Abstract
It has been suggested that clec14a may be involved in tumor angiogenesis. However, a molecular mechanism has not been clearly identified. In this study, we show for the first time that C-type lectin-like domain (CTLD) of clec14a may be important for regulating cell migration and filopodia formation. Using phage display technology, recombinant human antibodies specific to the CTLDs of human and mouse clec14a (clec14a-CTLD (immunoglobulin G) IgG) were selected. Functional assays using the antibodies showed that clec14a-CTLD IgGs specifically blocked endothelial cell migration and tube formation without affecting cell viability or activation. Further, clec14a-CTLD IgGs inhibited clec14a-mediated cell–cell contact by blocking interaction between CTLDs. Finally, clec14a cross-linking by the clec14a-CTLD IgGs significantly downregulated clec14a expression on the surface of endothelial cells. These results strongly suggest that the clec14a-CTLD may be a key domain in angiogenesis, and that clec14a-CTLD IgGs specifically inhibit angiogenesis by modulating CTLD-mediated cell interactions and clec14a expression on the surface of endothelial cells.
Keywords: Clec14a, C-type lectin-like domain, antibody, angiogenesis, endothelial cell, regulation
Introduction
The rapid recent development of recombinant antibody technology has produced ∼30 antibodies approved for human therapy, with more than 270 currently in clinical development for a range of diseases.1, 2 However, conventional antibody selection methods remain time- and labor-intensive and expensive. Traditionally, target protein extracellular regions were used to select antibodies. Consequently, most selected antibodies bind to cells, but are not functional antibodies with therapeutic potential. Owing to recent advances in molecular biology and protein biochemistry, large amounts of information on protein domains and motifs that could link these structures to cell functions are available. Use of a functional domain to select recombinant antibodies may be an effective means of identifying functional antibodies and investigating underlying modes of action.
Tumor angiogenesis has an important role in tumor progression. Vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) are key factors in angiogenesis,3, 4 and targeting angiogenesis is a promising strategy for cancer treatment. The anti-VEGF antibody bevacizumab is used to treat patients with metastatic colorectal cancer, renal cell carcinoma, non-small-cell lung cancer and malignant brain glioma.5, 6 Cetuximab, an anti-EGFR antibody, may inhibit endothelial cell–cell contact and expression of angiogenic factors, such as VEGF, interleukin-8 and basic fibroblast growth factor (bFGF).7, 8, 9, 10 However, because of redundancy of tumor-secreted angiogenic factors, including placental growth factor, angiopoietin, bFGF and hepatocyte growth factor, these drugs generate a resistant phenotype in tumors.11, 12 Identification of new therapeutic targets may provide viable alternatives for treating cancer patients by inhibition of angiogenesis.
Clec14a is a type I transmembrane protein whose extracellular domain consists of a C-type lectin-like domain (CTLD), a series of epidermal growth factor-like domains, and a sushi-like domain. Rho et al.13 reported that clec14a is endothelial cell-specific and may have a key role in cell–cell contact in angiogenesis. Mura et al.14 showed that clec14a is critical for regulating pro-angiogenic phenotypes associated with filopodia formation, cell migration and endothelial tube formation, and identified clec14a as a tumor endothelial cell marker not expressed on the endothelium of normal tissues.
In this study, we identified CTLD functions in cell migration and filopodia formation, key events of angiogenesis. Using phage display technology, we selected recombinant human antibodies against human and mouse clec14a-CTLDs. Functional assays showed that the antibodies specifically inhibited endothelial cell migration and tube formation without affecting viability or activation. We proposed a mechanism of action whereby the antibodies may inhibit angiogenesis by modulating CTLD-mediated cell–cell interaction and downregulating clec14a expression on the surface of endothelial cells. In summary, this study uses human clec14a-CTLD immunoglobulin G (IgGs) to validate CTLD functions in endothelial cells and provides the first insights into the potential of CTLD as a therapeutic angiogenic target during cancer progression.
Results
Clec14a-CTLD may have a key role in cell migration and filopodia formation
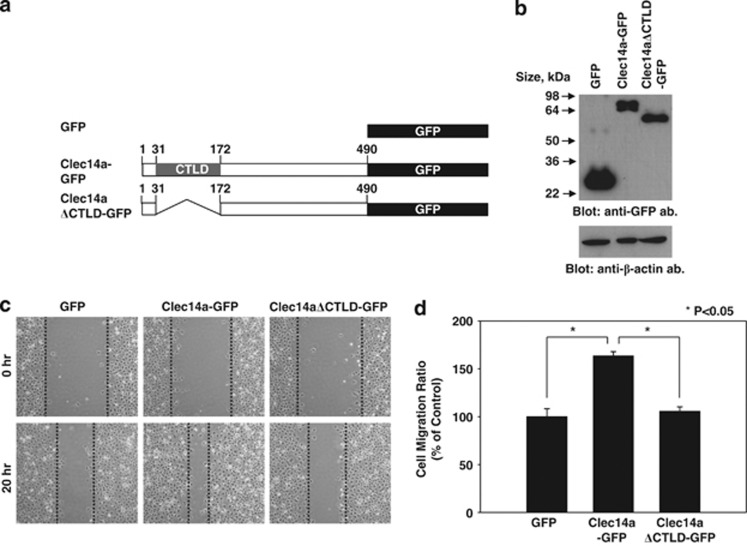
To elucidate the role of CTLD in clec14a-mediated cell migration, we transfected COS-7 cells with green fluorescent protein (GFP), wild-type clec14a fused to GFP (clec14a-GFP) or a clec14a-CTLD deletion mutant fused to GFP (clec14aΔCTLD-GFP) (Figure 1a). After confirming expression of the transfected plasmids with immunoblot analysis (Figure 1b), we assayed migration in a wound healing assay 0 and 20 h. after transfection. The extent of migration of cells expressing clec14a-GFP was ∼1.6-fold greater than that of cells expressing GFP alone. Cells expressing clec14aΔCTLD-GFP displayed only minor changes in migration (Figures 1c and d).
Figure 1.
Effect of clec14a-CTLD in cell migration. (a) Schematic representation of GFP, clec14a-GFP and clec14aΔCTLD-GFP. (b) Lysates of COS-7 cells transfected with GFP, clec14a-GFP or clec14aΔCTLD-GFP were analyzed by immunoblotting with anti-GFP or anti-β-actin antibodies. (c) Migration of COS-7 cells transfected with GFP, clec14a-GFP or clec14aΔCTLD-GFP in the wound healing assay was monitored under a light microscope. Images were captured at 0 h (top) and 20 h (bottom). (d) Distance migrated is expressed as percent of control migration. Values represent mean±s.d. of triplicate measurements from one of three independent experiments.
