cells. Antigen-presenting cells in the skin also have an instrumental role in innate immunity, initiating inflammatory responses on exposure to stress-induced danger signals (Piccinini and Midwood, 2010). Thus, we next generated bone marrow–derived dendritic cells (BMDCs; Supplementary Figure S2 online) from WT and CD1d−/− mice and analyzed if the innate immune status of these cells are dependent on CD1d function. Following lipopolysaccharide (LPS) stimulation of WT and CD1d−/− BMDCs, both the absolute (Figure 5d and e) and relative induction (data not shown) of CXCL1 and IL-6 protein secreted by CD1d−/− cells were significantly less compared with WT control cells, suggesting that CD1d expressed by dendritic cells controls the induction of pro-inflammatory mediators following TLR4 activation by LPS. Interestingly, as no difference in their relative mRNA fold induction levels was detected (Supplementary Figure S3 online), CD1d expressed by dendritic cells may in part control CXCL1 and IL-6 gene induction post-transcriptionally. This control was not just specific to TLR4 receptor signaling because CXCL1 induction by other TLR agonists acting via different TLR receptors was also significantly reduced (Supplementary Figure S4 online). The ability of CD1d to control the expression of pro-inflammatory mediators was not just limited to dendritic cells since WT and CD1d−/− bone marrow–derived macrophage cells gave the same results under the same conditions (data not shown). An analysis of the basal gene expression levels of CXCL1 in CD1d-deficient BMDCs and macrophages revealed that these were significantly lower compared with WT cells (Figure 5f), as was observed in CD1d−/− keratinocytes. Thus, collectively these results suggest, to our knowledge and unreported previously, that the innate immune status of epithelial cells and differentiated myeloid cells is dependent on CD1d expression, and independent of NKT cell involvement.
Figure 5.
CXCL1 expression is reduced in CD1d−/− mouse epithelial keratinocyte (MEK) and differentiated myeloid cells. (a) ELISA of UVB dose-dependent release of CXCL1 by wild-type (WT) and CD1d knockout (CD1d−/−) MEK 24 hours after exposure (triplicates, means±SD), representative experiment performed three times independently. (b) ELISA of CXCL1 protein and (c) mRNA levels in resting WT and CD1d −/− MEKs. t-Test n=9, means; *P<0.05. Kinetic of CXCL1 (d) and mIL-6 (e) proteins released in the medium of WT and CD1d −/− bone marrow–derived dendritic cell (BMDC) stimulated with 100 ng ml–1 lipopolysaccharide (LPS) over 24 hours (ELISA triplicates; means±SD), representative experiment performed three times independently. (f) CXCL1 mRNA levels expressed in resting WT and CD1d −/−-deficient BMDCs and bone marrow–derived macrophages (BMDM); t-test n=6 for BMDC, n=8 for BMDM; means±SEM; *P<0.05.
Discussion
The sunburn reaction is a complex inflammatory process in response to acute UVB-induced tissue injury, with the mechanisms involved being still largely unresolved (Johnson, 1978; Cavallo and DeLeo, 1986; Clydesdale et al., 2001). Although mouse and human CD1d proteins are largely conserved in amino acid sequence and 3D structure (Brigl and Brenner, 2004; Pellicci et al., 2009), and are expressed by keratinocytes (Canchis et al., 1993; Bonish et al., 2000; Fishelevich et al., 2006; Sikder et al., 2009), the role of cutaneous CD1d in regulating sunburn development is unknown. Using different transgenic knockout mice, this study provides evidence that the CD1d-NKT cell arm of innate immunity has a critical role in the pathogenesis of the sunburn reaction. We show that compared with WT controls, mice deficient in CD1d and NKT cells resist UVB-induced cutaneous tissue injury and inflammation. However, although apoptotic cells arise during sunburn development (Brash et al., 1996; Herrlich et al., 2008), no significant difference in the levels and kinetics of apoptotic sunburn cell formation were observed between acute UVB-irradiated WT and CD1d−/− mice, suggesting that CD1d-dependent skin inflammatory events are regulated by UVB-induced apoptotic-independent pathways. It should be noted that in a previous study, the levels of acute UVB-induced apoptosis induced in the skins of WT and CD1d−/− mice were considerably higher, and in CD1d−/− mice were more prolonged (Matsumura et al., 2004). However, as UVB-induced apoptosis is UVB dose dependent (Bernerd et al., 2000), the fact that higher UVB doses were used in this previous study most likely account for the differences observed.
