Summary
Background
Re-establishing epithelial integrity and biosynthetic capacity is critically important following tissue damage. The adult Drosophila abdominal epithelium provides an attractive new system to address how post-mitotic diploid cells contribute to repair.
Results
Puncture wounds to the adult Drosophila epidermis close initially by forming a melanized scab. We found that epithelial cells near the wound site fuse to form a giant syncytium, which sends lamellae under the scab to re-epithelialize the damaged site. Other large cells arise more peripherally by initiating endocycles and becoming polyploid, or by cell fusion. Rac GTPase activity is needed for syncytium formation, while the Hippo signaling effector Yorkie modulates both polyploidization and cell fusion. Large cell formation is functionally important because when both polyploidization and fusion are blocked, wounds do not re-epithelialize.
Conclusions
Our observations indicate that cell mass lost upon wounding can be replaced by polyploidization instead of mitotic proliferation. We propose that large cells generated by polyploidization or cell fusion are essential because they are better able than diploid cells to mechanically stabilize wounds, especially those containing permanent acellular structures, such as scar tissue.
Introduction
Drosophila uses multiple mechanisms to heal wounds, including some that appear to have been conserved during evolution [1]. Immediately following a lesion to the larval or adult epidermis, a plug is formed that limits the escape of blood and the entry of microorganisms [2–4]. The plug matures into a melanin-rich scab due to the crosslinking of oxidized phenols mediated by the hemolymph enzymes and blood cells [5]. Subsequently, the wound is closed with a new epithelial layer during a period of hours to days. The powerful genetics and relative simplicity of Drosophila tissues provide exceptional opportunities to better address how tissue repair is coordinated and controlled.
Drosophila epithelial cell behaviors that contribute to wound closure and permanent healing appear well conserved. In embryos, re-epithelialization is driven by an actomyosin cable at the wound edge whose contractions pull the epithelium back together like a purse string [6, 7]. The actin cytoskeleton also plays an important role in repairing injuries to the larval epidermis [8–10]. Injury triggers release of PDGF and VEGF-related factor (Pvf) to drive actin-based cell migration [11], similar to the known role RTK ligands in mammalian skin repair [12, 13]. There is also conservation in the activation of a transcription factor, Grainy head, which turns on genes involved in cuticle synthesis in flies and stratum corneum synthesis in mammals [14–16]. The JNK pathway is activated at the wound site and is required for wound healing in both flies and mammals [1–3, 17]. The Hippo, BMP and Wnt pathways are also active in some wounded tissues, but their roles remain less clear [1, 18–20].
In mammals, lesions often stimulate mitotic cell proliferation to generate new cells that migrate to the wound site and participate in repair [21]. New cells may arise by increasing the activity of stem cells, expanding the number of transit amplifying divisions, or by activating quiescent tissue cells to re-enter the cell cycle. Drosophila adult contain active stem cells [22], and at least in the intestine both stem cells and downstream daughters increase proliferation in response to tissue damage [23]. Wounding stimulates imaginal disc cells to proliferate and quiescent diploid hindgut cells to re-enter the cell cycle [24, 25]. However, the functional significance of induced cell proliferation for healing wounds within quiescent tissues remains unclear.
Here we show that the adult abdominal epidermis responds to wounding by inducing large cell formation using two distinct mechanisms, polyploidization and cell fusion. Polyploidization replaces lost cell mass, whereas cell fusion provides rapid repair of the epithelium. We propose that large cells help to mechanically stabilize wounds and their organization around the scar may be required to support this acellular structure.
Results
Adult abdominal epithelium repairs after injury
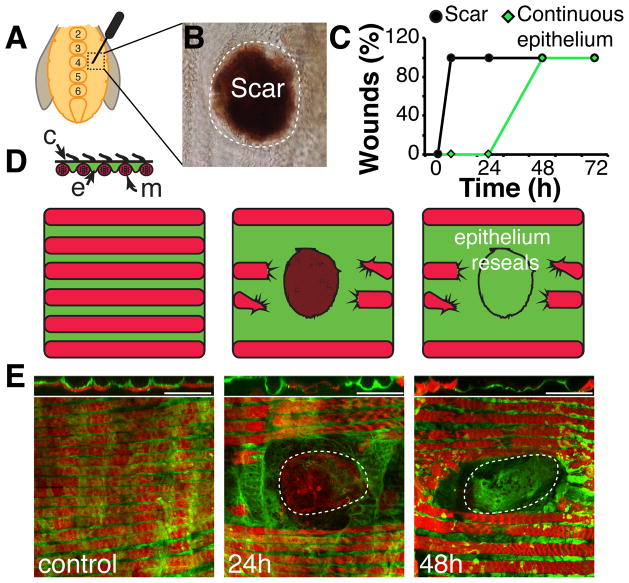
Epithelial repair was induced by puncturing the ventral abdominal tissue of adult female flies lateral to the midline with a sharp needle to generate wounds averaging 4,000 μm2 (Figure 1A). Like other wounded Drosophila epithelia, the first visible response was the formation of a melanized scab within 6 hours (Figure 1B, 1C, and S1A). Over the next two days, epithelial integrity was restored under the scab, but unlike wounds to the larval epidermis, the scab remained as a permanent scar. We followed the process of epithelial repair in detail using a line expressing GAL4 in adult epidermis (Epithelial-Gal4, see Experimental Procedures) to drive UAS-tubGFP. The wound severed several lateral muscle fibers that lie directly below the abdominal epithelium, and these never reconnected or regenerated (Figure 1D and 1E). Initially, the epithelial sheet retracted from the wound site after which a complete epithelial sheet gradually reappeared underneath the melanin scab, growing from the periphery toward the center to close the wound by 48 hours (Figure 1C and 1E). Electron microscopy (Figure S1) revealed only cellular debris below the scab at 1 day post injury (Figure S1C). However, by 2 days post injury (Figure S1D), a complete epithelial sheet was restored below the scar confirming our observations using GFP-marked epithelial cells. Hemocytes were recruited to the wound site as early as 1 hour after wounding, peaked in number at 24 hours post injury, and then disappeared by the time of wound closure (Figure S2).
Figure 1. Re-epithelialization occurs following abdominal injury.