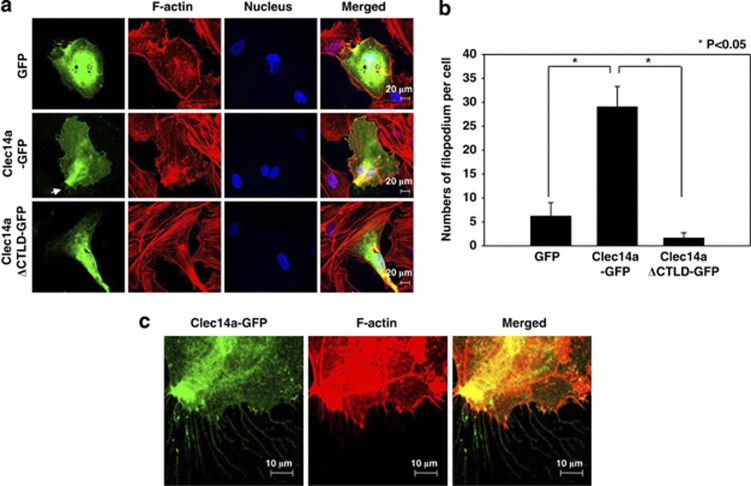
To determine the role of CTLD in filopodia formation in endothelial cells, we transfected human umbilical vein endothelial cells (HUVECs) with GFP, clec14a-GFP, or clec14aΔCTLD-GFP and performed immunocytochemistry. Clec14a-GFP significantly increased filopodia formation, whereas GFP and clec14aΔCTLD-GFP had minimal effect, suggesting that clec14a-CTLD may contribute to filopodia formation in HUVECs (Figures 2a–c). We obtained similar results with COS-7 cells overexpressing GFP, clec14a-GFP or clec14aΔCTLD-GFP, supporting the notion that clec14a-CTLD is critical for promoting filopodia formation (data not shown).
Figure 2.
Effect of clec14a-CTLD on filopodia formation. (a) HUVECs transfected with GFP, clec14a-GFP or clec14aΔCTLD-GFP were fixed, stained with rhodamine–phalloidin and Hoechst, and examined by confocal microscopy ( × 600). (b) The numbers of filopodia per cell were counted and expressed in a bar graph. (c) The magnified images ( × 2.5) represent the regions of filopodia formation and are indicated by arrows in panel a. Results are representative of three independent experiments.
These results suggest that CTLD may have a crucial role in endothelial cell migration and filopodia formation.
Isolation of CTLD-specific single-chain variable fragments
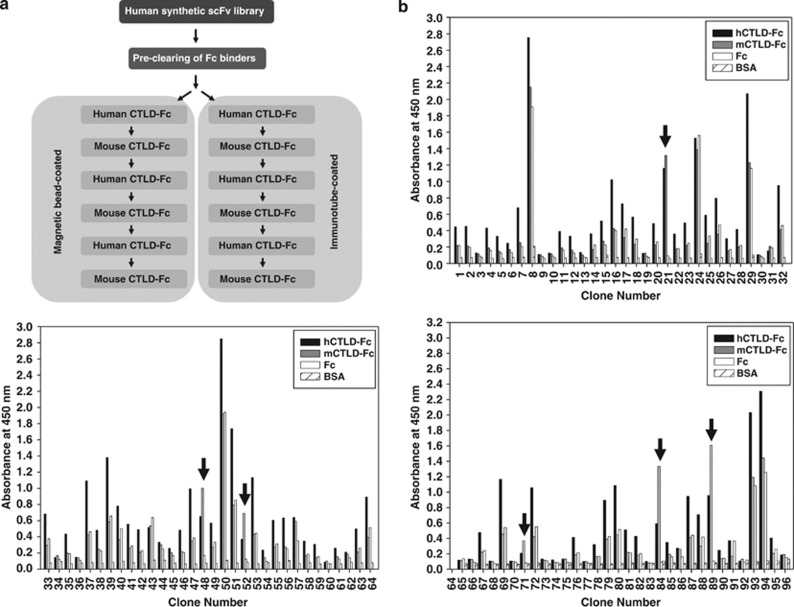
A human synthetic single-chain variable fragments (scFv) library was precleared to remove Fc binders; removal of ∼90% was confirmed by phage enzyme-linked immunosorbent assay (ELISA; data not shown). The library was alternately biopanned with human (hCTLD-Fc) or mouse (mCTLD-Fc) CTLD fusion proteins, using CTLD-Fc-coated immunotubes and magnetic beads, to isolate clones with cross-species CTLD reactivity (Figure 3a); several were identified only with beads (Figures 3b–d). Ninety-six phage clones were randomly selected, rescued by helper phage infection, and tested for reactivity to human and mouse CTLDs in phage enzyme immunoassay. Clone DNA was sequenced and four clones,1, 2, 3, 4 recognizing both human and mouse CTLDs, and with different complementarity-determining-region sequences, were selected (Supplementary Figure 1).
Figure 3.
Isolation of scFv clones specific to human and mouse CTLDs. (a) A human synthetic scFv antibody library was precleared of Fc binders and screened by alternative biopanning with recombinant hCTLD-Fc or mCTLD-Fc. (b) Ninety-six phage clones (1–96) displaying scFv were randomly selected and the supernatants were analyzed by phage ELISA. Reactivity of the selected scFv clones to human and mouse CTLDs was assayed by measuring absorbance at 450 nm. Arrows indicate scFv clones reactive to hCTLD-Fc (▪) and mCTLD-Fc (▪), but not Fc (□). BSA ( ) served as a background control.
) served as a background control.
Clec14a-CTLD IgGs specifically recognized human and mouse clec14a-CTLDs
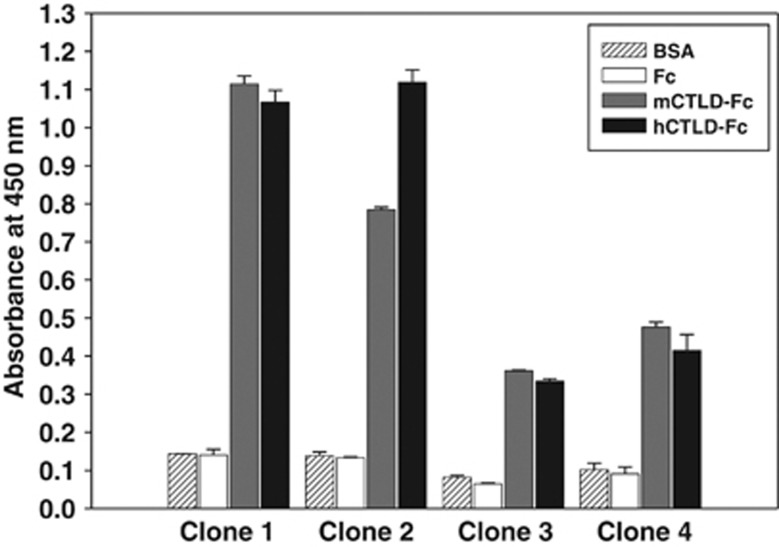
The scFv clones were converted to IgG, expressed in human embryonic kidney (HEK) 293F cells and purified. The four IgG clones were shown to be >90% pure by SDS–polyacrylamide gel electrophoresis and Coomassie staining (data not shown). ELISA showed that the purified CTLD-specific IgGs (clec14a-CTLD IgGs) specifically bound both human and mouse CTLD-Fc, and not Fc alone. Clones 1 and 2 showed much greater affinity than 3 and 4 (Figure 4).