A possible mechanism by which CD1d regulates the sunburn reaction is via the activation of NKT cells. However, Jα18−/− mice that express CD1d but are deficient in CD1d-dependent NKT type 1 cells were as susceptible to UVB-induced tissue injury and inflammation as WT mice. This was also the case for TCRα−/− mice that are deficient in all types of CD1d-dependent NKT cells suggesting that CD1d promotes sunburn development independently of CD1d-dependent NKT cells. These findings are supported by studies showing that mice deficient in CD4-positive T cells, which represent the majority of CD1d-restricted NKT cells, are not diminished in their capacity to induce a cutaneous inflammatory response following acute UVB exposure (Hatton et al., 2007), and human CD1d-bearing keratinocytes are unable to activate NKT cells to secrete cytokines (Bonish et al., 2000; Gober et al., 2008). Although the CD1d-mediated sunburn reaction is likely to be independent of NKT cells, we cannot rule out here the role of other CD1d-mediated T cells like γδT cells, which were recently proposed to be activated by CD1d (Dieude et al., 2011; Bai et al., 2012). Interestingly, CD1d has been shown to be involved in the modulation of metabolic functions through an invariant NKT-independent mechanism (Kotas et al., 2011), suggesting an alternative function for CD1d other than antigen presentation to T cells in metabolic or lipid-enriched tissues like the skin. Our finding that the innate immune status of CD1d-deficient keratinocytes and differentiated myeloid cells cultured in the absence of NKT cells was diminished in the absence or presence of a stressor lends further support to this notion.
Exactly how CD1d mediates UVB-induced tissue injury and inflammation and at which molecular level, remains to be elucidated. In mammals, UVB radiation is of biological relevance primarily for skin epithelial cells, which express CD1d. Thus, it is reasonable to suspect that CD1d may mediate at least some of these effects directly at the level of the keratinocyte. In this regard, we have observed that the epidermal layer of CD1d-deficient skin resists erosion, and has a higher capacity to proliferate in response to UVB overexposure compared with WT control mice, suggesting that CD1d may mediate UVB-induced epithelial tissue injury and regeneration directly at this cellular level. It is known that keratinocytes exposed to UVB also have an essential role in initiating inflammatory responses via their production of cytokines, chemokines, and release of UVB-damaged RNA (Kock et al., 1990; Luger and Schwarz, 1990; Barker et al., 1991). Thus, the innate immune function of keratinocytes may also be dependent on their direct expression of CD1d, because the levels of pro-inflammatory mediators as well as inflammatory cell infiltrates were drastically reduced in UVB-irradiated CD1d-deficient skin compared with WT control mice. The observation that CXCL1 gene expression was diminished in cultured CD1d-deficient keratinocytes and myeloid cells under resting conditions is intriguing. Similarly, it is also of interest that CD1d-deficient myeloid cells had a reduced capacity to induce CXCL1 expression following TLR activation, regardless of the TLR agonist. Several reports have shown that CD1d can directly reverse signal in epithelial or differentiated myeloid cells inducing IL-12 or IL-10 secretion when the receptor is ligated at the cell surface by anti-CD1d antibodies (Colgan et al., 1999; Yue et al., 2005; Kawana et al., 2008). However, our data allude more to an indirect mechanism, whereby CD1d regulates basal and stress-induced innate immunity independently of cell extrinsic factors (e.g., CD1d ligation or TCR ligation). Lipid metabolism and subsequently lipid trafficking and distribution between various intracellular organelles must be tightly regulated to ensure appropriate membrane function including lipid raft formation and cell signaling. Recent studies suggest an association between the cellular machinery that loads CD1d molecules with glycolipids and several key proteins that regulate lipid metabolism (Brozovic et al., 2004; Kang and Cresswell, 2004; Winau et al., 2004; Zhou et al., 2004; Dougan et al., 2005; Yuan et al., 2007). Interestingly, Muindi et al. (2010) reported that the repertoire of self-glycosphingolipids bound to mouse CD1d can be altered by intracellular trafficking and changes after lipopolysaccharide stimulation. Thus, one mechanism by which CD1d could regulate basal innate immunity and cell signaling events induced by environmental stressors such as UV or TLR agonists is by regulating cellular lipid metabolism and lipid-mediated cell signaling pathways. In this regard, it was recently reported that phospholipase C knockout mice exhibit a markedly suppressed UVB-induced neutrophil-associated skin inflammation because of a reduction in CXCL1 gene expression (Oka et al., 2011).