(A) Adult female flies were wounded on either side of the central sternites, between numbers 2–6 with a tungsten needle. (B) A melanin scab forms rapidly at the site of injury and persists after healing as a scar (shown 15 days post injury). (C) Time course of scab/scar formation (black) and re-epithelialization (green) (N=10–20 wounds/ time point). (D) Cross section model of ventral tissue organization. A continuous epithelial sheet (e, green) is in direct contact with outer cuticle (c, black), which is lined with small hairs. Lateral muscle fibers (m, red) lie below epithelium; organizing the tissue into alternating rows of muscle and epithelial cells. (E) Immunofluorescent images of abdominal tissue prior to and during re-epithelialization are shown below, with interpretive diagrams above. Epithelium (green, Epithelial-Gal4/ UAS-tubGFP). Muscle (red, Phalloidin). Scar (dashed white line). Scale bar= 50μm. See also Figure S1 and Figure S2.
Wounding induces local cell cycle entry
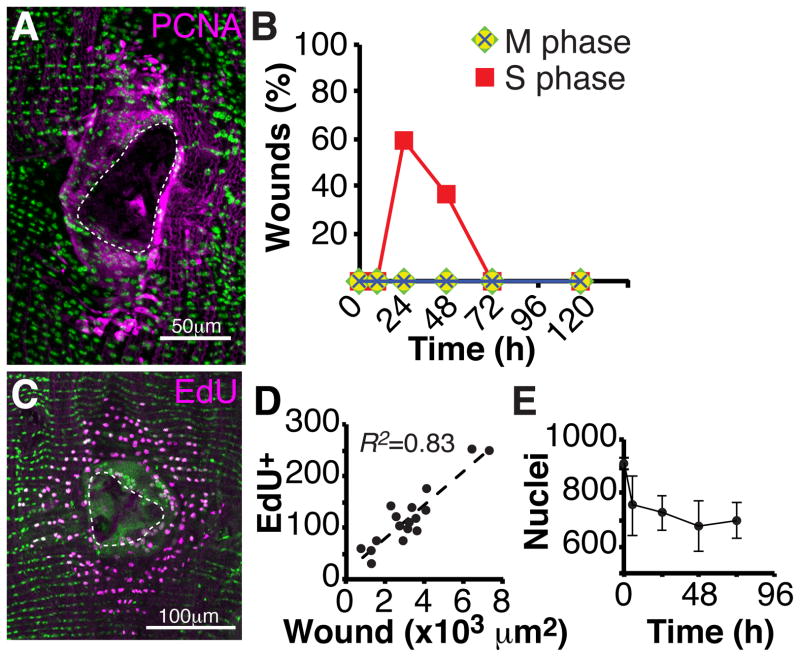
Normally, cells in the adult abdominal wall are quiescent. However, within 24 hours after wounding, epithelial cells, but not other abdominal cell types, re-entered the cell cycle as shown by PCNA-GFP expression and EdU incorporation (Figure 2A–C). DNA replication continued for another day as judged by PCNA-GFP expression, but thereafter was not detectable. Only cells located close to the wound re-entered S phase (Figure 2A and 2C). Up to about 250 nuclei responded and the number of EdU-positive nuclei correlated strongly with wound area (Figure 2D; R2= 0.83). Surprisingly, the reactivated cells did not divide. M phase markers including anti-phospho Histone 3 (PH3), Cyclin B (CycB-GFP), and Polo kinase (Polo)-GFP were not detected at any time point before or after injury (Figure 2B). Consistent with these observations, there was no increase in epithelial cell number during wound healing (Figure 2E). Within a standard region extending 100μm around the wound and containing 915 +/− 12 cells, an average of 233 +/− 90 epithelial cells were lost immediately upon wounding, and cell number was never restored (Figure 2E). Thus, epithelial cells located near the wound re-enter S phase at high frequency but do not divide.
Figure 2. Epithelial cells re-enter the cell cycle, but do not divide.
(A) Immunofluorescent image of PCNA-GFP (magenta) expression at 24 hours post injury. DAPI (green); Scar (dashed white line). (B) S phase, but not M phase cell cycle markers are expressed post injury. Time course of the percentage of wound zones with cells expressing the indicated cell cycle markers. S phase marker (PCNA-GFP, red square) and M phase markers (PH3, green diamond; CycB-GFP, yellow circle; Polo kinase-GFP, blue cross). N=30 wounds/ time point. (C) Immunofluorescent image of EdU-positive nuclei (magenta) within the wound zone following continuous EdU labeling and analysis at 3 days post injury. Epithelial nuclei (green, flpout nlsGFP; Epithelial-Gal4/ UAS-Flp); Scar (dashed white line). (D) The number EdU-positive nuclei correlates with wound area (N=17). (E) Nuclei number is not restored following epithelial repair. Time course of the abdominal epithelial nuclear number within a fixed zone (7.5 × 104 μm2) following injury at the zone’s center (N=5, mean ± SD).
Enlarged cells form by cell fusion and cell growth during re-epithelialization
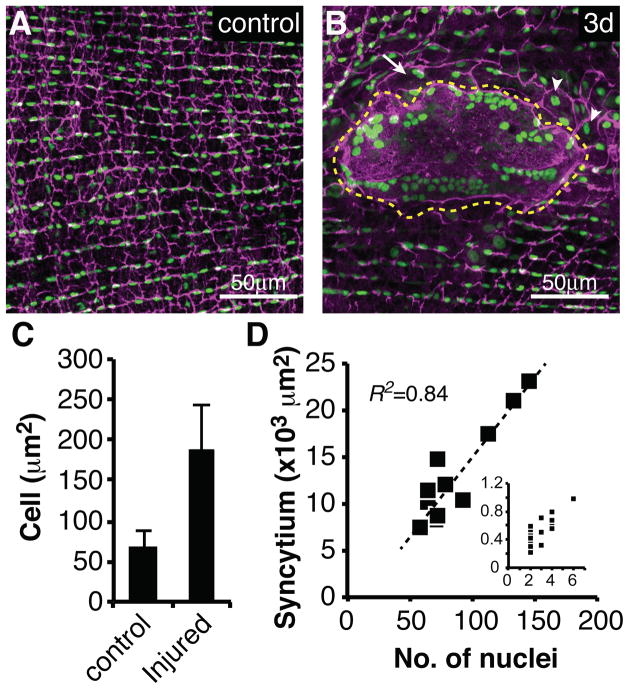
Since cell division was absent, we investigated whether epithelial cell size or shape changed following wound induction. Epithelial cells were marked using a specific Gal4 line to drive flpout nlsGFP (see Experimental Procedures). Prior to injury epithelial cell junctions (visualized by Fasciclin III (FasIII) staining) were arranged in regular rows (Figure 3A and S3). Every epithelial cell contained a single nucleus and covered an area of 65μm2 ± 20μm2 (Figure 3A and 3C). After injury, this regular organization was drastically altered in the vicinity of the wound (Figure 3B and S3).