Figure 4.
Cross-species reactivity of clec14a-CTLD IgGs to human and mouse CTLDs. ELISA was performed with purified, selected IgG scFv clones (clones 1–4) on 96-well microtiter plates coated with hCTLD-Fc (▪), mCTLD-Fc (□) and Fc (□). BSA ( ) served as a background control. Values represent mean±s.d. of triplicate measurements from one of two independent experiments.
) served as a background control. Values represent mean±s.d. of triplicate measurements from one of two independent experiments.
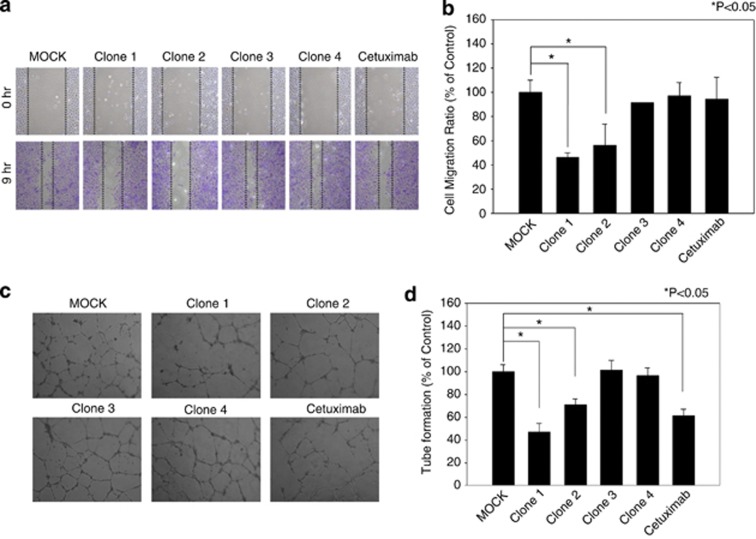
Clec14a-CTLD IgGs specifically suppressed HUVEC cell migration and tube formation
To investigate the inhibitory effect of clec14a-CTLD IgGs on endothelial cell migration, we performed wound healing assays with HUVECs in the absence or presence of clec14a-CTLD IgGs. Cetuximab, an anti-EGFR antibody, was used as control IgG. Of the selected clec14a-CTLD IgGs, clones 1 and 2 significantly suppressed HUVEC cell migration to ∼44% and 54%, respectively, whereas clones 3 and 4, and cetuximab alone had little effect (Figures 5a and b).
Figure 5.
Effect of clec14a-CTLD IgGs on endothelial cell migration and tube formation. (a) After wounding, migration of HUVECs incubated in the absence (MOCK) or presence of clec14a-CTLD IgG (clones 1–4) or cetuximab was monitored by light microscopy. Images were captured at 0 h (top) and 9 h (bottom). (b) Distance migrated is expressed as percent of control (MOCK) migration. Values represent mean±s.d. of triplicate measurements from one of three independent experiments. (c) Tube formation was assayed in the absence (MOCK) or presence of clec14a-CTLD IgG (clones 1–4) or cetuximab. (d) Extent of tube formation is expressed as percent of control (MOCK) tube formation. Values represent mean±s.d. of triplicate measurements from one of three independent experiments.
To determine the effect of clec14a-CTLD IgGs on tube formation, tube formation was assayed in the absence or presence of the clec14a-CTLD IgGs and cetuximab. Clones 1 and 2, and cetuximab10 specifically blocked HUVEC tube formation, whereas 3 and 4 did not have a significant effect (Figures 5c and d), suggesting that clec14a-CTLD IgGs specifically inhibit endothelial cell migration and tube formation.
Effect of Clec14a-CTLD IgGs on endothelial cell viability and activation
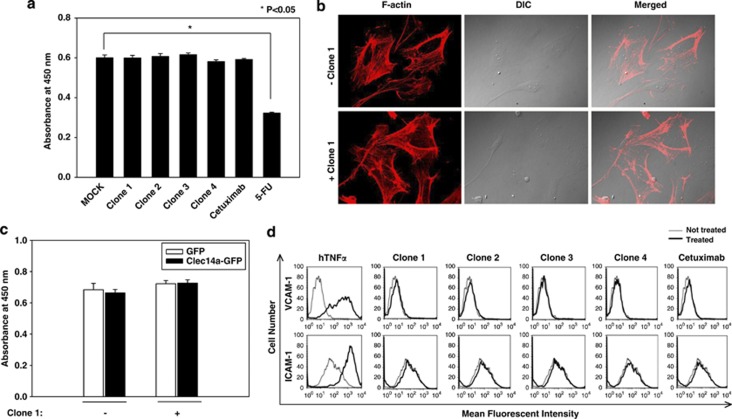
To investigate the effect of clec14a-CTLD IgGs on endothelial cell viability, HUVECs were cultured in the absence or presence of clec14a-CTLD IgGs, cetuximab or 5-fluorouracil (5-FU), an apoptosis inducer, for 2 days, and cell viability was checked using a cell counting kit. The antibodies had little effect on HUVEC viability, whereas 5-FU specifically decreased viability (Figure 6a).
Figure 6.
Effect of clec14a-CTLD IgGs on endothelial cell proliferation and activation. (a) HUVECs were incubated in the absence (MOCK) or presence of clec14a-CTLD IgGs, cetuximab or 5-FU (positive control) for 2 days. Cell viability was assessed by measuring absorbance at 450 nm. Values represent mean±s.d. of triplicate measurements from one of two independent experiments. (b) HUVECs cultured in the absence or presence of clec14a-CTLD IgG (clone 1) were stained with rhodamine–phalloidin and examined by confocal microscopy. (c) COS-7 cells transfected with GFP or clec14a-GFP were incubated in the absence or presence of clec14a-CTLD IgG (clone 1). Cell viability was assessed by measuring absorbance at 450 nm. Values represent the mean±s.d. of triplicate measurements from one of two independent experiments. (d) HUVECs were cultured in the absence (dashed line) or presence (solid line) of hTNFα, clec14a-CTLD IgGs or cetuximab; stained with anti-VCAM-1 (upper) or ICAM-1 (lower) polyclonal antibody and analyzed by flow cytometry. hTNFα served as a positive control for endothelial cell activation. Results are representative of three independent experiments.