Similar to microbial-induced inflammation, sterile inflammation is marked by the production of pro-inflammatory cytokines and chemokines, which lead to the recruitment of neutrophils and macrophages to the site of tissue damage (Chen and Nunez, 2010). The main function of sterile inflammation is tissue and wound repair, and to return to a state of homeostasis (Chen and Nunez, 2010). Here we present evidence that the highly evolutionary conserved major histocompatibility complex class 1-like molecule, CD1d (Brigl and Brenner, 2004; Pellicci et al., 2009), has an instrumental role in initiating UVB-induced sterile inflammation by regulating neutrophil recruitment, cytokine, and chemokine production, most likely for cutaneous tissue and wound repair. Although sterile inflammation is important in tissue and wound repair, unresolved, chronic inflammation that occurs when the offending agent is not removed or contained can be detrimental to the host leading to different inflammatory diseases depending on the nature of the stimuli (Coussens and Werb, 2002; de Visser et al., 2006). Similarly, in the case of UVB overexposure or chronic exposure, this can be detrimental to the host resulting in sunburn, and eventually skin cancer. Previously, we reported that the incidence of UV-induced skin tumors were reduced in the skin of chronically UVB-irradiated CD1d−/− mice indicating a critical role for the CD1d-NKT cell arm of innate immunity in the development of UVB-induced skin tumors (Matsumura et al., 2004). Thus, it seems reasonable to speculate that sunburn and skin tumor development are the aberrant consequence of this arm of the innate immune system attempting to maintain epithelial tissue homeostasis in the face of excessive and/or constant UVB challenges over the course of a life time.
In conclusion, this study has provided to our knowledge previously unreported experimental evidence that CD1d has an essential function in the pathogenesis of sunburn reactions. As UVB-induced skin tumor development is also dependent on CD1d function (Moodycliffe et al., 2000; Matsumura et al., 2004) our findings also introduce the concept that sunburn and UVB-induced skin tumor development maybe mechanistically linked at the level of CD1d-NKT cell innate immune protection against UV-induced epithelial injury. Thus, indicating an original strategy for skin cancer prevention by targeting this arm of the immune system to treat sunburn.
Materials and Methods
Animals
CD1d-knockout (CD1d−/−) mice have been described (Mendiratta et al., 1997) and were kindly provided Dr Luc Van Kaer (Vanderbilt University School of Medicine, Nashville, TN). Both CD1d−/− and littermate control mice maintained on a mixed background (C57BL/6 × 129) and C57BL/6 CD1d−/− and C57BL/6 littermate control mice backcrossed to a B6 background were used. Jα18−/− mice backcrossed to B6 were kindly provided by Dr Masaru Taniguchi (Chiba University, Chiba, Japan). C57BL/6 TCRα−/− and C57BL/6 control mice were obtained from Charles River Laboratories, L'Arbresle, France. All mice were housed under specific pathogen-free conditions. UVB irradiation of mice was performed with a bank of four Philips Ultraviolet-B TL40W/12 sunlamps (Philips, Eindhoven, The Netherlands) see Supplementary Materials. The experiments were approved by the Swiss Cantonal Institutional Animal Care and Use Committee and are in conformance with the Swiss Law of Animal Protection.