Figure 3. Cell fusion and increased cell size are induced by wounding.
Immunofluorescent images of wound zones reveal epithelial organization: (A) Uninjured or (B) 3 days post injury. Cell-cell junctions (magenta, αFasIII). Epithelial nuclei (green, flpout nlsGFP; Epithelial-Gal4/ UAS-Flp). Giant syncytium (dashed yellow line), small syncytium (white arrow), enlarged cells (white arrowheads). (C) Average cell size (μm2) increases after injury. (N=28, mean ± SD). (D) Syncytial area (×103 μm2) versus nuclear number in the giant syncytium, or the small peripheral syncytia (inset, same units). See also Figure S3.
Wounding caused large epithelial cells to arise by two distinct processes. Epithelial cells began to spread, move towards the wound edge, and fuse as early as 15 hours post injury. By 48 hours, a giant syncytium containing 87 +/− 30 nuclei on average encompassed the entire region below the melanin scab (Figure 3B, dashed yellow line). Multiple smaller syncytia formed at more peripheral locations and contained 2–7 nuclei (Figure 3D, inset). Changes in epithelial organization were complete by 48 hours post-wounding and no further alterations were observed even after one week (168 hours) (Figure S3).
3D reconstruction confirmed that the giant central syncytium extends beneath the melanin-rich scar, which it likely stabilizes and supports (Movie S1). Consistent with this idea, FasIII staining increased along the edges of the syncytium and at the interface with the scar. The nuclei of the central syncytium were highly clustered and were distributed asymmetrically near its outer border along the scar periphery (Figure 3B). The syncytium may be thicker in these locations and better able to accommodate nuclei, or clustering may serve a mechanical purpose.
Epithelial cells polyploidize in response to injury
A second class of enlarged cells also arose near the wound (Figure 3B, arrowheads). These cells averaged 187μm2 ± 56μm2 in area, more than twice that of epithelial cells prior to injury, and contained a single large nucleus (Figure 3C and 4A), suggesting that they were formed by polyploidization. Measurements of DNA content showed that adult abdominal epithelial nuclei from unwounded animals are diploid (Figure 4B). However, by 3 days post injury about 50% of the nuclei located within 100μm of a wound contained an elevated DNA content (Figure 4A and 4B). Most were close to 4c, suggesting that they had re-entered the cell cycle and completed S phase.
Figure 4. Polyploidization is induced by wounding.
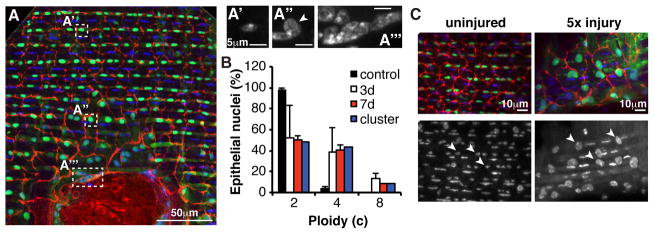
(A) Immunofluorescent images of wound zone showing location (white boxes) of particular nuclei shown stained with DAPI in panels to the right. A′, diploid nucleus distant from wound; A″, polyploid nucleus proximal to wound; A‴, cluster of polyploid nuclei from giant syncytium. Cell-cell junctions (red, αFasIII). Epithelial nuclei (green, flpout nlsGFP; Epithelial-Gal4/ UAS-Flp). DAPI (blue/ white, DNA). (B) Percentage of individual nuclei at indicated times post injury in the wound periphery (white and red bars) or cluster averages from the giant syncytium (blue bars) with DNA content in three ploidy classes (N=3 wounds, mean ± SD); blue bar (N=23 clusters, pooled from 3 and 7 day wounds). (C) Re-injury enhances polyploidy. A wound zone prior to injury (left panels; arrowheads = diploid nuclei) and after 5 wounds to the same site during a two-week period (right panels; arrowheads = highly polyploid nuclei). Markers are the same as in A.
About 10% of the nuclei had a DNA content of approximately 8c DNA values, indicating that they had undergone two endocycles. The increased DNA content was stable for at least 7 days post injury (Figure 4B). Thus, wounding induces previously quiescent cells to undergo one or more endocycles.
The magnitude of the polyploidization response suggested that it served to replace the synthetic capacity of the diploid nuclei lost as a result of the wound. Since 233 diploid cells were destroyed on average, they represent a loss of 466 genomes. Our counts indicated that 40% of 688 remaining cells became tetraploid and 10% reached 8c, representing approximately 850 replacement genomes, slightly more than what was lost. Consistent with cell replacement, we found that re-injuring flies multiple times (cells are killed by each wound) enhanced the polyploidization response. Surviving epithelial cells close to the wound edge dramatically increased their DNA content and cell size when the abdomen was re-injured 5 times at the same site (Figure 4C).
DNA content measurements revealed that many of the nuclei within the giant syncytium were also polyploid. The clustered nuclei could only be measured accurately in groups, but the average DNA content of these grouped nuclei fell into ploidy categories like those of single nuclei (Figure 4B, “cluster”). This suggested that nuclei with similar DNA content are spatially clustered. The heterogeneity in DNA content within syncytial nuclei would be most easily explained if the some epithelial cells entered the endocycle prior to fusion.
Yorkie controls cell fusion and polyploidization
We next investigated genes that may be involved in the response of adult epithelial cells to wounding. The puckered-lacZ (puc-lacZ) construct serves as a sensitive JNK reporter a7nd as expected was activated by wounding [2, 3]. Puc-lacZ expression was induced at 5 hours post injury in cells located near the wound (Figure S4A) and continued to be expressed for the next 3 days (Figure S4B).
Hippo (Hpo) signaling was also induced by wounding but the magnitude of the increase was modest. We monitored Yorkie (Yki) activation, the transcription factor that mediates Hpo signaling, using two reporters, expanded-lacZ (ex-lacZ) and dIAP-lacZ [26]. Both reporters were detected in the abdominal epithelium before wounding (Figure S4C–D). By 48 hours after wounding both ex-lacZ and dIAP-lacZ expression were further increased 2–3 fold after correction for increased nuclear ploidy (Figure S4D).