To exclude the possibility that the antibodies were toxic, HUVECs were cultured in the absence or presence of clec14a-CTLD IgGs (clone 1), stained with rhodamine–phalloidin, and the cell morphology and F-actin structures were observed using confocal microscopy. No morphological changes to indicate cell death in the presence of the antibodies were observed (Figure 6b).
To further confirm that the effect of clec14a-CTLD IgGs on clec14a-mediated cell viability was not due to antibody toxicity, COS-7 cells overexpressing GFP or clec14a-GFP were cultured in the absence or presence of clec14a-CTLD IgGs (clone 1) for 2 days and cell viability was measured. No changes in the cell viability of COS-7 cells overexpressing GFP or clec14a-GFP were observed in the presence of antibodies (Figure 6c).
To determine the effect of clec14a-CTLD IgGs on endothelial cell activation, HUVECs were cultured in the absence or presence of clec14a-CTLD IgGs, cetuximab, or human tumor necrosis factor alpha (hTNFα). Activation was determined based on the expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular CAM-1 (ICAM-1) measured by flow cytometry. Cells were treated with hTNFα that induces upregulation of VCAM-1 and ICAM-1, as a positive control. The antibodies and cetuximab had no effect on HUVEC activation (Figure 6d), suggesting that clec14a-CTLD IgGs have little effect on endothelial cell viability or activation.
Clec14a-CTLD IgGs specifically inhibited clec14a-mediated cell–cell contact
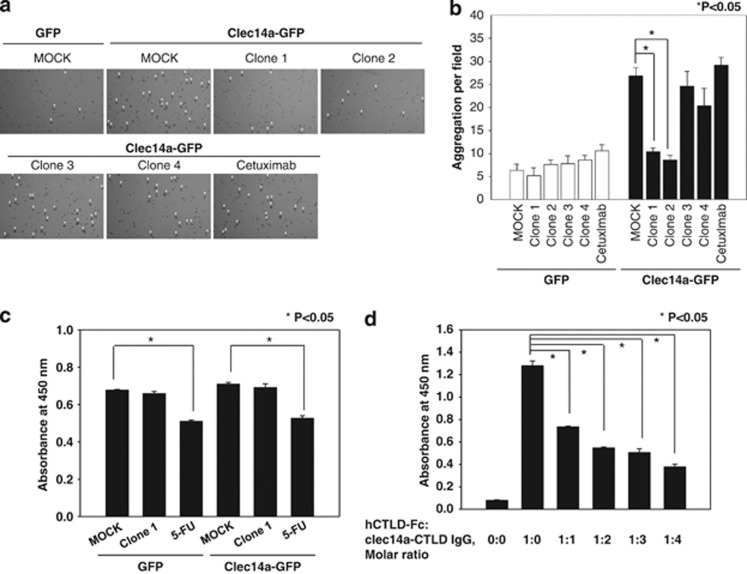
A recent study suggested that clec14a-CTLD is important for endothelial cell–cell contact.13 To elucidate whether clec14a-CTLD IgGs have an inhibitory effect on endothelial cell–cell contact, the number of cell aggregates, an indicator of clec14a-mediated cell–cell contacts,13 formed by HEK293F cells transfected with GFP or clec14a-GFP, and grown in the absence or presence of clec14a-CTLD IgGs or cetuximab was determined. The number of cell aggregates was ∼fourfold greater in cells expressing clec14a-GFP than in those expressing GFP alone (Figures 7a and b). Furthermore, clones 1 and 2 substantially suppressed the aggregation of cells transfected with clec14a-GFP, whereas the other clones and cetuximab had little effect.
Figure 7.
Effect of clec14a-CTLD IgGs on clec14a-mediated cell–cell contact. (a) HEK293F cells transfected with GFP and clec14a-GFP were incubated in the absence (MOCK) or presence of clec14a-CTLD IgGs or cetuximab for 8 h. Cell aggregates (mass >4 cells; arrowheads) were counted under a light microscope. The number of aggregates per field is shown in. b. Values represent mean±s.d. of triplicate measurements from one of three independent experiments. (c) HEK293F cells transfected with GFP and clec14a-GFP were subjected to a cell viability assay with a CCK-8 cell counting kit. (d) Following preincubation of hCTLD-Fc with HUVECs, the extent of hCTLD-Fc binding to HUVECs in the absence or presence of increasing concentrations of clec14a-CTLD IgG (clone 1) was measured by cell ELISA. Values represent the mean±s.d. of triplicate measurements from one of three independent experiments.
To exclude the possibility of antibody toxicity in this assay, we measured the cell viability of HEK293F cells transfected with GFP or clec14a-GFP at the same experimental settings detailed in Figures 7a and b. 5-FU was used as a positive control for cell death. As shown in Figure 7c, clec14a-CTLD IgG had little cytotoxicity in cell–cell contact assays.
To further confirm that clec14a-CTLD IgGs specifically inhibited clec14a-mediated endothelial cell–cell contact, we performed a cell ELISA in the presence or absence of clec14a-CTLD IgGs (clone 1) on HUVECs that had been incubated with hCTLD-Fc. clec14a-CTLD IgGs specifically inhibited hCTLD-Fc binding to HUVECs in a concentration-dependent manner. We also noted that Fc alone was virtually unable to bind HUVECs (data not shown). These results suggest that clec14a-CTLD IgGs have an inhibitory role in clec14a-mediated endothelial cell–cell contact.
Clec14a-CTLD IgG specifically blocked clec14a-CTLD–CTLD interactions
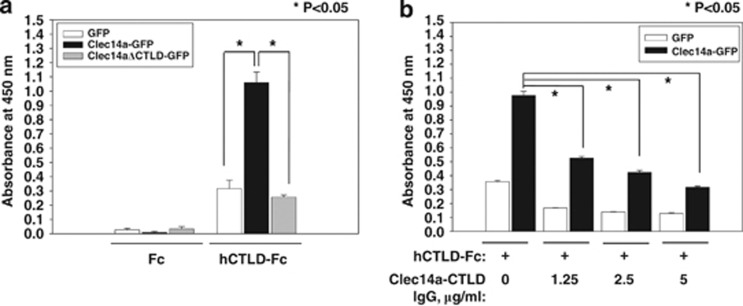
To better understand the interaction between clec14a-CTLDs, we performed ELISAs on COS-7 cells transfected with GFP, clec14a-GFP or clec14aΔCTLD-GFP. Lysates were incubated with hCTLD-Fc or Fc to observe interactions between CTLDs. Although hCTLD-Fc may interact with other proteins in COS-7 cell lysates, it bound strongly to clec14a-GFP but not to clec14aΔCTLD-GFP, suggesting a specific CTLD–CTLD interaction in endothelial cells (Figure 8a).