Histology and immunostaining
Skin punch biopsies (one per mouse) taken from the UVB-exposed site of individual mice (4–9 mice per experimental group) at different times after irradiation or from untreated mice were fixed in formaldehyde and paraffin embedded. Two skin sections (5 μm) derived from a single punch biopsy were stained with hematoxylin–phloxine–safrin and a complete histopathological examination performed blind. Sections were stained with primary anti-mouse myeloperoxidase (Thermo Fisher Scientific, Rockford, IL), anti-mouse Ki67 (Thermo Fisher Scientific), and anti-vertebrate cytokeratin (clone Lu5; BMA Biomedicals, Augst, Switzerland) antibodies; revealed using appropriate Alexa secondary antibodies (Invitrogen, Bleiswijk, The Netherlands) and co-stained with 4,6-diamidino-2-phenylindole before mounting. Images were captured by a Zeiss fluorescence microscope (Feldbach, Switzerland) coupled and quantified using ImageJ (Open Source Software, http://rsb/info.nih.gov/ij).
Quantitative Real-Time PCR
Eight millimeter punch biopsies of dorsal skin were harvested and homogenized using a FastPrep (Q-Biogen, Illkirch, France) instrument, centrifuged and supernatants frozen at –20 °C until RNA extraction. RNA was extracted from supernatants using a totally RNA kit (Ambion Invitrogen, Bleiswijk, The Netherlands) based on the manufacturer's recommendations. After DNase1 (Invitrogen) treatment, each total RNA sample (1 μg) were reverse transcribed based on the manufacturer's instructions (Superscript, Invitrogen). Resulting complementary DNA samples were quantified by quantitative PCR (Model 5700; Applied Biosystems, Bleiswijk, The Netherlands) using Taqman probes and primers (Applied Biosystems, assays on demand) for IL-6, TNFα, CXCL2, CCL3, COX-2, and CXCL1. Calculations were made according to the delta-delta ct method (Schmittgen and Livak, 2008) after normalization to glyceraldehyde-3-phosphate dehydrogenase. The reference gene glyceraldehyde-3-phosphate dehydrogenase was selected after the comparison of the data sets with other references genes for beta-actin (Actb), hypoxanthine–guanine phosphoribosyltransferase (Hprt), and TATA-binding protein (Tbp) (Applied Biosystems, assays on demand). For cell culture experiments, RNA isolation, synthesis of complementary DNA, and quantitative real-time reverse-transcriptase–PCR experiments were carried out as performed by (Almeida et al., 2011). mKC/CXCL1 (QT00115647, Qiagen, Hombrechtikon, Switzerland) or mIL6 (Roger et al., 2011) mRNA expression were quantify using the delta–delta ct method, normalized to gene expression values of either mRPL13 (QT00147840, Qiagen) or hypoxanthine-guanine phosphoribosyltransferase (Roger et al., 2011).
ELISA
Skin punches measuring 8 mm were homogenized in 1.5 ml extraction buffer (containing 10 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100) per gram of tissue using a FastPrep (Q-Biogen) instrument. The homogenates were centrifuged at 15,000 r.p.m. for 15 minutes at 4 °C to remove the debris. CCL3, IL-6, and TNFα concentrations were measured with ELISA kits (MIP-1α Duo set, R&D Systems, Abingdon, UK, IL-6 OptEIA kit and TNF BD Cytometric Bead Array kit, Becton-Dickinson Biosciences, Franklin Lakes, NJ) according to the manufacturer's instructions. Cytokines and chemokines levels in the medium of mouse epithelial keratinocyte, BMDCs and bone marrow–derived macrophages culture were quantified by MSD ELISA following the manufacturer instructions (MULTI-SPOT 96-well-7-spot plate #K15012C, MSD, Meso Scale Discovery, Rockville, MD).
TUNEL assay
Detection of apoptotic cells in paraffin-embedded tissues were performed using the DeadEnd Fluorometric Tunel system (Promega, Madison, WI). Mouse skin sections (5 μm) were stained with TUNEL-reactive compounds as described in the protocol. Before mounting the slides, the sections were counterstained with propidium iodide to visualize all nuclei. Quantification of TUNEL-positive cells was performed using ImageJ.
Cell culture
Neonatal mouse primary keratinocyte were isolated and cultivated as described by Pirrone et al. (2005). Culture of BMDCs were generated as described in Lutz et al. (1999) and bone marrow–derived macrophages as described in Roger et al. (2011). UVB irradiation of primary keratinocyte was performed with a single medisun HF-54 (Schulze & Böhm, Brühl, Germany) sunlight lamp (see Supplementary Materials online).