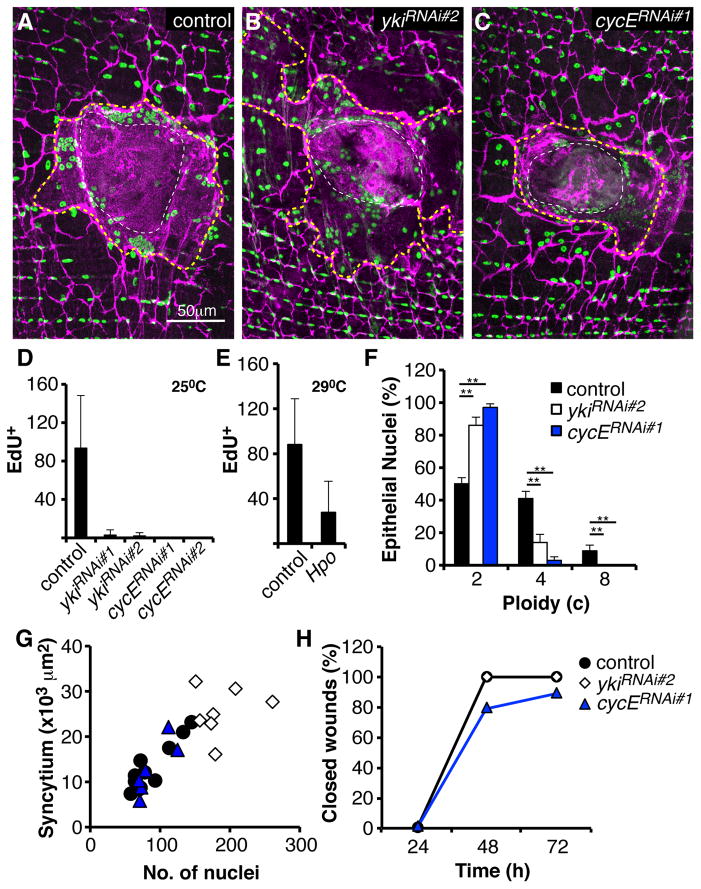
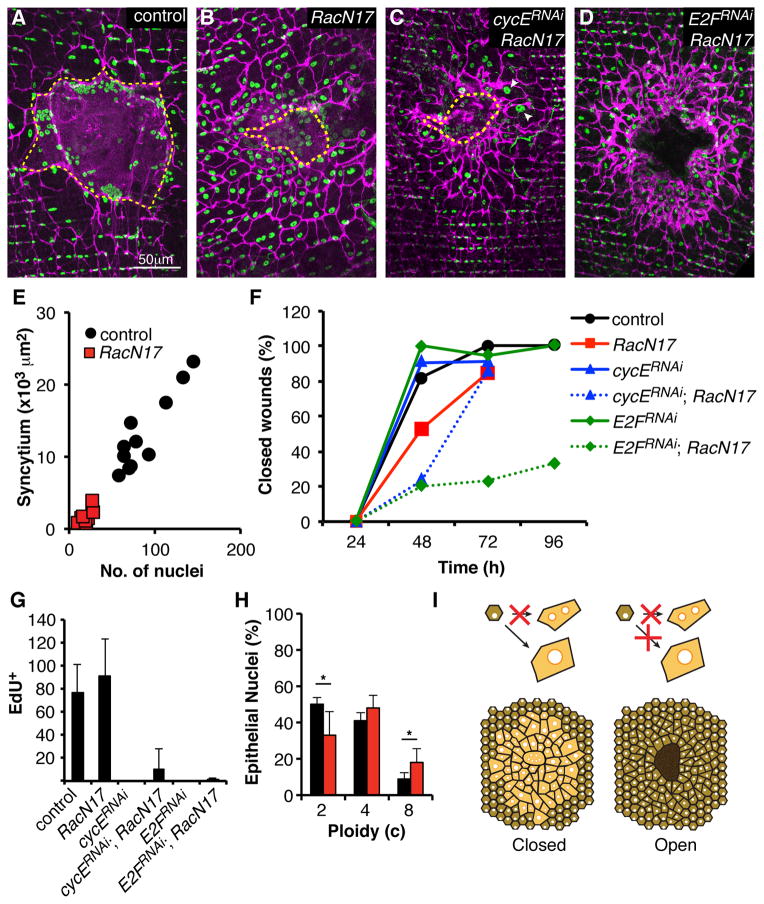
Disrupting yki strongly altered the wound response (Figure 5A and 5B), despite its relatively modest modulation at the level of target gene expression. When yki was knocked down using two different RNAi constructs (Figure 5D, S4E, and S4F), both polyploidization and cell fusion were drastically altered (Figure 5F and 5G). Whereas more than 80 cells normally incorporate EdU in response to wounding, virtually no such cells were observed after yki knockdown (Figure 5D). Knocking down cyclin E (cycE), a known Yki target [26], also blocked S phase re-entry (Figure 5D). Overexpressing Hpo in epidermal cells also reduced S phase entry (Figure 5E). In cells expressing ykiRNAi only 717% (instead of 50%) showed increased DNA content, and virtually none did when cycE was knocked down (Figure 5F). These results indicate that Yki function is important for cell cycle re-entry and polyploidization in response to wounding, probably as a mediator of Hippo signaling.
Figure 5. Yki regulates polyploidization and cell fusion.
(A–C) Immunofluorescent images of wound zones at 3 days post injury reveal polyploidization and cell fusion in control (no transgene) (A), yki knockdown (B), or in cycE knockdown (C) flies. Epithelial-Gal4 drove expression of the indicated transgene. Cell-cell junctions (magenta, αFasIII). Epithelial nuclei (green, αGrh). Giant syncytium (dashed yellow line). Scar (dashed white line). (D) Yki and CycE are required for S phase re-entry. Number of EdU-positive nuclei/ wound zone following continuous labeling and analysis at 3 days post injury in controls and flies expressing the indicated transgenes driven by Epithelial-Gal4 at 25°C (N=30, mean ± SD). (E) Same as D, except UAS-Hpo was driven in the presence of Gal80ts, and flies were shifted to 29°C prior to wounding (N=7, mean ± SD). (F) Yki and CycE are required for cells to polyploidize in response to injury. Percentage of nuclei from the indicated genotypes in the wound periphery at 7 days post injury with DNA content in three ploidy classes. Epithelial-Gal4 drove the indicated transgenes (N=3, mean ± SD). (**) T-test, p ≤ 0.01. (G) Knocking down yki enhances syncytium size. Giant syncytial area (×103 μm2) versus nuclear number at 3–4 days post injury. (H) Knocking down yki or cycE has little effect on wound closure. Indicated transgenes were expressed with Epithelial-Gal4, UAS-mCD8-RFP (N=10 wounds/ time point). mCD8-ChRFP was used to visualize wound closure. See also Figure S4.