Figure 8.
Effect of clec14a-CTLD IgG on CTLD–CTLD interaction. (a, b) COS-7 cells expressing GFP (white), clec14a-GFP (black) or clec14aΔCTLD-GFP (light gray) were incubated with 0.15 μg hCTLD-Fc or Fc and CTLD–CTLD interaction was measured by ELISA. Values represent mean±s.d. of triplicate measurements from one of three independent experiments.
To determine if clec14a-CTLD IgG blocks CTLD–CTLD interactions, hCTLD-Fc was incubated with increasing concentrations of clec14a-CTLD IgG (clone 1), and the protein complexes were incubated with lysates of GFP- or clec14a-GFP-transfected cells. Competitive ELISA showed that clec14a-CTLD IgG specifically inhibited CTLD–CTLD interactions in a concentration-dependent manner (Figure 8b), suggesting that clec14a-CTLD IgG may specifically block clec14a-CTLD–CTLD interactions in endothelial cells.
Cross-linking with clec14a-CTLD IgG downregulated clec14a on the surface of HUVECs
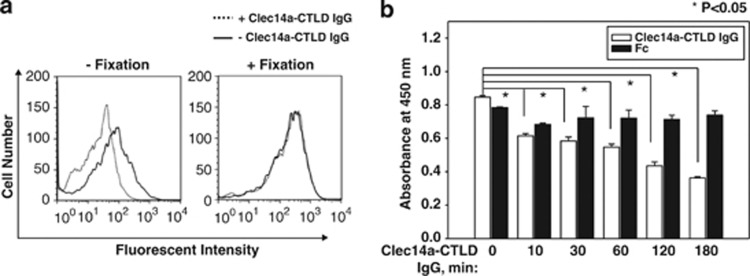
To investigate a possible role of clec14a-CTLD IgG on down-regulating the expression level of clec14a on the surface of HUVECs, we used flow cytometry to compare the level of clec14a expression on HUVEC membranes before and after treatment with clec14a-CTLD IgG (clone 1). IgG treatment significantly reduced clec14a on live, but not paraformaldehyde-fixed, cells (Figure 9a).
Figure 9.
Effect of clec14a-CTLD IgG on downregulation of clec14a on the surface of endothelial cells. (a) Fixed and unfixed HUVECs were incubated in the presence (dotted line) or absence (solid line) of a clec14a-CTLD IgG (clone 1) and analyzed by flow cytometry. (b) Clec14a on the surface of HUVECs incubated with clec14a-CTLD IgG (white) or Fc (black) for the indicated time was assayed by cell ELISA. Values represent mean±s.d. of triplicate measurements from one of three independent experiments.
To confirm downregulation of clec14a on HUVEC membranes by clec14a-CTLD IgG, cells were treated with IgG (clone 1) or Fc, and membrane clec14a was measured by cell ELISA. Clec14a-CTLD IgG downregulated clec14a on HUVEC membranes in a time-dependent manner (Figure 9b). These results strongly suggest that clec14a-CTLD IgG cross-linking of clec14a specifically downregulates clec14a on endothelial cell membranes.
Discussion
Angiogenesis is a hallmark of cancer. Increasing attention is paid to the mechanisms by which tumors promote new blood vessel formation,3, 15 and angiogenesis is a promising target for cancer treatment.5, 6 However, an angiogenic phenotype is complex and results from the coordinated action of many pro- and anti-angiogenic molecules.4 In this study, we developed recombinant human clec14a-CTLD-specific IgGs and evaluated their efficacy and mechanism of action. We propose that clec14a-CTLD may be a key factor in angiogenesis, and clec14a-CTLD-specific antibodies may have therapeutic potential.
Several reports have suggested that clec14a has a key role in angiogenesis.13, 14 However, the molecular mechanisms underlying this function have not been clearly identified. We suggest that clec14a-CTLD may be a unique domain that regulates angiogenesis in a CTLD–CTLD interaction-dependent manner. First, clec14a-CTLD, particularly amino acids 31–172, had a key role in clec14a-mediated cell migration by regulating filopodia dynamics. Second, consistent with our observation that clec14a-CTLD IgG specifically suppresses clec14a-mediated cell–cell contacts, Rho et al.13 demonstrated that CTLD is important for endothelial cell–cell contact. Third, clec14a modulation by clec14a-CTLD IgG specifically inhibited HUVEC migration and tube formation. Finally, formation of clec14a-CTLD–CTLD complexes was specifically inhibited by clec14a-CTLD IgG. In this study, we used GFP fusion proteins to elucidate the specific roles of clec14a-CTLD on angiogenic functions in vitro and thus cannot exclude the possibility that the fusion proteins may not have identical activities to those of wild-type clec14a.
The human VEGF antibody bevacizumab is currently used to treat patients with a variety of cancers.5, 6 However, because the VEGF receptor is also expressed on normal cells, its use is likely to be associated with adverse effects, including hypertension, proteinuria and gastrointestinal perforation.16, 17, 18 Adverse effects may also limit the therapeutic use of many antibodies against pro-angiogenic factors, such as VEGF receptor-2 and angiopoietin-2.19, 20, 21 Consequently, identification of cancer-specific targets is critical for developing therapeutic antibodies with fewer adverse effects.
The human antibodies to clec14a-CTLD we developed in this study recognize human and mouse CTLD. Broad cross-species reactivity is critical for use in preclinical studies to gain an understanding of antibody function and mode of action prior to clinical trials. Although real-time interaction analysis with BIA-CORE 2000 indicated that the KD constant for the interaction between clec14a-CTLD IgG (clone 1 and clone 2) and hCTLD-Fc was ∼1.7 × 10−7 and 6.1 × 10−7, respectively (data not shown), the clec14a-CTLD IgGs specifically inhibited endothelial cell migration and tube formation without affecting cell viability and activation. Recently, others reported that clec14a is expressed exclusively on endothelial cells13 and may be a specific tumor endothelial cell marker.14 Although further optimization such as affinity maturation is needed, it is reasonable to speculate that the clec14a-CTLD antibodies might have fewer adverse effects in normal endothelium, target clec14a expressed exclusively on tumor endothelium and suppress angiogenesis during clec14a-mediated tumor progression.