Statistical analysis
A Mann–Whitney test was performed for histological scoring. For real-time PCR analysis, Fisher's least significant difference was used on a 5% significance level for multiple comparisons. On the figures, dots represent means of log-transformed normalized individual data points (log 2−ΔCT) where ΔCT=(CT gene of interest−CT internal control) and intervals represent ±½ least significant difference; as a consequence, if two intervals do not cross, the means are significantly different on a 5% significance level. ELISA data were analyzed using an unpaired Student's t-test, probabilities <0.05 (P<0.05) were considered significant. Myeloperoxidase and Ki67 staining, epidermal thickness (Lu5), and apoptosis (TUNEL) quantification were analyzed by one-way analysis of variance.
Acknowledgments
This work is supported by grants awarded by the Swiss National Science Foundation (SNSF) 116840 and the Placide Nicod Foundation. We thank L van Kaer and M Taniguchi for the CD1d and Jα18-knockout mice.
Glossary
- BMDC
bone marrow–derived dendritic cell
- NMSC
non-melanoma skin cancer
- NKT
natural killer T
- TNFα
tumor necrosis factor α
- WT
wild type
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Almeida S, Ryser S, Obarzanek-Fojt M, et al. The TRAF-interacting protein (TRIP) is a regulator of keratinocyte proliferation. J Invest Dermatol. 2011;131:349–357. doi: 10.1038/jid.2010.329. [DOI] [PubMed] [Google Scholar]
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Bai L, Picard D, Anderson B, et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol. 2012;42:2505–2510. doi: 10.1002/eji.201242531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balato A, Unutmaz D, Gaspari AA. Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J Invest Dermatol. 2009;129:1628–1642. doi: 10.1038/jid.2009.30. [DOI] [PubMed] [Google Scholar]
- Barker JN, Mitra RS, Griffiths CE, et al. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Bernerd F, Vioux C, Asselineau D. Evaluation of the protective effect of sunscreens on in vitro reconstructed human skin exposed to UVB or UVA irradiation. Photochem Photobiol. 2000;71:314–320. doi: 10.1562/0031-8655(2000)071<0314:EOTPEO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Blumberg RS, Terhorst C, Bleicher P, et al. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol. 1991;147:2518–2524. [PubMed] [Google Scholar]
- Bonish B, Jullien D, Dutronc Y, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000;165:4076–4085. doi: 10.4049/jimmunol.165.7.4076. [DOI] [PubMed] [Google Scholar]
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136–142. [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Brozovic S, Nagaishi T, Yoshida M, et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- Canchis PW, Bhan AK, Landau SB, et al. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d. Immunology. 1993;80:561–565. [PMC free article] [PubMed] [Google Scholar]
- Cavallo J, DeLeo VA. Sunburn. Dermatol Clin. 1986;4:181–187. [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Hershberg RM, Furuta GT, et al. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc Natl Acad Sci USA. 1999;96:13938–13943. doi: 10.1073/pnas.96.24.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Dieude M, Striegl H, Tyznik AJ, et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted gammadelta T cells in the normal murine repertoire. J Immunol. 2011;186:4771–4781. doi: 10.4049/jimmunol.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan SK, Salas A, Rava P, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishelevich R, Malanina A, Luzina I, et al. Ceramide-dependent regulation of human epidermal keratinocyte CD1d expression during terminal differentiation. J Immunol. 2006;176:2590–2599. doi: 10.4049/jimmunol.176.4.2590. [DOI] [PubMed] [Google Scholar]
- Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- Gober MD, Fishelevich R, Zhao Y, et al. Human natural killer T cells infiltrate into the skin at elicitation sites of allergic contact dermatitis. J Invest Dermatol. 2008;128:1460–1469. doi: 10.1038/sj.jid.5701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, et al. NKT cells: what's in a name. Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Hatton JL, Parent A, Tober KL, et al. Depletion of CD4+ cells exacerbates the cutaneous response to acute and chronic UVB exposure. J Invest Dermatol. 2007;127:1507–1515. doi: 10.1038/sj.jid.5700746. [DOI] [PubMed] [Google Scholar]