Disrupting yki also affected syncytium formation. When yki action was blocked, larger syncytia containing an increased number of cells formed in response to wounding (Figure 5B and 5G), but knocking down yki had no effect on epithelial cells in the absence of wounding (Figure S4G). The syncytia distal to the scab were also larger after yki knockdown (Figure 5B). Increased syncytial size was not caused by enhanced cell death, since ykiRNAi did not affect apoptosis (Figure S4H). It did not result from attenuated polyploidization, since knocking down cycE had no effect on cell fusion (Figure 5C and 5G) despite completely eliminating polyploidization (Figure 5D and 5F). Neither yki nor cycE knockdown changed the rate of wound closure (Figure 5H). T hus, Yki activity negatively regulates cell fusion independently of its effects on polyploidization.
Large cell formation is essential for re-epithelialization
We investigated whether the formation of large cells via polyploidization and/ or fusion is functionally important by disrupting them separately and in combination. No functional defects in wound healing were observed in our previous experiments when polyploidization was blocked by disrupting CycE function (Figure 5). To investigate the role of syncytium formation, we noted that expression of a dominant negative mutation in the Rac GTPase (RacN17) can block myoblast fusion and muscle development [27]. Consequently, we overexpressed RacN17 in the abdominal epithelium to look for effects on the wound response. These flies developed to adulthood and did not show gross defects (data not shown). However, upon injury, epithelial cell fusion was drastically reduced (Figure 6A and 6B). Only a small central syncytium made up of 10–30 nuclei formed (Figure 6B and 6E), and single epithelial cells now moved under the scab and carried out most of the re-epithelialization (Figure 6B). RacN17 expressing epithelial cells still labeled with EdU post injury (Figure 6G) and ploidy measurements indicated that injury-induced polyploidization was similar, except that nearly twice as many 8c nuclei were observed (Figure 6H). Strikingly, when syncytium formation was reduced by RacN17 expression, wound closure was significantly slowed, requiring an extra day for completion (Figure 6F). Perhaps due the slower action of the single cells, the thickness of the epithelial layer below the scar was increased (Figure S5).
Figure 6. Cell fusion and polyploidization are both required for wound closure.
(AD) Immunofluorescent images of wound zones reveal affects of the indicated transgenes driven by Epithelial-Gal4 on cell fusion and wound healing at 3 days post injury. Cell-cell junctions (magenta, αFasIII). Epithelial nuclei (green, αGrh). Giant syncytium (dashed yellow line). (E) Epithelial expression of RacN17 reduces the size and nuclear content of the giant syncytium at 3–4 days post injury. (F) Blocking cell fusion alone or in combination with S phase inhibitory factors delays wound closure. Indicated transgenes were expressed with Epithelial-Gal4, UAS-mCD8-RFP. mCD8-ChRFP was used to visualize wound closure. (N=10–20 wounds/ time point). (G) Number of nuclei incorporating EdU following wounding of flies expressing the indicated transgenes as in F (N=7, mean ± SD). (H) Epithelial expression of RacN17 enhances polyploidization. Percentage of nuclei in the wound periphery from control (black bars, Epithelial-Gal4, no transgene) or Epithelial-Gal4, UAS-RacN17 flies at 7 days post injury with DNA content in three ploidy classes (N=3, mean ± SD). (*) T-test, p ≤ 0.05. (I) Models illustrating how blocking cell fusion (left) or fusion and polyploidization (right) affects wound closure. Only when both pathways are blocked do wounds remain open. The epithelial layer does not reseal under the scar. Scar is shown in dark brown. See also Figure S5.
We next investigated whether polyploidization was functionally important when an alternative means of generating large cells, cell fusion, was not available. Both fusion and S phase re-entry were reduced simultaneously by expressing either cycERNAi or E2FRNAi in combination with RacN17 in the abdominal epithelium (Figure 6C, 6D, and 6G). When both processes of cell enlargement were affected simultaneously, an even larger delay in wound closure was observed than when RacN17 was expressed alone, with 80% of wounds remaining open at 2 days post injury (Figure 6F). The cycERNAi; RacN17 expressing flies, which still had some residual EdU incorporation (Figure 6G), managed to generate some polyploid cells (Figure 6C, arrowheads) and closed their wounds by day 3 (Figure 6F). In contrast, E2FRNAi, RacN17 expressing flies, which completely blocked EdU incorporation (Figure 6G), failed to re-epithelialize 67% of their wounds even at 4 days post injury (Figure 6D and 6F). These results show that polyploidization and cell fusion work redundantly to support re-epithelialization during the normal wound closure process (Figure 6I). Thus, the formation of large cells by one process or the other is necessary for wound healing in the adult epidermis.
The Drosophila hindgut also exhibits damage-induced polyploidization
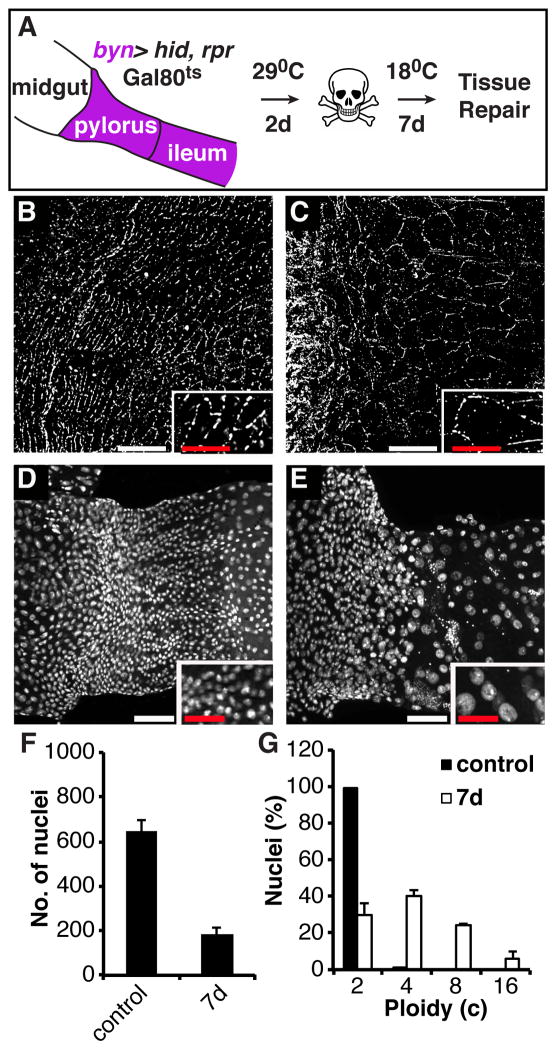
We next examined whether polyploidization in response to tissue damage is a general response by studying a different tissue, the pyloric region of the hindgut. The normally quiescent pylorus, which lacks active stem cells, is known to undergo a cell cycle response to injury [24]. The tissue was injured by expressing the apoptotic genes hid and rpr for 2-days driven by a hindgut-Gal4 driver (byn), followed by a 7-day recovery period (Figure 7A), a procedure that kills more than half of the pyloric cells [24]. Similar to the abdomen, cell number did not recover following acute injury, however, both pyloric cells (Figure 7B and 7C) and their nuclei (Figure 7D and 7E) appeared larger following the recovery period. Under the conditions used, cell number decreased about 3-fold in the distal pylorus (Figure 7F). We measured the nuclear DNA contents of the remaining cells and found that ploidy levels had increased dramatically following damage (Figure 7G). Most of the cells were now polyploid, with DNA contents as high as 16c. Thus, enhancing cell size by returning quiescent cells to the endocycle instead of inducing mitotic cell proliferation appears to be a widespread mechanism to compensate for cell loss in Drosophila tissues.