Bevacizumab is a therapeutic antibody9, 11, 18 that suppresses angiogenesis by inhibiting interaction between soluble VEGF and its receptors. However, long-term use of bevacizumab generates a resistant tumor phenotype due to redundancy of tumor cell-secreted pro-angiogenic growth factors.11, 12 This may pose the greatest challenge to using antibodies against soluble growth factors in patients requiring long-term therapy. Clec14a is a type I transmembrane protein critical for endothelial cell–cell contact13 with a different mechanism of action than bevacizumab. The clec14a-CTLD IgG developed in this study appears to have dual mechanisms of action to suppress angiogenic properties in vitro. It specifically blocked CTLD–CTLD interaction in a concentration-dependent manner and clec14a cross-linking by IgG induced clec14a downregulation on endothelial cell membranes. Within 2 h of cross-linking, clec14a-CTLD IgG appeared in a dot-like pattern inside HUVECs (data not shown), suggesting antibody-induced endocytosis. Although this study focused on identifying the mechanisms underlying clec14a-CTLD IgG as a blockade of clec14a in angiogenesis, we cannot exclude the possibility that endogenous clec14a ligands exist and other CTLD-binding partners may be involved in clec14a-mediated angiogenesis.
On the basis of the currently available evidence, we propose a mode of action for clec14a-CTLD in endothelial cells during angiogenesis. The clec14a-CTLD domain is critical for regulation of filopodia dynamics in migrating endothelial cells and contributes to endothelial cell–cell contact via CTLD–CTLD interaction. Furthermore, clec14a-CTLD IgGs specifically inhibit pro-angiogenic activities such as endothelial cell migration, cell–cell contact and tube formation by simultaneously blocking CTLD–CTLD interaction and inducing clec14a downregulation on the surface of endothelial cells. Although the current study is based on in vitro results, in near future we plan to test this hypothesis by examining the in vivo functional relevance of clec14a-CTLD using optimized clec14a-CTLD IgGs.
Materials and methods
Cell culture and transfection
HUVECs (Lonza, Walkersville, MD, USA) were maintained in endothelial growth medium-2. COS-7 cells were grown in Dulbecco's modified Eagle medium containing 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin. Cells were maintained in a humidified, CO2-controlled incubator (Sanyo, Panasonic Healthcare Company, Secaucus, NJ, USA) at 37 °C and 5% CO2. HEK293F cells were maintained in Freestyle 293 expression media (Invitrogen, Carlsbad, CA, USA) supplemented with 1% (v/v) penicillin/streptomycin in a humidified Multitron incubation shaker (Infors HT, Bottmingen, Switzerland) at 37 °C and 8% CO2. HUVECs and COS-7 cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Wound healing and cell migration assay
A CytoSelect 24-well wound healing assay kit (Cell Biolabs Inc., San Diego, CA, USA) was used according to the manufacturer's instructions. Briefly, COS-7 cells transfected with GFP, clec14a-GFP, or clec14aΔCTLD-GFP were added to each well of the wound healing insert. When cells were confluent, inserts were removed from the wells, and cells were washed twice with phosphate-buffered saline (PBS). Images were captured 20 h after wounding.
To analyze endothelial cell migration, HUVECs cultured in the insert until monolayer formation were incubated in the absence or presence of 20 μg/ml clec14a-CTLD IgG or cetuximab for 9 h at 37 °C. Cells were washed twice with PBS and stained with crystal violet (Sigma, St Louis, MO, USA). For quantitative analysis, five fields per plate were photographed under a light microscope (Nikon TS 100, Melville, NY, USA), and distance migrated was measured manually.
Immunocytochemistry
Immunocytochemistry was performed as described previously.22 To measure filopodia formation, HUVECs were grown on collagen-coated coverslips for 1 day and transfected with GFP, clec14a-GFP or clec14aΔCTLD-GFP. To measure cell morphology, HUVECs were incubated in the absence or presence of 20 μg/ml clec14a-CTLD IgG (clone 1) for 1 day. Following fixation with 4% (w/v) paraformaldehyde for 30 min at 37 °C, cells were washed twice with PBS and blocked by incubation in PBS containing 5% (w/v) bovine serum albumin (BSA) and 0.1% Triton X-100 for 1 h at 37 °C. Cells were incubated with 0.1 unit/well rhodamine–phalloidin and/or 2 μg/ml Hoechst for 1 h, and viewed under a confocal microscope (Olympus LX80 FV300, Olympus, Tokyo, Japan).
Construction and preparation of human and mouse CTLD Fc fusion proteins
DNA encoding amino acid residues 31–172 of hCTLD was amplified using primers 5′-TCGCGCGGCCGCTGCTCGGCCTCGGGGGCCTGC-3′ and 5′-TCGCCTCGAGC TTGCACAGGTAGCCGTTGG-3′. DNA encoding the same residues of mCTLD was amplified using primers 5′-TCGCGGCCCAGGCGGCCTGTTCGGCCTCGGGG GCTTG-3′ and 5′-TCGCGGCCGGCCTGGCCCTTGCATAGGTAGCCATCGG-3′. PCR fragments were digested with SfiI (NEB, Ipswich, MA, USA) and cloned into the modified mammalian expression vector pCEP4 (Invitrogen) encoding the hinge and CH2–CH3 domain of human IgG1 in the 3′ region of the cloning site. Ligated products were transformed into competent Escherichia coli DH5α cells and plasmid DNA was prepared.23 HEK293F cells (6 × 108 cells) were transfected with 0.75 mg DNA each using 1.5 mg polyethylenimine (Polysciences, Inc., Warrington, PA, USA). After 7 days in culture, culture medium was collected and fusion proteins purified by affinity chromatography on protein A Sepharose (RepliGen, Waltham, MA, USA).24 Protein concentration was quantified using a NanoDrop spectrophotometer (Thermo, Wilmington, DE, USA). Samples were dialyzed against PBS and analyzed by SDS–polyacrylamide gel electrophoresis and Coomassie brilliant blue staining.25 Aliquots of the final pooled fraction were stored at −80 °C.
Preclearing of human synthetic scFv library
A human synthetic scFv library was re-amplified.26 To preclear Fc binders, human immunoglobulin-G (Green Cross Corp., Yong-in, South Korea) was immobilized on protein A Sepharose and the antibody–protein A complexes were incubated with the library at 37 °C for 2 h. Following brief centrifugation, the supernatant was collected and the pellet discarded, and this procedure was repeated twice.
Selection of CTLD-specific scFvs using phage display
Three rounds of biopanning were carried out with immunotubes (Immunotube maxisorp, Nunc, Rochester, NY, USA) or magnetic beads (Dynabeads M-270 epoxy, Invitrogen) coated with 4 μg recombinant hCTLD-Fc or mCTLD-Fc to select clones with cross-species reactivity, as described previously.26, 27 Ninety-six phage clones were randomly selected from colonies grown on output plates and tested for reactivity to human and mouse CTLDs by phage enzyme immunoassay.28 DNA of the final scFv clones was sequenced and classified as four scFv clones with different complementarity-determining-region sequences.