- Herrlich P, Karin M, Weiss C. Supreme EnLIGHTenment: damage recognition and signaling in the mammalian UV response. Mol Cell. 2008;29:279–290. doi: 10.1016/j.molcel.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BE. Formation of thymine containing dimers in skin exposed to ultraviolet radiation. Bull Cancer. 1978;65:283–297. [PubMed] [Google Scholar]
- Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- Kawana K, Matsumoto J, Miura S, et al. Expression of CD1d and ligand-induced cytokine production are tissue-specific in mucosal epithelia of the human lower reproductive tract. Infect Immun. 2008;76:3011–3018. doi: 10.1128/IAI.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Bajdik CD, Willemze R, et al. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087–1093. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- Kock A, Schwarz T, Kirnbauer R, et al. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas ME, Lee HY, Gillum MP, et al. Impact of CD1d deficiency on metabolism. PLoS One. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger TA, Schwarz T. Evidence for an epidermal cytokine network. J Invest Dermatol. 1990;95:100S–104S. doi: 10.1111/1523-1747.ep12874944. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Moodycliffe AM, Nghiem DX, et al. Resistance of CD1d−/− mice to ultraviolet-induced skin cancer is associated with increased apoptosis. Am J Pathol. 2004;165:879–887. doi: 10.1016/S0002-9440(10)63350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Mendiratta SK, Martin WD, Hong S, et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- Moodycliffe AM, Nghiem D, Clydesdale G, et al. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- Muindi K, Cernadas M, Watts GF, et al. Activation state and intracellular trafficking contribute to the repertoire of endogenous glycosphingolipids presented by CD1d [corrected] Proc Natl Acad Sci USA. 2010;107:3052–3057. doi: 10.1073/pnas.0915056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor MF. Erythema, skin cancer risk, and sunscreens. Arch Dermatol. 1997;133:373–375. [PubMed] [Google Scholar]
- Nickoloff BJ, Wrone-Smith T, Bonish B, et al. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes: detection of T cells expressing receptors typically present on natural killer cells, including CD94, CD158, and CD161. Arch Dermatol. 1999;135:546–552. doi: 10.1001/archderm.135.5.546. [DOI] [PubMed] [Google Scholar]
- Oka M, Edamatsu H, Kunisada M, et al. Phospholipase Cvarepsilon has a crucial role in ultraviolet B-induced neutrophil-associated skin inflammation by regulating the expression of CXCL1/KC. Lab Invest. 2011;91:711–718. doi: 10.1038/labinvest.2011.10. [DOI] [PubMed] [Google Scholar]
- Pellicci DG, Patel O, Kjer-Nielsen L, et al. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinini AM, Midwood KS.2010DAMPening inflammation by modulating TLR signalling Mediators Inflamm(e-pub ahead of print 13 July 2013, doi: 10.1155/2010/672395 [DOI] [PMC free article] [PubMed]
- Pirrone A, Hager B, Fleckman P. Primary mouse keratinocyte culture. Methods Mol Biol. 2005;289:3–14. doi: 10.1385/1-59259-830-7:003. [DOI] [PubMed] [Google Scholar]
- Porcelli SA. The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- Roger T, Lugrin J, Le Roy D, et al. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood. 2011;117:1205–1217. doi: 10.1182/blood-2010-05-284711. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sikder H, Zhao Y, Balato A, et al. A central role for transcription factor C/EBP-beta in regulating CD1d gene expression in human keratinocytes. J Immunol. 2009;183:1657–1666. doi: 10.4049/jimmunol.0900057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, et al. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- Van Rhijn I, Young DC, Im JS, et al. CD1d-restricted T cell activation by nonlipidic small molecules. Proc Natl Acad Sci USA. 2004;101:13578–13583. doi: 10.1073/pnas.0402838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winau F, Schwierzeck V, Hurwitz R, et al. Saposin C is required for lipid presentation by human CD1b. Nat Immunol. 2004;5:169–174. doi: 10.1038/ni1035. [DOI] [PubMed] [Google Scholar]
- Yuan W, Qi X, Tsang P, et al. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci USA. 2007;104:5551–5556. doi: 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue SC, Shaulov A, Wang R, et al. CD1d ligation on human monocytes directly signals rapid NF-kappaB activation and production of bioactive IL-12. Proc Natl Acad Sci USA. 2005;102:11811–11816. doi: 10.1073/pnas.0503366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Cantu C, 3rd, Sagiv Y, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.