Figure 7. Cell growth and polyploidy occur in response to hindgut tissue damage.
(A) Scheme for genetic cell ablation in the adult hindgut. At 18°C, the Gal80ts repressor inhibits Gal4, preventing it from binding to UAS elements upstream of the pro-apoptotic genes hid and rpr. At 29°C, Gal80ts is inactivated, and Gal4 drives apoptotic gene expression specifically in the hindgut, under control of brachyenteron (byn) expression. (B–E) Changes in cell and nuclear size after injury, insets show magnified views of same image. (B) Cell-cell junctions and cell size in the undamaged adult pylorus are visualized with the adherens junction protein DE-Cadherin. (C) Two weeks after acute injury, cell-cell junction contacts appear intact around the now larger pyloric cells. (D) Nuclei in the undamaged adult pylorus. (E) Nuclei in the repairing adult pylorus. (F) Cell number does not recover in the adult pylorus after acute damage (control: N=6, mean ± SD; 7d: N=12, mean ± SD). (G) Measurements of nuclear ploidy in the adult pylorus (N=3, mean ± SD). Scale bars (white=25μm, red=12.5 μm).
Discussion
Polyploidization of quiescent cells in response to wounding replaces lost cell mass
Puncture wounds of the adult epithelium such as those studied here present multiple challenges. Biosynthetic capacity has been reduced by cell loss, the epithelial barrier has been breached, and regional mechanical stability has been compromised by irreversible muscle damage. We found that the epithelium employs two novel processes, polyploidization and cell fusion, rather than cell proliferation, to respond to these challenges.
One function of polyploidization is to restore the tissue mass destroyed during wounding. The number of nuclei induced to leave quiescence in the adult abdomen and re-enter S phase correlates closely with the wound size (Figure 2D). Furthermore, the levels of epithelial polyploidy observed are sufficient to generate approximately the same number of new genomes as were initially lost. More severe cell losses in the abdomen caused by repeated wounding induce higher levels of endoreplication (Figure 4C). This correspondence even holds in the case of the severe damage to the hindgut pyloric region. Here, we observed that a region of 300 cells (600 genomes) was reduced to 100 cells following damage, but their ploidy increased sufficiently to restore a total of 550 genomes (Figure 7G), approximately the starting number. Thus, previously quiescent diploid cells can sense the severity of tissue damage, re-enter the cell cycle, and endoreplicate to levels that replace the lost cells.
During development animals and their component organs are able to precisely control their size [28]. Following injury, as after liver resection, cells proliferate until the normal organ size is again attained. In many tissues, certain cell types complete differentiation while undergoing endocycles [29], hence mechanisms to modulate endocycling as well as cell proliferation in response to tissue size must exist. Indeed, during development the ploidy level of cells can increase beyond that normally attained to compensate for overall reductions in cell number caused by mutation or damage, both in Drosophila embryos, ovarian follicles, rectum, and in the mammalian liver [30–34]. Here we extend the known versatility of endocycle regulation by showing that polyploidization can also be induced in previously differentiated, quiescent diploid cells and then terminated at an appropriate level as a repair response.
Fusion of quiescent cells is induced by wounding and speeds re-epithelialization
Another striking response to adult epidermal wounding is the generation of a large syncytium by cell fusion. Barrier function is transiently restored by scab formation after which a continuous epithelium must be regenerated. Our studies suggest that syncytium formation at the site of the scab accelerates this process. When we blocked cell fusion by expressing RacN17, wound re-epithelialization was significantly slowed. Previous studies described cell fusion and syncytium formation at the site of larval epidermal wounds [2]. Larval epidermal cells facilitate wound closure by sending lamellae under the scab using actin treadmilling and myosin II-dependent crawling [8, 9, 11]. The giant syncytia we studied contain 5–10 times as many cells as in these larval epidermal wounds, but may utilize many of the same processes to speed re-epithelialization.
The giant syncytium may provide several additional advantages. Concentrating most nuclei at the periphery of the scar may allow thin cytoplasmic lamellae to rapidly move under the scab to seal the wound while the cell itself remains firmly fixed at the wound periphery. Individual diploid cells would have to migrate under the scab, a zone that is probably not conducive to organized cellular movement due to the absence of a basement membrane, polarity signals, and supporting muscle. Even after the epithelium is restored, the large syncytial cell may continue to function by stabilizing the scar, a large rigid structure susceptible to motions that could damage the tissue and re-open the wound.
Hippo signaling and its effector Yorkie regulate cell fusion and polyploidization
How does the injured abdomen induce polyploidization and cell fusion at the appropriate levels and locations? Both JNK and Hippo signaling are upregulated at the wound site, suggesting that these pathways regulate the wound response. Hippo signaling, in conjunction with the TOR, and insulin/IGF pathways, plays an important role in organ size control in both mammals and insects [26, 28]. Yki, the major effector of Hippo signaling, is required for the polyploidization response. Hippo signaling in response to wound damage may activate cycE, a gene known to be regulated by Yki in other tissues to stimulate S phase re-entry. The modest 2–3 fold increase in the Yki regulated genes, expanded and dIAP, may reflect the relative mild nature of these wounds, which only caused a 25% reduction in cell number (233/913) within the wound region. Thus, Hippo signaling via Yki may specify how much polyploidization ensues in response to the magnitude of the wound, as well as its spatial location.