Preparation of clec14a-CTLD IgG
The variable heavy chain gene of selected scFv clones (clones 1–4) was amplified using the primers 5′-CGGGAATTCGCCGCCACCATGGAATGGAGCTGGGTCTTTCTCTTCTTCCTGCTGTCAGTAACTACAGGTGTCCTCTCCGAGGTGCAGCTGTTGGAGTCTG-3′ and 5′-GGCGGGCCCTTG GTGGAGGCTGAGCTCACGGTGACCAGTGCCCTTGGCCCC-3′. The variable light chain gene of the clones was amplified using the primers 5′-CCCAAGCTTGCCGCCACCATGGAGACACATTCTCAGGTCTTTGTATACATGTTGCT GTGGTTGTCTGGTGTTGAAGGACCAGTCTGTGCTGACTCAGCC-3′ and 5′-GGCCGTACGTAGGACCGTCAGCTTGGTGCCTCCGCCTAAGACATAACCACC-3′. The variable heavy chain primers were designed to add EcoRI and ApaI restriction sites to both the 5′ and 3′ ends. The variable light chain primers were designed to add HindIII and BsiWI sites to both the 5′ and 3′ ends. PCR fragments were digested with the appropriate restriction enzymes (NEB) and cloned into the bicistronic mammalian expression vector pCDNA3.1 (Invitrogen), encoding the hinge and CH2–CH3 domain of human IgG1 3′ of the variable heavy chain cloning site.23 Clec14a-CTLD IgG was produced and purified as described previously.29
ELISA
Recombinant hCTLD-Fc, mCTLD-Fc or Fc fragment of IgG1 (25 nM) in PBS was added to 96-well plates. Plates were incubated overnight at 37 °C, washed three times with PBS containing 0.05% (v/v) Tween 20 (PBST), and incubated with 3% (w/v) BSA in PBST for 1 h at 37 °C. Plates were incubated with 67 nM clec14a-CTLD IgG in PBST containing 3% (w/v) BSA for 3 h at 37 °C. Following two times washings with PBST, horseradish peroxide (HRP)-conjugated anti-human lambda light chain antibody (1:1000; Bethyl Laboratories, Montgomery, TX, USA) in PBST containing 3% (w/v) BSA was incubated for 1 h at 37 °C.
To measure CTLD–CTLD interaction, lysates (20 μg) of COS-7 cells transfected with GFP, clec14a-GFP or clec14aΔCTLD-GFP in 96-well plates were preincubated with 0.15 μg of hCTLD-Fc or Fc in 3% (w/v) BSA in PBST for 2 h at 37 °C. To assay competition with clec14a-CTLD IgG, the increasing concentrations of clec14a-CTLD IgG in PBST containing 3% (w/v) BSA was added to the wells for 2 h at 37 °C. Then, HRP-conjugated donkey anti-human Fc IgG (1:5000; Jackson ImmunoResearch, West Grove, PA, USA) in PBST containing 3% (w/v) BSA was incubated for 1 h at 37 °C. A concentration of 100 μl 3,3′,5,5′-Tetramethylbenzidine substrate solution (BD Biosciences, San Jose, CA, USA) were added to each well. Optical density was measured at 450 nM using a microtiter plate reader (VICTOR X4, Perkin Elmer, Waltham, MA, USA).
Flow cytometry
HUVECs (3 × 105) grown in six-well plates were incubated in the absence or presence of 20 ng/ml hTNFα (Millipore, Billerica, MA, USA), or 20 μg/ml clec14a-CTLD IgG or cetuximab for 24 h. Cells were harvested and stained with 20 μg/ml anti-VCAM-1 or ICAM-1 polyclonal antibody in flow cytometry buffer for 1 h at 37 °C. Cells were washed three times with flow cytometry buffer and incubated for 1 h at 37 °C with Alexa Fluor 488-labeled anti-rabbit antibody (1:1000; Jackson ImmunoResearch) in flow cytometry buffer.
HUVECs (3 × 105) grown in six-well plates were fixed with 4% (w/v) paraformaldehyde. Fixed and unfixed cells were washed twice with PBS, and incubated in the absence or presence of 20 μg/ml clec14a-CTLD IgG for 2 h at 37 °C. Cells were stained with 7.5 μg/ml sheep anti-clec14a polyclonal antibody (R&D Systems, Minneapolis, MN, USA) for 2 h at 37 °C. Cells were washed three times with flow cytometry buffer, incubated with Northern Lights 493 Fluorochrome (R&D Systems) (NL493)-labeled anti-sheep antibody (1:200; R&D Systems) in flow cytometry buffer, and analyzed by flow cytometry (BD FACSCalibur, BD Biosciences, Miami, FL, USA).
Tube formation
Tube formation assays were performed as described previously13 and counted manually. Briefly, 250 μl Matrigel (BD Biosciences) was added to 24-well plates and allowed to polymerize for 20 min at 37 °C. HUVECs cultured in endothelial growth medium-2 were harvested, resuspended in endothelial growth medium-2 and seeded onto the Matrigel (1 × 105 cells/well). Cultures were incubated in the absence or presence of 20 μg/ml clec14a-CTLD IgGs or cetuximab at 37 °C and photographed at 21 h.
Cell viability assay
HUVECs (104) were seeded in 96-well plates and incubated with 20 μg/ml clec14a-CTLD IgGs, cetuximab, or 5-FU (Sigma) for 2 days at 37 °C. COS-7 cells (5 × 103) overexpressing GFP or clec14a-GFP were cultured in 96-well plates and incubated in the absence or presence of 20 μg/ml clec14a-CTLD IgG (clone 1) for 2 days at 37 °C. 1 × 107 HEK293F cells overexpressing GFP or clec14a-GFP were cultured in Freestyle 293 expression medium overnight and seeded in 96-well plates (1 × 104 cells/well). Cells were maintained in the absence or presence of 20 μg/ml clec14a-CTLD IgG or 5-FU for 8 h. Cell viability was measured using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to manufacturer's instructions. Absorbance was measured at 450 nm with a VICTOR X4 spectrophotometer (Perkin Elmer).
Measurement of cell–cell contact
Cell–cell contact assays were performed as described previously.13 Briefly, 1.5 × 107 HEK293F cells in suspension were transfected with plasmids expressing GFP, clec14a-GFP and clec14aΔCTLD-GFP, cultured in Freestyle 293 expression medium overnight and seeded in six-well plates (5 × 105 cells/well). Cells were maintained in the absence or presence of 20 μg/ml clec14a-CTLD IgG or cetuximab for 8 h. Cell aggregates (mass >4 cells) were counted in at least 10 fields and mean±s.d. were determined.