In order to fulfill this role, Hippo signaling would have to be activated locally within the tissue in proportion to the magnitude of the damage. Changes in cell polarity, actin cytoskeletal dynamics, and cell density can all induce the expression of Yki regulated genes [26, 35]. In addition, other signals may play Hippo-independent roles in controlling abdominal wound repair. Hemocytes are known to be recruited to the wound site to help clear debris and microorganisms [36]. We found that hemocytes are also recruited to adult abdominal wound and are present at the 24 hours when cells are both undergoing cell fusion and re-entering S phase (Figure S2). This suggests that blood cells could liberate factors that facilitate either of these wound healing processes. Another possibility is DNA damage from genotoxic stress, such as that produced from reactive oxygen species (ROS). ROS are known to be released after injury to many tissues [37], and these products can also induce the endocycle in plant cells [38]. The syncytial and polyploid cells induced by wounding might themselves send both local and long range signals that participate in sensing when organ size and stability has been restored.
Production of large cells by fusion or polyploidization is required for wound healing
Our experiments provided insight into the distinct and overlapping roles of polyploidy and cell fusion in the healing process. Polyploidization appears to be solely responsible for replacing the lost cells and restoring the tissue back to its initial mass. However, the 25% reduction in synthetic capacity generated by the wounds studied here is probably just too small to register in our assays when polyploidization alone is blocked. A 25% reduction in the time required for re-epithelialization or in the thickness of the lamella under the scar would not have been detected. Despite this, we detected a distinct function for polyploidization by analyzing its role in conjunction with that of cell fusion.
Blocking cell fusion clearly perturbs wound healing, as an extra day is now required to complete wound closure, but healing is not prevented. In the absence of fusion polyploidization still takes place (Figure 6I, left). Indeed, the level of ploidy is slightly increased under these conditions (Figure 6H). However, when polyploid cells cannot form and cell fusion is also blocked, wounds usually fail to heal (Figure 6I, right). This indicates that the polyploid cells, which are located near the edge of the scab and extend several cell diameters away, contribute something critically important to the wound repair in addition to restoring cell mass, but that this function is redundant when cell fusion can operate.
Generating large cells may help mechanically stabilize wounds, especially those with scar tissue
We propose that large cells, whether syncytial or polyploid in origin, provide a unique mechanical function that helps organize and control the healing process, and which cannot be provided by the surviving diploid cells. The large uninterrupted cytoplasmic size of either type of cell allows much more robust cytoskeletal structures to form and function than is easily possible in diploid cells. This is likely the reason that muscle cells fuse into large syncytia prior to organizing myofibrils. However, other large cytoplasmic mechanical structures are present and function in many other types of polyploid cells that are less familiar. For example, megakaryocytes, which extrude segments of their cytoplasm as platelets, contain long branching β1-tubulin-based processes that are required for platelet release [39]. Polyploid jump reflex neurons in Drosophila produce exceptionally long and thick axons that allow signals to be transmitted with great speed [40]. Trophoblast giant cells accumulate stress fibers and specialized podsomes, which may structurally support placenta development [41, 42].
Mechanical tension is already known to play a critical role in mammalian epithelial wound healing [43]. Cells tend to migrate toward regions of higher ECM rigidity (“durotaxis”) [44]. Large cells may be necessary at the wound site to generate an appropriate mechanical environment for migrating cells to complete their movements under the scar and close the wound. In the abdomen, the transverse muscle bands that normally span the abdomen did not undergo repair. Large cells may also be particular advantageous for dealing with mechanical stability issues that require balancing forces over a substantial area due the size of the damaged region and the presence of altered structures such as scar tissue. The central syncytium may not only be advantageous in rapidly closing the wound, but also because a large cell can better stabilize the scar and prevents it from breaking loose. The enlarged peripheral cells, whether polyploid or syncytial, may more easily generate stabilizing forces to protect the wounded region during the normal flexure and stress on the abdomen.
These same considerations would apply equally to mammalian as well as Drosophila tissue. In many damaged mammalian tissues, extracellular matrix deposits of fibrin and collagen initially form a fibrin clot/ scab to hold edges of the wound together, but collagen protein deposits can persist in a lasting mark at the injury site, in the form of a scar [45]. In tissues where cell proliferation is limited, such as our heart, scar formation is necessary to maintain tissue integrity, but also lead to stiffness and reduced heart function [46]. Polyploidization may frequently take place in response to mammalian tissue damage that repairs imperfectly and leaves scar tissue, such as in the heart, but have received little attention. Cardiomyocytes re-enter the cell cycle after injury leading to a low level of cell division, as well as polyploidy and multinucleation at the scar periphery [47, 48]. Consequently, the establishment of a model system for studying the control of polyploidization and syncytium formation in response to wounding is likely to provide insight to questions of wide significance.
Experimental Procedures
Fly strains
Drosophila melanogaster strains used in this study were reared on standard cornmeal agar yeast food at 25°C, unless otherwise noted. Epithelial-GAL4 (R51F10-Gal4) was identified in screen of Janelia Farm Research Campus collection [49]. Epithelial nuclei were labeled, with high specificity, by combining: Epithelial-GAL4 with flpout nlsGFP (Ubi-p63E(FRT.STOP)Stinger9F6) and UAS-Flp [50]. Other fly strains used in this study are described in Supplemental Experimental Procedures along with remaining experimental procedures.
Supplementary Material
Highlights.