Immunoblot analysis
Immunoblot analysis were performed as described previously.25 Briefly, proteins were separated by electrophoresis in a 12% polyacrylamide gel and transferred to nitrocellulose membranes using a wet transfer system (GE Healthcare Life Sciences, Piscataway, NJ, USA). After blocking in tris-buffered saline and tween 20 (TTBS) buffer (10 mM Tris/HCl, pH 7.5, 150 mM NaCl, and 0.05 (v/v)% Tween 20) containing 5% (w/v) skimmed milk, the membranes were incubated with anti-GFP monoclonal antibody (1:5000; Santa Cruz Biotechnology, Dallas, TX, USA) or anti-β-actin monoclonal antibody (1:5000; Applied Biological Materials, Richmond, BC, Canada) overnight at 4 °C. The membrane was washed with TTBS and incubated with HRP-conjugated Affinipure goat anti-mouse IgG (1:5000; Jackson ImmunoResearch) at room temperature for 1 h. Following several washes with TTBS, protein bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA) according to manufacturer's instructions.
Cell ELISA
For the competition assay with clec14a-CTLD IgG, HUVECs (104) grown in 96-well plates were preincubated with 0.66 μg of hCTLD-Fc and incubated in the absence or presence of increasing concentrations of clec14a-CTLD IgG (clone 1). Following three washes with PBS, bound hCTLD-Fc was measured using HRP-conjugated donkey anti-human Fc IgG (1:5000; Jackson ImmunoResearch).
To measure clec14a downregulation in HUVECs, HUVECs (104) plated on 96-well plates were incubated with 20 μg/ml clec14a-CTLD IgG or Fc for 0, 10, 30, 60, 120, or 180 min at 37 °C. Cells were washed twice with ice-cold PBS, blocked in 3% (w/v) BSA in PBS for 1 h at 4 °C, and incubated with sheep anti-clec14a polyclonal antibody (1:1000) in the blocking solution for 2 h at 4 °C. Then, cells were incubated with HRP-conjugated anti-sheep IgG (1:5000; Santa Cruz Biotechnology) for 1 h at 4 °C. After several washes with PBS, 100 μl 3,3′,5,5′-Tetramethylbenzidine substrate solution was added to each well. Optical density was measured at 450 nm using a microtiter plate reader.
Acknowledgments
This work was supported by grants from Scripps Korea Antibody Institute and partly by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012R1A2A1A01002916).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- Teillaud JL. From whole monoclonal antibodies to single domain antibodies: think small. Methods Mol Biol. 2012;911:3–13. doi: 10.1007/978-1-61779-968-6_1. [DOI] [PubMed] [Google Scholar]
- Zilian O. Scope for innovation in immunotherapy from the financial market's point of view: Phacilitate Immunotherapy Leaders' Forum. Hum Vaccin Immunother. 2012;2012;8:1370–1372. doi: 10.4161/hv.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora G, Melisi D, Ciardiello F. Angiogenesis: a target for cancer therapy. Curr Pharm Des. 2004;10:11–26. doi: 10.2174/1381612043453595. [DOI] [PubMed] [Google Scholar]
- Bergsland E, Dickler MN. Maximizing the potential of bevacizumab in cancer treatment. Oncologist. 2004;9 (Suppl 1:36–42. doi: 10.1634/theoncologist.9-suppl_1-36. [DOI] [PubMed] [Google Scholar]
- Dienstmann R, Markman B, Tabernero J. Application of monoclonal antibodies as cancer therapy in solid tumors. Curr Clin Pharmacol. 2012;7:137–145. doi: 10.2174/157488412800228929. [DOI] [PubMed] [Google Scholar]
- Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- Vincenzi B, Schiavon G, Silletta M, Santini D, Tonini G. The biological properties of cetuximab. Crit Rev Oncol Hematol. 2008;68:93–106. doi: 10.1016/j.critrevonc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Huang SM, Li J, Harari PM. Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol Cancer Ther. 2002;1:507–514. [PubMed] [Google Scholar]
- Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- Rho SS, Choi HJ, Min JK, Lee HW, Park H, Kim YM, et al. Clec14a is specifically expressed in endothelial cells and mediates cell to cell adhesion. Biochem Biophys Res Commun. 2011;404:103–108. doi: 10.1016/j.bbrc.2010.11.075. [DOI] [PubMed] [Google Scholar]
- Mura M, Swain RK, Zhuang X, Vorschmitt H, Reynolds G, Durant S, et al. Identification and angiogenic role of the novel tumor endothelial marker CLEC14A. Oncogene. 2012;31:293–305. doi: 10.1038/onc.2011.233. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Gordon MS, Margolin K, Talpaz M, Sledge GW, Jr., Holmgren E, Benjamin R, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Leow CC, Coffman K, Inigo I, Breen S, Czapiga M, Soukharev S, et al. MEDI3617, a human anti-angiopoietin 2 monoclonal antibody, inhibits angiogenesis and tumor growth in human tumor xenograft models. Int J Oncol. 2012;40:1321–1330. doi: 10.3892/ijo.2012.1366. [DOI] [PubMed] [Google Scholar]
- Lee SH. Tanibirumab (TTAC-0001): a fully human monoclonal antibody targets vascular endothelial growth factor receptor 2 (VEGFR-2) Arch Pharm Res. 2011;34:1223–1226. doi: 10.1007/s12272-011-0821-9. [DOI] [PubMed] [Google Scholar]
- Spratlin J. Ramucirumab (IMC-1121B): Monoclonal antibody inhibition of vascular endothelial growth factor receptor-2. Curr Oncol Rep. 2011;13:97–102. doi: 10.1007/s11912-010-0149-5. [DOI] [PubMed] [Google Scholar]
- Lee S, Chung J, Ha IS, Yi K, Lee JE, Kang HG, et al. Hydrogen peroxide increases human leukocyte adhesion to porcine aortic endothelial cells via NFkappaB-dependent up-regulation of VCAM-1. Int Immunol. 2007;19:1349–1359. doi: 10.1093/intimm/dxm104. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW.Molecular cloning: a laboratory manual3rd ed.Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY; 2001 [Google Scholar]
- Shukla AA, Hubbard B, Tressel T, Guhan S, Low D. Downstream processing of monoclonal antibodies—application of platform approaches. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:28–39. doi: 10.1016/j.jchromb.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Van Meter KE, Stuart MK. A monoclonal antibody that inhibits translation in Sf21 cell lysates is specific for glyceraldehyde-3-phosphate dehydrogenase. Arch Insect Biochem Physiol. 2008;69:107–117. doi: 10.1002/arch.20271. [DOI] [PubMed] [Google Scholar]
- Barbas CF. Phage display: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY; 2001. [Google Scholar]
- Vestweber D. Lymphocyte trafficking through blood and lymphatic vessels: more than just selectins, chemokines and integrins. Eur J Immunol. 2003;33:1361–1364. doi: 10.1002/eji.200324011. [DOI] [PubMed] [Google Scholar]
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- Kim HY, Tsai S, Lo SC, Wear DJ, Izadjoo MJ. Production and characterization of chimeric monoclonal antibodies against Burkholderia pseudomallei and B. mallei using the DHFR expression system. PLoS One. 2011;6:e19867. doi: 10.1371/journal.pone.0019867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.