Polyploidization is induced by wounding and regenerates cell mass
Cell fusion depends on Rac GTPase and speeds re-epithelialization
Hippo signaling via Yorkie regulates cell fusion and polyploidization
Large cells may help mechanically stabilize wounds with scar tissue
Acknowledgments
We would like to thank Michael Sepanski for EM sample preparation and imaging, as well as Rafael Villagaray and Madelyn Goodman for assistance with data processing. We are grateful to Ming-Chia Lee, Matt Sieber, and other members of the Spradling lab for helpful comments on the manuscript. We are thankful for the generosity of fly community in particular Bloomington Drosophila Stock Center, Developmental Studies Hybridoma Bank, VDRC, TRiP center at Harvard Medical School (NIH/NIGMS R01-GM084947), J. Rubin, D. Pan, and W. McGinnis for providing transgenic stocks or additional reagents used in this study. Support for this research was provided by the Howard Hughes Medical Institute, by a Jane Coffin Childs postdoctoral fellowship received by V.P.L., and by grants from the March of Dimes and Pew Charitable Trust to D.T.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Razzell W, Wood W, Martin P. Swatting flies: modelling wound healing and inflammation in Drosophila. Dis Model Mech. 2011;4:569–574. doi: 10.1242/dmm.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramet M, Lanot R, Zachary D, Manfruelli P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev Biol. 2002;241:145–156. doi: 10.1006/dbio.2001.0502. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Wilhelmsson C, Hyrsl P, Loof TG, Dobes P, Klupp M, Loseva O, Morgelin M, Ikle J, Cripps RM, et al. Pathogen entrapment by transglutaminase--a conserved early innate immune mechanism. PLoS Pathog. 2010;6:e1000763. doi: 10.1371/journal.ppat.1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang H. Regulation and function of the melanization reaction in Drosophila. Fly (Austin) 2009;3:105–111. doi: 10.4161/fly.3.1.7747. [DOI] [PubMed] [Google Scholar]
- 6.Abreu-Blanco MT, Verboon JM, Liu R, Watts JJ, Parkhurst SM. Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string. J Cell Sci. 2012;125:5984–5997. doi: 10.1242/jcs.109066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 8.Baek SH, Cho HW, Kwon YC, Lee JH, Kim MJ, Lee H, Choe KM. Requirement for Pak3 in Rac1-induced organization of actin and myosin during Drosophila larval wound healing. FEBS Lett. 2012;586:772–777. doi: 10.1016/j.febslet.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 9.Baek SH, Kwon YC, Lee H, Choe KM. Rho-family small GTPases are required for cell polarization and directional sensing in Drosophila wound healing. Biochem Biophys Res Commun. 2010;394:488–492. doi: 10.1016/j.bbrc.2010.02.124. [DOI] [PubMed] [Google Scholar]
- 10.Lesch C, Jo J, Wu Y, Fish GS, Galko MJ. A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics. 2010;186:943–957. doi: 10.1534/genetics.110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Brock AR, Wang Y, Fujitani K, Ueda R, Galko MJ. A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae. Curr Biol. 2009;19:1473–1477. doi: 10.1016/j.cub.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363–2370. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- 13.Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994;266:819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- 14.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- 15.Pearson JC, Juarez MT, Kim M, Drivenes O, McGinnis W. Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proc Natl Acad Sci U S A. 2009;106:2224–2229. doi: 10.1073/pnas.0810219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ting SB, Caddy J, Hislop N, Wilanowski T, Auden A, Zhao LL, Ellis S, Kaur P, Uchida Y, Holleran WM, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308:411–413. doi: 10.1126/science.1107511. [DOI] [PubMed] [Google Scholar]
- 17.Angel P, Szabowski A, Schorpp-Kistner M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 2001;20:2413–2423. doi: 10.1038/sj.onc.1204380. [DOI] [PubMed] [Google Scholar]
- 18.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2012;70s:2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernascone I, Martin-Belmonte F. Crossroads of Wnt and Hippo in epithelial tissues. Trends Cell Biol. 2013;23:380–389. doi: 10.1016/j.tcb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23:946–953. doi: 10.1016/j.semcdb.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H, Edgar BA. Intestinal stem cells in the adult Drosophila midgut. Exp Cell Res. 2011;317:2780–2788. doi: 10.1016/j.yexcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5:290–297. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worley MI, Setiawan L, Hariharan IK. Regeneration and transdetermination in Drosophila imaginal discs. Annu Rev Genet. 2012;46:289–310. doi: 10.1146/annurev-genet-110711-155637. [DOI] [PubMed] [Google Scholar]
- 26.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–83. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes JJ, Atreya KB, Desai KM, Hall RE, Patel MD, Desai AA, Benham AE, Mable JL, Straessle JL. A dominant negative form of Rac1 affects myogenesis of adult thoracic muscles in Drosophila. Dev Biol. 2005;285:11–27. doi: 10.1016/j.ydbio.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Andersen DS, Colombani J, Leopold P. Coordination of organ growth: principles and outstanding questions from the world of insects. Trends Cell Biol. 2013;23:336–44. doi: 10.1016/j.tcb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Fox DT, Duronio RJ. Endoreplication and polyploidy: insights into development and disease. Development. 2013;140:3–12. doi: 10.1242/dev.080531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar BA, O’Farrell PH. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989;57:177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King RC. Ovarian development in Drosohila melanogaster. New York: Academic Press; 1970. [Google Scholar]
- 32.Tamori Y, Deng WM. Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Dev Cell. 2013;25:350–363. doi: 10.1016/j.devcel.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox DT, Gall JG, Spradling AC. Error-prone polyploid mitosis during normal Drosophila development. Genes Dev. 2010;24:2294–2302. doi: 10.1101/gad.1952710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diril MK, Ratnacaram CK, Padmakumar VC, Du T, Wasser M, Coppola V, Tessarollo L, Kaldis P. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci U S A. 2012;109:3826–3831. doi: 10.1073/pnas.1115201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23:803–811. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Brock AR, Babcock DT, Galko MJ. Active cop, passive cop: developmental stage-specific modes of wound-induced blood cell recruitment in Drosophila. Fly. 2008;2:303–305. doi: 10.4161/fly.7395. [DOI] [PubMed] [Google Scholar]
- 37.Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol. 2013;14:249–262. doi: 10.1038/nrm3541. [DOI] [PubMed] [Google Scholar]
- 38.Adachi S, Minamisawa K, Okushima Y, Inagaki S, Yoshiyama K, Kondou Y, Kaminuma E, Kawashima M, Toyoda T, Matsui M, et al. Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108:10004–10009. doi: 10.1073/pnas.1103584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machlus KR, Italiano JE., Jr The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen MJ, Godenschwege TA, Tanouye MA, Phelan P. Making an escape: development and function of the Drosophila giant fiber system. Semin Cell Dev Biol. 2006;17:31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Parast MM, Aeder S, Sutherland AE. Trophoblast giant-cell differentiation involves changes in cytoskeleton and cell motility. Dev Biol. 2001;230:43–60. doi: 10.1006/dbio.2000.0102. [DOI] [PubMed] [Google Scholar]
- 42.Patel A, Dash PR. Formation of atypical podosomes in extravillous trophoblasts regulates extracellular matrix degradation. Eur J Cell Biol. 2012;91:171–179. doi: 10.1016/j.ejcb.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B, Wang JHC. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J Tissue Viability. 2011;20:108–120. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trepat X, Fredberg JJ. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 2011;2:638–646. doi: 10.1016/j.tcb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 46.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 47.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, Patananan AN, Sitz D, Tran P, Do MT, et al. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods. 2009;6:603–605. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.